
Deep vein thrombosis after arthroplasty: Nanjing deep vein thrombosis study
Introduction
Deep vein thrombosis (DVT) remains a life-threatening complication of arthroplasty, despite the development of preventive measures. DVT is caused by both acquired and genetic risk factors. The identification of persons at high risk of DVT clearly aids prevention of the same. Age, sex, pregnancy, hormone therapy, an increase in body-mass index (BMI), history of thromboembolism, and surgery are risk factors for DVT (1). Despite effective prophylaxis, subclinical DVT develops soon after surgery in 15% to 20% of patients who undergo total hip replacement (THR) (2); symptomatic venous thromboembolism develops within 3 months after surgery in 2% to 4% of patients (3). The incidence of venographically detected DVT in Asian patients undergoing major orthopedic surgery without pharmacological prophylaxis is as high as 50% after hip fracture surgery (4) and ranged from 9.8% to 64.3% after THR (4-8).
Multi-center clinical trials have been conducted during the past few years. Separated into single centers, subjects with limited cases were included (9-11). Several family and twin studies indicated that genetic factor accounts for 60% of DVT risk (12). A number of genetic variants were shown to increase susceptibility to DVT. However, only FVL, PT, MTHFR and CYP4V2 seem consistently associated with DVT (12-14).
Calf vein thrombosis accounts for the great proportion of DVT after arthroplasty and most of the DVT cases are asymptomatic. As indicated, contrast venography (CV) is the gold standard technique for the diagnosis of DVT, while compression ultrasound with venous imaging (real-time B-mode imaging) has been proved to be accurate for diagnosing only acute, symptomatic proximal DVT. Therefore, the anti-coagulation effects of referred drugs confirmed by multi-center clinical trials need to be examined (9-11). Similar conditions were shown in the genetic study of DVT (12-14).
We examined all patients who underwent joint surgery by venography and detailed potential clinical risks. Studies were conducted to determine whether clinical risk factors and/or genetic variants might predict the development of DVT and find efficient anticoagulation strategies.
Methods
Participants
From January 2007 to April 2010, 908 subjects of Nanjing DVT study (NJDVTS) received CV after hip or knee surgery. Revision hip replacement, total knee replacement, revision knee replacement, semi-hip replacement and cemented THR were excluded. A total of 328 subjects (215 women and 113 men) who underwent uncemented total hip arthroplasty (THA) using lateral approach performed by two skilled orthopedic surgeons were studied. The prostheses are from the same company. All the subjects were enrolled consecutively at Center of Diagnosis and Treatment for Joint Disease, Drum Tower Hospital Affiliated to Medical School of Nanjing University. All subjects included in the study were Han Chinese living in and around Nanjing. No subjects dropped out during the study. The study was approved by the ethical committee of Medical School of Nanjing University, and informed consent was obtained from the subjects included.
Clinical management and assessment of DVT
In the anticoagulation group, patients received 0.3 mL (38 International factor Xa inhibitory units per kilogram of body weight) of low-molecular-weight heparin from the same company subcutaneously once daily. The dosage level used as prophylaxis is considered to be moderate. The first injection was given 12 to 16 hours before surgery if there was no clinically evident bleeding. Prophylaxis was continued until venography was performed. All the patients were examined by CV 3–5 days after operation and diagnosed by three experienced doctors. Five patients had CV performed during surgery with and without retractors. DVT was diagnosed according to the Robinov group’s criterion (15). If DVT was detected, conventional thrombolysis treatment was to be started. If not, patients would not receive any further anticoagulation treatment.
Measurements
Age, sex, DVT related history, diabetes mellitus (DM), hypertension, cancer, hormone therapy, drug, and smoking history were recorded. We measured clinical and biochemical data (weight, height, ABO blood type, PT, APTT, INR, fibrinogen, RBC, PLT, D-dimmer, triglyceride, cholesterol), surgery-related data (blood loss, duration of surgery, EF, Hb, drainage, symptoms of DVT). The duration of surgery, blood loss, anesthesia and drainage all were described.
