Managing bone loss in revision total knee arthroplasty
Introduction
Despite continuous technical innovation and a newer understanding of biomechanics, revision rates after total knee arthroplasty (TKA) have remained steady (1,2). Multiple studies predict that there will be an increase in the number of patients requiring revision TKA in the near future (3). It is also estimated that as the volume of revisions rises, re-revisions will become increasingly common, as well (3). A comparison of quality reports and national prosthetic joint registers revealed a 12% to 12.8% revision rate, with 78,600 revisions in the U.S. in 2010 and 17,200 in Germany in 2012 (1,4).
Mechanisms of failure include aseptic loosening, infection, osteolysis, instability, component malposition, periprosthetic fracture, extensor mechanism complication and arthrofibrosis (5,6). Identifying a clear preoperative cause for failure prior to revision surgery is necessary for surgical success (7,8). When performing revision TKA for aseptic loosening, osteolysis, or two-stage reimplantation after placement of an antibiotic cement spacer for infection eradication, there is often bone loss. These bone defects can occur as a result of prior bone preparation, polyethylene wear, stress shielding, and bone loss during implant removal (3,9).
Various strategies have been described to manage these bone defects (10,11). Selection of reconstructive techniques are often based on surgeon’s preference or experience, the integrity of the ligaments, the location and magnitude of the bone loss, and patient factors, including the potential for additional revision, functional demand, and comorbidities (6,12). In this article, general principles for revision TKA with bone loss are presented, with a focus on the size of the defect, available augments, and utilizing the correct degree of implant constraint.
General principles
Bone defects range from small bone loss that do not compromise component stability, to significant bone loss compromising a major portion of the femoral condyles or proximal tibia with ligamentous instability. Physical exam and radiographic analysis of implants are not enough to assess bone loss; computed tomography (CT) can help assess bone loss, but bone loss can only truly be assessed with intraoperative evaluation after the implant and cement have been removed. Dr. Charles Engh who developed the first bone loss classification system stated that “the surgeon should anticipate the worst scenario because often the defects turn out to be more severe than predicted from radiographs” (13).
There are many classification systems for bone loss after revision TKA and they depend on the size, symmetry, morphology, location, and containment of the bone defect. The Anderson Orthopedic Research Institute (AORI) classification is the most widely adopted system for categorizing the severity of bone deficiency encountered during revision TKA and for predicting the most appropriate method of reconstruction (6,13). The femur and tibia are considered separately, but with the same distinct deficit (2). Bone loss is classified as type 1–3 and different reconstruction methods are recommended depending on the amount of bone loss present. However, treatment options can overlap between type 1 and 2, or type 2 and 3. Therefore, two main categories (small and/or contained defects versus large and/or uncontained defect) are presented in this review.
Management of small and/or contained bone defect
Small and/or contained bone defects can be defined as bone that is intact in the metaphysis and there are minor defects that do not affect the stability of the implant; these correspond well with AORI type 1 defects. These defects can be up to 10 mm in breadth and depth. In general, these types of defects are managed with morselized bone graft, cement, or cement and screw fixation. If bone defects are less than 5 mm, the best surgical choice may be cement to fill the bone loss, as this allows one to get stability comparable to impaction bone grafting and structural allograft (14). In cases of >5 mm but <10 mm defect, cement may be reinforced with embedded screws. However, some authors recommend using bone graft when the cement mantle is greater than 5 mm thick below the tibia plateau (2).
If possible, it is preferable to use primary TKA implants, but revision components may be necessary. In principle, fixation and long-term durability of implants are inversely proportional to prosthetic constraint (15). Therefore, it is generally recommended that an implant with the least constraint required for satisfactory knee stability is selected to reduce stress on the implant-fixation interface with compromised bone (6).
Management of large and/or uncontained bone defect
Large and/or uncontained bone defects can be defined as metaphyseal bone that is damaged, which should be reconstructed in order to provide stability to the prosthetic components. These are >10 mm uncontained bone defects in breadth and depth and correspond with AORI type 2 or 3. There are many options for reconstructing these defects, including utilizing cement, metal augments (cones and sleeves), allograft, and impaction grafting, while using semi-constrained or fully constrained prostheses with or without stems (10). Multiple techniques have been described to restore the metaphysis and optimize the results of bone loss in revision TKA (6,16-18).