After discharge, patients were followed for up to 3 months after surgery. All subjects received physiotherapy from the same physiotherapist. CV was performed for the patients without DVT in hospital to assess the late onset of DVT.
Genotyping
We checked two mutations (FVL, PT) in DVT subjects and association of two SNPs (rs1801133 of MTHFR, rs13146272 of CYP4V2) with DVT by a case-control study.
DNA was obtained from all the subjects from peripheral blood using the Chelex-100 method (16) or buccal swabs using the DNA IQ System (Promega, Madison, WI, USA) according to manufacturer’s instructions. The SNP rs1801133 and rs13146272 were genotyped using Taqman assay (Applied Biosystems 7500, ABI, Foster City, CA, USA) and the two mutations were detected by sequencing. Genotyping and sequencing were performed by laboratory personnel who were not informed of the case status, and two authors independently reviewed the genotyping results, data entry and statistical analyses.
Statistical analysis
For the comparison of independent factors between the two groups, t-test was used. For the evaluation of predicting ability of each covariate and genetic factor, logistic regression analysis was performed. We also used logistic regression analysis with variable selection for obtaining the prediction model of DVT. In a logistic regression analysis, the risk score S for a patient is represented as,
where β0 is the constant, βi is the regression coefficient of each predictive variable Xi, and number of predictive variables is p. The variables which showed a significant P value in univariate analysis were used in the variable selection. The forward selection method with Akaike Information Criteria (AIC) was used for the detection of the prediction model. The risk score S was used to calculate probability of developing DVT, P(x). Given the risk score S, the P(x) is given as 1/(1+e−s). For the evaluation of prediction, ROC curve and area under curve (AUC) was used. The statistical analysis was performed entirely by statistical software R (17) and ROCR package (18).
Results
The demographic of the studied population was shown in Table 1.
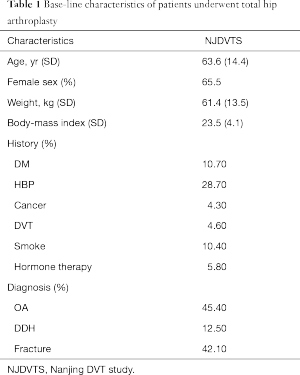
Full table
In total, 31.7% (104/328) of the patients (33.5% and 28.3% in female and male subjects) developed DVT. Central DVT accounted for 9.6% (10/104). The incidence of subjects with an age of over 60 was 34.5% (67/194), while the remainder was 27.6% (37/134). When stratified by diagnosis, the incidence of patients with osteoarthritis, development dysplasia of the hip and fracture of femoral neck was 28.9% (43/149), 19.5% (8/41), and 38.4% (53/138), respectively. Stratified by blood type, incidence of patients with blood type A, B, AB, O was 32.6% (29/89), 33.7% (32/95), 39.4% (13/33), 27.0% (30/111). Smoking patients had an incidence of 35.3% (12/34), while for non-smoking subjects this was 31.3% (92/294).
By univariate analysis, greater age, increased duration of surgery, increased blood red cell count after operation, and symptoms after operation were independent predictors of DVT. The significant level was set to 0.05 (Table 2).
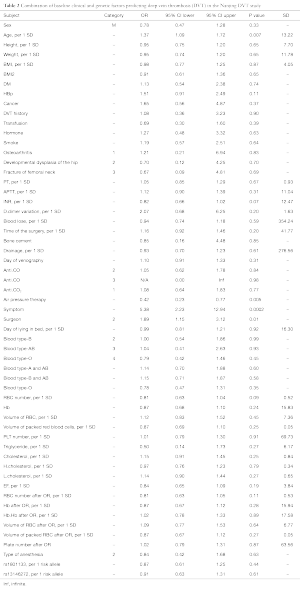
Full table
Models for personal factors (gender, age, height, weight), demographic factors (age, sex, weight, height, DVT related history, DM, hypertension, cancer, hormone, drug, and smoke history), biochemical factors [ABO blood type, PT, APTT, INR, fibrinogen, red blood cell (RBC), PLT, D-dimmer, triglyceride, cholesterol] and surgery related factors [blood loss, duration of surgery, left ventricular ejection fraction (EF), Hb, drainage, symptoms of DVT] were constructed for the risk factor of DVT. Age, surgeries with longer duration and symptoms of DVT were strong signs of DVT and mechanical compression was a good inhibitor of DVT (Table 2). We used these covariates to obtain the prediction equation of DVT. The risk score for the prediction was as follows,
All of the significant covariates were selected in the prediction model. The ROC curves for estimated probability P(x) are show in Figure 1A. The AUC is the measure of the accuracy of the prediction. The AUC of DVT versus the non-DVT group was 0.66, suggesting this prediction model was applicable. The sensitivity and specificity curves were shown in Figure 1B. We determined an optimal cutoff value of P(x) as 0.28 from these curves.

No statistical difference was found for the incidence of DVT in anticoagulation (32.3%, 76/235) and un-anticoagulation groups (30.1%, 28/93). No significant difference was detected for drainage between anticoagulation (329.4±239.1 mL) and un-anticoagulation groups (297.9±232.6 mL) (P=0.28). Blood lose in the anticoagulation group (472.6±412.4 mL) was more than in the un-anticoagulation group (418.8±288.0 mL) (P=0.26). The value of Hb, number of plate and RBC after operation was similar in the two groups (all P>0.05).
All THA were performed by two skilled orthopedic surgeons (A and B groups). Significant differences were detected for the incidences of the DVT [group A, 25.3% (46/182); group B, 39.7% (58/146)] (P=0.049). In group A, with anticoagulation regimen (27.2%, 28/103), no significant incidence decrease was detected compared with un-anticoagulation group (22.8%, 18/79). However, in group B, a significant decrease was detected in the anticoagulation group (38.0%, 46/121) as compared to the non-anticoagulation group (48.0%, 12/25). Significant difference was detected in duration of surgery between the two groups (group A, 95.9±33.6 min; group B, 126.7±43.6 min, P<0.001). And in group A, greater height and H-cholesterol were included (P=0.01 and 0.04, respectively). Lower RBC number after operation was detected in group A (P=0.039). No significant difference was detected in other recorded factors (age, Hb, PT, APTT, INR, fibrinogen, RBC, PLT, D-dimmer, triglyceride, cholesterol) (all P>0.05).
We compared triglyceride, cholesterol, H-cholesterol, L-cholesterol in DVT and non-DVT groups. The concentration of triglyceride was much higher in the DVT group (2.28±8.90 mmol/L) than in the non-DVT group (1.43±0.97 mmol/L). The concentrations of cholesterol (DVT group, 4.53±0.85 mmol/L; non-DVT group, 4.46±0.87 mmol/L), H-cholesterol (DVT group, 1.26±0.32 mmol/L; non-DVT group, 1.27±0.35 mmol/L), and L-cholesterol (DVT group, 2.48±0.66 mmol/L; non-DVT group, 2.41±0.67 mmol/L) were similar between the two groups. No significant results were found for all hyperlipoidemia-related factors (all P>0.05).
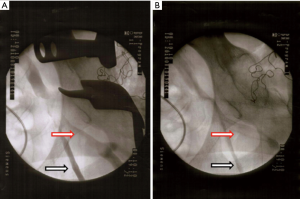
By CV during the operation, iliaca externa vein stenosis was detected when the retractors were placed around acetabulum compared with CV after operation. The radiopaque contrast medium was stagnated around the stenosis (Figure 2).
All the patients were followed for up to 3 months. Non-DVT patients (n=42, 18.8% of all non-DVT patients) in hospital received second CV. Late onset DVT was not detected in any of these patients.
Common variants in four genes were genotyped in all the cases. No mutation was detected in any of the subjects. In the case-control study, no significant association was detected (P>0.05). When we stratified by age, gender, type of DVT, surgeons, BMI and diagnosis, no significant results were found (all P>0.05).
Discussion
We conducted a large scale DVT study in a single center. All patients received CV after arthroplasty to examine DVT. A single center study reduced the influence of difference of diagnosis criterion, surgeons, environmental factors, etc. Our study provides insight into the relative importance of clinical risk factors and DNA variants associated with DVT after THA.