Impaction grafting
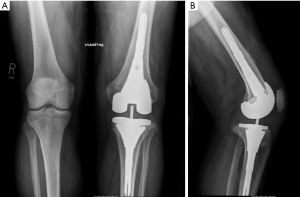
Traditionally, morselized allograft was widely utilized to manage bone loss and uncontained defects required the use of mesh for containment of graft (Figure 1) (6). The purpose of using morselized allograft was to restore host bone stock, which was particularly beneficial in younger patients where there is potential for future reconstructive surgeries (2,6). Impaction bone graft is cost-effective and obviates the need for excessive bone resection and the use of large metal augments, bulk structural allografts, or custom prostheses (2). Disadvantages include the time-consuming and technically demanding nature of this reconstruction, particularly when wire mesh is required (19). The use of bone graft also carries the risks of nonunion, malunion, graft resorption, graft collapse, and a minimal risk of disease transmission (12). Some authors consider the graft size should be 5–10 mm because chips less than 5 mm can be reabsorbed by the inflammatory process, while chips bigger than 10 mm have a slow integration (20,21).
Structural allograft
Structural allografts can be used to replace deficient segments of the femur and tibia, and can be used to address central or peripheral defects without the need for additional wire mesh (22). It can be effective used in AORI type 2 and 3 defects that are too large to be managed with prosthetic augments (12). Generally, this technique is used in the treatment of segmental defects <15 mm for the femur and >20–45 mm for the tibia. The femoral head, distal femoral segments, and proximal tibial segments are the most commonly used allografts (2,23). For the management of large bone defects, bulk or structural allografts in combination with long-stemmed prostheses have been historically used. Advantages of this technique include the ability to create any shape or size of construct, excellent support of the revision implant, the potential for long-term biologic integration of the graft restoring host bone stock, and the potential for ligamentous reattachment (6). However, using bulk allograft has a number of disadvantages, including late graft resorption, graft fracture, graft nonunion with native bone, and the risk of disease transmission (23-26). A recent systematic review of 551 structural allografts in revision TKA with an average follow-up of 5.9 years reported a 6.5% rate of graft failure, a 3.4% rate of aseptic component loosening, and a 5.5% rate of deep infection (10,22). The increased failure rate with structural allografts leads to the development of other substitutes.
Prosthetic augmentation
Augments are most frequently used for uncontained unicondylar or bicondylar defects of moderate size (12). In most revision systems, there are a variety of shapes and sizes of both tibial and femoral augments that can be used to facilitate joint line restoration, balancing, and placement of the component in the correct rotation. Tibial augments are block- or wedge-shaped and femoral augments are typically block-shaped and of variable thicknesses, ranging from approximately 5 to 20 mm (6). Disadvantages of prosthetic augments are that they are expensive, limited in size and shape options, do not restore bone stock, and usually require additional bone removal to match the pattern of the augment (6,27). In early designs of prosthetic augments, they showed frequent radiolucent lines which theoretically increased the risk of fretting and corrosion, although they showed satisfactory mid-term follow-up (28-30). In addition, the use of these augments resulted in stress shielding and increased potential bone loss because of the difference in the elasticity between metal and bone (31). Therefore, current designs strive to maintain a high volumetric porosity for bone ingrowth, with a low modulus of elasticity and high frictional characteristics, making this metal conductive to biologic fixation (32).
Metaphyseal sleeve (titanium sleeve)
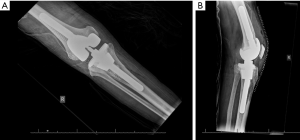
Titanium sleeves were designed to fill large contained cavitary and combined cavitary-segmental metaphyseal defects in the femur and tibia (Figure 2). They are shaped in a stepwise manner and coated with titanium beads to provide an interconnected porous surface for bone ingrowth (6,22). This metaphyseal sleeve is bonded to the implant by a morse taper. For the sleeve to be uncemented, sufficient axial and rotational stability must be achieved in the metaphyseal bone at time of implantation (22). Femoral sleeves are especially advantageous when there is significant posterior femoral condyle bone loss, as they can add rotational stability to the implant (2). The tibial sleeve can be impacted onto the tibial tray in as much as 20 degrees of internal or external rotation. After using an opening reamer, the smallest broach is first used and the broaches are sequentially increased until a tight fit is achieved (2). The most common complication from the use of metaphyseal sleeve is fracture when broaching the sleeves or impacting the final stem-sleeve in the tibia or femur (2). Fractures are commonly fixed by cerclage wires to provide adequate fixation (2). There are no offset stems in this system, which limits placement of the sleeve, and the size of the stem that can be used is limited by the maximum diameter of the sleeve. Current tribology of metaphyseal sleeves limit bony ingrowth and require long and uncemented diaphyseal stems that may be undersized because of stem pain (33). However, favorable mid-term outcomes are also being reported using metaphyseal sleeves (5,16).
Highly porous tantalum cone
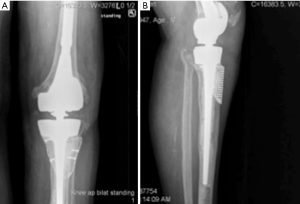
Highly porous tantalum cones were also developed to address the weaknesses of structural allograft and have been used in revision TKA (Figure 3). The high porosity of tantalum and its scaffolding abilities for osteoblastic activity enables bone ingrowth and makes it a suitable material for these augments (6,9,22,34). Tantalum cones are often inserted with short or intermediate length cemented stems to ensure adequate initial stability needed for osseointegration. While the cement interface between the cone and the implant can be considered a site of junctional mechanical failure, this has not been observed clinically (18,22). The high variability of sizes and shapes allows for good adaptability of these cones to the metaphyseal bone deficiency, primarily for those types of cavities in which a reliable cortical shell is present. The option of symmetric or asymmetric cones allows for reconstruction of segmental defects, not just extended cavitary defects (2). In a recent systematic review comparing porous implants to structural allograft, porous implants showed significantly decreased loosening rates when used in AORI type 2 and 3 defects, and overall failure rates were substantially lower in the porous metal group (10). After the initial description of tantalum cone use by Mayo clinic, mid-term results are being reported in the literature with very promising results (10,17,35,36). These cones are now widely used both in North America and Europe.
Other considerations
In addition to restorating metaphyseal defects, other considerations should be evaluated when performing revision TKA, including restoration of the anatomical joint line, ligamentous stability, and knee kinematics. If the joint line is not restored, augmentation should be considered using augments, metaphyseal cones or sleeves, or allograft bone. If ligamentous stability is not achieved, a posterior stabilized implant may be used in revision surgery. However, for cases with ligamentous insufficiency and above moderate bone loss, a semi-constrained TKA design that supplements the collateral ligaments may be appropriate (6). In cases of massive bone deficiency with loss of collateral ligamentous support or gross flexion-extension gap mismatch, a hinged prosthesis may be necessary (6,12). However, if satisfactory meta-diaphyseal fixation can be achieved, this may reduce the mechanical stress at the level of the joint line and decrease the need for high levels of TKA constraint (37).
Stems are often used during revision TKA when a bone defect is present, and their use is indicated whenever existing condylar bone support is compromised (15,38). Stems can offload the stress from the implant-fixation interface, provide increased surface area for fixation, and help restore optimal implant alignment (15). Offset stem extensions can assist with implant alignment on the metaphysis if there is an offset diaphysis, can avoid medial-lateral or anterior-posterior component overhang, can reduce the incidence of coronal or sagittal malalignment, and can help balance the flexion and extension spaces by effectively translating the components (39). The two options for stem fixation include fully cemented with cement restrictors or partially cemented fixation (hybrid fixation). There have been no significant differences with regard to survival between the two fixation methods (40). Fully cemented stems are generally shorter and metaphyseal engaging, and there must be at least 2 mm of a cement mantle around the stem. However, it is recommended in the case of bone loss during revision TKA that hybrid fixation (diaphyseal engaging press-fit plus proximal cementing in the tibia and distal cementing in the femur) is critical to producing a stable construct. In this technique, vertical cementing 10–20 mm above the stem-implant junction is recommended for stability (41). However, when a revision TKA with bone loss is being performed after two-stage exchange arthroplasty for infection, it is recommended to use antibiotic cement to prevent infection recurrence.