Ageing is one of the strongest and most prevalent risk factors for DVT. It increases to ~1% per year in very old age. Increased age was a strong risk factor for onset of DVT after THA (1). In our study, ageing leads to a high attributable risk. Ageing may be associated with an increased prevalence of conventional risk factors, development of new, age-specific risk factors and accumulation of risk factors with age. In the elder subjects, women were the higher proportion. Female patients also had a higher prevalence in our study, although no statistical sex-difference was detected. Asymptomatic DVT played a larger part in our study, causing diagnosis to be easily overlooked. Patients with symptoms had over a 5-fold risk of suffering DVT. Symptoms were a strong sign of DVT. Blood type also affected the prevalence of DVT. As factor VIII and VWF concentrations are higher in the non-O individuals than in the O group, patients with the O alleles of the ABO genotype had the lowest incidence of DVT (1).
In the 1970s, some clinicians reported the important role of less intense warfarin sodium in the prophylactic treatment of DVT after THA. With the absence of prophylactic anticoagulation, this disorder occurs in 40% to 60% of patients receiving hip implants (19-23). Currently, subcutaneous low-molecular-weight heparin with warfarin sodium and an inhibitor of factor Xa are popular for prophylactic anticoagulation (20-24). In our study, no significant difference was detected between the anticoagulation and the non-anticoagulation groups. In the surgeon A group with shorter surgery duration, the prophylactic anticoagulation regimen was not effective. However, it was useful in the group surgeon B with longer surgery duration. This observation suggested that prophylactic anticoagulation regimen was helpful for longer surgeries, and, in the worst case scenario, it increased the amount of blood lose.
Incidence of DVT was different in the two skilled surgeons’ groups. Longer duration of surgery was responsible for the difference, since the iliaca externa vein was mechanically compressed by the retractor during surgery. Vascular stenosis was detected by CV during surgery by two skilled surgeons and the duration of surgery determined how long the vascular clogging occurred for. Therefore, a longer duration surgery resulted in slower blood flow in the veins and more facile formation of DVT (23). No difference was detected for other conventional risk factors. In the multi-center trials, the interesting and appealing results should be questioned according to limited number in some groups with different surgeons, different ethnic background and un-sensitive diagnosis strategy (9-11).
In the patients without further medical intervention who were followed, no late-onset DVT was detected by 3 months afterwards. By the conventional method, extended thromboprophylaxis after THA was suggested reducing the incidence of late onset DVT (24). Incidence of late-onset DVT, symptomatic pulmonary embolism or sudden death was rare. The role of extended prophylaxis for late-onset DVT is controversial, and at the same time, extended prophylaxis accounts for a major portion of the cost and contributes to high risk of bleeding. In our study, for non-DVT patients, it was not necessary tocarry out further anticoagulation for at least 3 months. Strategies for extended prophylaxis should be tailored. A large scale multi-center randomized trial is needed to confirm the perspective results.
In our study, no mutation of FVL and PT20210 was found in any DVT subjects. No significant association of MTHFR, and CYP4V2 with DVT was detected even when stratified by gender and type of DVT. No significant association of SNPs with PT, APTT, INR was detected. DVT after THA was an acute complication. Genetics might play a subtle or otherwise role in the development of DVT.
Conclusions
DVT after THA was mostly affected by personal (age) and clinical factors (mechanical compression, duration of surgery). Short duration THA did not require prophylactic anticoagulation. Extended anticoagulation was unnecessary for non-DVT patients after discharge from the hospital.