Conclusions
The most optimal management method of bone loss differs for each patient. For small cavitary defects, morselized allograft can be used as well as cement. Structural allografts for larger defects have been replaced with new materials, such as augments for limited segmental defects, and sleeves to restore the metaphysis. However, the use of both augments and sleeves may require supplementation with long and uncemented diaphyseal stem, and bony ingrowth is limited to sleeves. Tantalum cones have some benefits including metaphyseal restoration, biologic fixation, and the ability to utilize shorter and cemented stems for the initial axial and rotational stability. These methods of meta-diaphyseal fixations may reduce the mechanical stress at the level of the joint line when restoring bone loss in revision TKA and reduce the need for high levels of implant constraint.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2016.08.03). AFC serves as an unpaid Associate Editor of Annals of Joint from Jun 2016 to May 2018. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thiele K, Perka C, Matziolis G, et al. Current failure mechanisms after knee arthroplasty have changed: polyethylene wear is less common in revision surgery. J Bone Joint Surg Am 2015;97:715-20. [Crossref] [PubMed]
- Panegrossi G, Ceretti M, Papalia M, et al. Bone loss management in total knee revision surgery. Int Orthop 2014;38:419-27. [Crossref] [PubMed]
- Nelson CL, Vanushkina M, Irgit K, et al. Stemmed femoral implants show lower failure rates in revision total knee arthroplasty. Knee 2015;22:429-34. [Crossref] [PubMed]
- Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res 2010;468:45-51. [Crossref] [PubMed]
- Dalury DF, Barrett WP. The use of metaphyseal sleeves in revision total knee arthroplasty. Knee 2016;23:545-8. [Crossref] [PubMed]
- Ponzio DY, Austin MS. Metaphyseal bone loss in revision knee arthroplasty. Curr Rev Musculoskelet Med 2015;8:361-7. [Crossref] [PubMed]
- Bieger R, Kappe T, Fraitzl CR, et al. The aetiology of total knee arthroplasty failure influences the improvement in knee function. Arch Orthop Trauma Surg 2013;133:237-41. [Crossref] [PubMed]
- Callaghan JJ, O'rourke MR, Saleh KJ. Why knees fail: lessons learned. J Arthroplasty 2004;19:31-4. [Crossref] [PubMed]
- Haidukewych GJ, Hanssen A, Jones RD. Metaphyseal fixation in revision total knee arthroplasty: indications and techniques. J Am Acad Orthop Surg 2011;19:311-8. [Crossref] [PubMed]
- Beckmann NA, Mueller S, Gondan M, et al. Treatment of severe bone defects during revision total knee arthroplasty with structural allografts and porous metal cones-a systematic review. J Arthroplasty 2015;30:249-53. [Crossref] [PubMed]
- Shen C, Lichstein PM, Austin MS, et al. Revision knee arthroplasty for bone loss: choosing the right degree of constraint. J Arthroplasty 2014;29:127-31. [Crossref] [PubMed]
- Daines BK, Dennis DA. Management of bone defects in revision total knee arthroplasty. Instr Course Lect 2013;62:341-8. [PubMed]
- Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect 1999;48:167-75. [PubMed]
- Dorr LD, Ranawat CS, Sculco TA, et al. Bone graft for tibial defects in total knee arthroplasty. 1986. Clin Orthop Relat Res 2006;4-9. [Crossref] [PubMed]
- Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty 2007;22:32-8. [Crossref] [PubMed]
- Bugler KE, Maheshwari R, Ahmed I, et al. Metaphyseal Sleeves for Revision Total Knee Arthroplasty: Good Short-Term Outcomes. J Arthroplasty 2015;30:1990-4. [Crossref] [PubMed]
- Jensen CL, Petersen MM, Schrøder HM, et al. Revision total knee arthroplasty with the use of trabecular metal cones: a randomized radiostereometric analysis with 2 years of follow-up. J Arthroplasty 2012;27:1820-1826.e2. [Crossref] [PubMed]
- Kamath AF, Lewallen DG, Hanssen AD. Porous tantalum metaphyseal cones for severe tibial bone loss in revision knee arthroplasty: a five to nine-year follow-up. J Bone Joint Surg Am 2015;97:216-23. [Crossref] [PubMed]