Acknowledgments
Funding: This work was supported by National Nature Science Foundation of China (No.30973046).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2016.04.01). DS serves as an unpaid Executive Editor-in-Chief of Annals of Joint from Mar 2016 to Feb 2021. QJ serves as an Editor-in-Chief of Annals of Joint from Mar 2016 to Feb 2021. QJ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethical committee of Medical School of Nanjing University, and informed consent was obtained from the subjects included.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Engbers MJ, van Hylckama Vlieg A, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost 2010;8:2105-12. [Crossref] [PubMed]
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338S-400S. [Crossref] [PubMed]
- White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446-55. [PubMed]
- Dhillon KS, Askander A, Doraismay S. Postoperative deep-vein thrombosis in Asian patients is not a rarity: a prospective study of 88 patients with no prophylaxis. J Bone Joint Surg Br 1996;78:427-30. [PubMed]
- Yoo MC, Kang CS, Kim YH, et al. A prospective randomized study on the use of nadroparin calcium in the prophylaxis of thromboembolism in Korean patients undergoing elective total hip replacement. Int Orthop 1997;21:399-402. [Crossref] [PubMed]
- Kim YH, Choi IY, Park MR, et al. Deep vein thrombosis after uncemented total hip replacement. Bull Hosp Jt Dis 1997;56:133-9. [PubMed]
- Moon KH, Kim WH, Lee JY. Deep vein thrombosis after cementless total hip replacement arthroplasty using Doppler ultrasound. J Korean Orthop Assoc 1998;33:1553-8.
- Song EK, Kim JK, Lee KB, et al. Deep Vein Thrombosis after Total Knee Replacement. Incidence and Correlation with Clinical Risk Factors. J Korean Knee Soc 1998;10:18-22.
- Turpie AG, Gallus AS, Hoek JA, et al. A synthetic pentasaccharide for the prevention of deep-vein thrombosis after total hip replacement. N Engl J Med 2001;344:619-25. [Crossref] [PubMed]
- White RH, Gettner S, Newman JM, et al. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med 2000;343:1758-64. [Crossref] [PubMed]
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. [Crossref] [PubMed]
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;301:2472-85. [Crossref] [PubMed]
- Bezemer ID, Bare LA, Doggen CJ, et al. Gene variants associated with deep vein thrombosis. JAMA 2008;299:1306-14. [Crossref] [PubMed]
- Lenicek Krleza J, Jakovljevic G, Bronic A, et al. Contraception-related deep venous thrombosis and pulmonary embolism in a 17-Year-old girl heterozygous for factor V leiden, prothrombin G20210A mutation, MTHFR C677T and homozygous for PAI-1 mutation: report of a family with multiple genetic risk factors and review of the literature. Pathophysiol Haemost Thromb 2010;37:24-9. [Crossref] [PubMed]
- Rabinov K, Paulin S. Roentgen diagnosis of venous thrombosis in the leg. Arch Surg 1972;104:134-44. [Crossref] [PubMed]
- Walsh PS, Metzger DA, Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 1991;10:506-13. [PubMed]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria, 2008. Available online: https://www.r-project.org
- Sing T, Sander O, Beerenwinkel N, et al. ROCR: visualizing classifier performance in R. Bioinformatics 2005;21:3940-1. [Crossref] [PubMed]
- Stamatakis JD, Kakkar VV, Sagar S, et al. Femoral vein thrombosis and total hip replacement. Br Med J 1977;2:223-5. [Crossref] [PubMed]
- Harris WH, Salzman EW, Athanasoulis CA, et al. Aspirin prophylaxis of venous thromboembolism after total hip replacement. N Engl J Med 1977;297:1246-9. [Crossref] [PubMed]
- Nillius AS, Nylander G. Deep vein thrombosis after total hip replacement: a clinical and phlebographic study. Br J Surg 1979;66:324-6. [Crossref] [PubMed]
- Evarts CM, Feil EJ. Prevention of thromboembolic disease after elective surgery of the hip. J Bone Joint Surg Am 1971;53:1271-80. [PubMed]
- López JA, Chen J. Pathophysiology of venous thrombosis. Thromb Res 2009;123:S30-4. [Crossref] [PubMed]
- Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31-9. [Crossref] [PubMed]
Cite this article as: Shi D, Pang Y, Yao C, Wang F, Xia N, Chen D, Xu Z, Dai J, Qin J, Lv Y, Chen H, Qiu X, Yuan T, Weng W, Ran F, Zhang M, Liu C, Zheng M, Nakamura T, Jiang Q. Deep vein thrombosis after arthroplasty: Nanjing deep vein thrombosis study. Ann Joint 2016;1:3.