- Mabry TM, Hanssen AD. The role of stems and augments for bone loss in revision knee arthroplasty. J Arthroplasty 2007;22:56-60. [Crossref] [PubMed]
- Whiteside LA, Bicalho PS. Radiologic and histologic analysis of morselized allograft in revision total knee replacement. Clin Orthop Relat Res 1998;149-56. [Crossref] [PubMed]
- Whittaker JP, Dharmarajan R, Toms AD. The management of bone loss in revision total knee replacement. J Bone Joint Surg Br 2008;90:981-7. [Crossref] [PubMed]
- Sculco PK, Abdel MP, Hanssen AD, et al. The management of bone loss in revision total knee arthroplasty: rebuild, reinforce, and augment. Bone Joint J 2016;98-B:120-4. [Crossref] [PubMed]
- Backstein D, Safir O, Gross A. Management of bone loss: structural grafts in revision total knee arthroplasty. Clin Orthop Relat Res 2006;104-12. [Crossref] [PubMed]
- Bauman RD, Lewallen DG, Hanssen AD. Limitations of structural allograft in revision total knee arthroplasty. Clin Orthop Relat Res 2009;467:818-24. [Crossref] [PubMed]
- Clatworthy MG, Ballance J, Brick GW, et al. The use of structural allograft for uncontained defects in revision total knee arthroplasty. A minimum five-year review. J Bone Joint Surg Am 2001;83-A:404-11. [PubMed]
- Sternheim A, Drexler M, Kuzyk PR, et al. Treatment of failed allograft prosthesis composites used for hip arthroplasty in the setting of severe proximal femoral bone defects. J Arthroplasty 2014;29:1058-62. [Crossref] [PubMed]
- Huff TW, Sculco TP. Management of bone loss in revision total knee arthroplasty. J Arthroplasty 2007;22:32-6. [Crossref] [PubMed]
- Brand MG, Daley RJ, Ewald FC, et al. Tibial tray augmentation with modular metal wedges for tibial bone stock deficiency. Clin Orthop Relat Res 1989;71-9. [PubMed]
- Gofton WT, Tsigaras H, Butler RA, et al. Revision total knee arthroplasty: fixation with modular stems. Clin Orthop Relat Res 2002;158-68. [Crossref] [PubMed]
- Patel JV, Masonis JL, Guerin J, et al. The fate of augments to treat type-2 bone defects in revision knee arthroplasty. J Bone Joint Surg Br 2004;86:195-9. [Crossref] [PubMed]
- Stuchin SA. Allografting in total knee replacement arthroplasty. Semin Arthroplasty 1993;4:117-22. [PubMed]
- Tigani D, Sabbioni G, Raimondi A. Early aseptic loosening of a porous tantalum knee prosthesis. Chir Organi Mov 2009;93:187-91. [PubMed]
- Huten D. Femorotibial bone loss during revision total knee arthroplasty. Orthop Traumatol Surg Res 2013;99:S22-33. [Crossref] [PubMed]
- Qiu YY, Yan CH, Chiu KY, et al. Review article: Treatments for bone loss in revision total knee arthroplasty. J Orthop Surg (Hong Kong) 2012;20:78-86. [PubMed]
- Jensen CL, Winther N, Schrøder HM, et al. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee 2014;21:1233-7. [Crossref] [PubMed]
- Parratte S, Abdel MP, Lunebourg A, et al. Revision total knee arthroplasty: the end of the allograft era? Eur J Orthop Surg Traumatol 2015;25:621-2. [Crossref] [PubMed]
- Indelli PF, Giori N, Maloney W, et al. Level of constraint in revision knee arthroplasty. Curr Rev Musculoskelet Med 2015;8:390-7. [Crossref] [PubMed]
- Patel AR, Barlow B, Ranawat AS. Stem length in revision total knee arthroplasty. Curr Rev Musculoskelet Med 2015;8:407-12. [Crossref] [PubMed]
- Baldini A, Balato G, Franceschini V. The role of offset stems in revision knee arthroplasty. Curr Rev Musculoskelet Med 2015;8:383-9. [Crossref] [PubMed]
- Wang C, Pfitzner T, von Roth P, et al. Fixation of stem in revision of total knee arthroplasty: cemented versus cementless-a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Ro du H. Extent of vertical cementing as a predictive factor for radiolucency in revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2016;24:2710-7. [Crossref] [PubMed]
Cite this article as: Lee YS, Chen AF. Managing bone loss in revision total knee arthroplasty. Ann Joint 2016;1:17.