
In vitro and in vivo kinematics of total knee arthroplasty—a review of the research at the Orthopaedic Bioengineering Laboratory of the Massachusetts General Hospital (MGH)
Introduction
The purpose of total knee arthroplasty (TKA) is to reduce pain, enhance function and improve quality of life in patients with severe osteoarthritis (OA) of the knee and other end-stage knee joint diseases (1,2). Recently, many innovative TKA component designs have been introduced with the aim to increase knee joint flexion and improve longevity of the prosthesis (3-7). Although many clinical follow-up data showed satisfactory post-operative outcomes (1,8), others have indicated that persistent pain or difficulties during functional activities and limited high-flexion in patients after TKA surgery (1,8-11). While many factors have been attributed to postoperative complications, such as component design and surgical implantation, altered knee kinematics following TKA surgery has been widely believed to affect knee joint biomechanics and lead to suboptimal patient satisfaction (9,10,12,13).
Numerous studies have investigated the knee joint kinematics after TKA (3,14-18). For example, cadaveric specimens have been used to compare the TKA kinematics with those of native knees under controlled conditions (19,20); motion analysis systems have been used to quantify the six degrees-of-freedom (DOF) kinematics of the TKA knees (21,22); and more recently, fluoroscopic imaging techniques have been used to study the in vivo articular contact locations in TKA knees (18,23-25). Our lab started of studying TKA kinematics using an in vitro robotic experimental setup (20), thereafter an in vivo dual fluoroscopic imaging system (DFIS) was developed (25). We have studied knee biomechanics of both posterior cruciate retaining (CR) and posterior substituting (PS) TKAs, including tibiofemoral joint kinematics, cartilage contact locations, posterior cruciate ligament (PCL) function in CR TKAs and cam-post contact biomechanics in PS TKAs (20). Furthermore, we investigated biomechanical factors that may affect knee flexion by comparing the biomechanics of conventional and high-flexion TKA designs. High-flexion TKA designs have been created to prevent edge loading on the posterior tibial articular surface and to increase the tibiofemoral contact area at high degrees of flexion. In this review, we will briefly introduce the in vitro and in vivo testing systems used for our TKA kinematics investigation and then summarize the major research on various TKA components under simulated physiological loads and during in vivo functional knee activities that was performed in our lab.
Experimental methodology
In vitro robotic testing system
The robotic testing system is composed of a six DOF robotic manipulator (Kawasaki Heavy Industries Ldt, UZ150®, Akashi, Japan) and a six DOF load cell (JR3 Inc., Woodland, CA, USA) (Figure 1A). The robotic manipulator is a position-control device with a maximum payload of 150 kg and an accuracy of <0.1 mm for slow speed rate tests as used in our TKA experiments. A control algorithm that uses a global convergence method that considers the coupling effects of the different DOF motions of the knee was custom-developed to link the robot and the load cell so that both displacement and force control modes can be obtained (26).
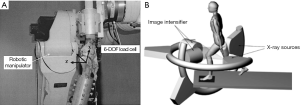
In an actual experiment, a fresh-frozen cadaveric knee is thawed overnight at room temperature prior to testing. The knee includes approximately 25 cm of bone proximal and distal to the knee joint. All soft tissues surrounding the knee joint are kept intact. The fibula is fixed to the tibia in an anatomic position by a cortical bone screw. The femoral and tibial shafts are then potted in thick-wall aluminum cylinders to enable secured mounting of the specimen on the robotic system (Figure 1A).
During experiment, the femur is fixed rigidly to a specially designed clamp that could be adjusted to allow six DOF positioning of the femur. The tibia is fixed rigidly to the robot arm through the six DOF load cell. A knee joint coordinate system is constructed using a digitizer (MicroScribe 3DX®, San Jose, CA, USA). This setup allows the tibia to move with the robot arm in six DOF about the femur. Therefore, the knee motion is measured by the relative position and orientation of the tibial coordinate system with respective to the femoral coordinate system. The robotic manipulator can learn the complex motion of the knee specimen in response to external loads and then can reproduce these motions in subsequent tests after specimen modifications such as removal of a ligament of the knee or replacing the knee joint with a TKA. Tibial translation and rotation with respect to the femur can be determined from full extension to the targeted maximum flexion angle under various loading conditions.
In vivo DFIS
The DFIS consists of two fluoroscopes (BV Pulsera; Philips, Bothell, WA, USA) (Figure 1B) (25,27). A subject is free to move within the common imaging zone of the two fluoroscopes (corresponding to a 315 mm × 315 mm field of view). Various motions can be imaged this way such as treadmill gait, squat, step-up, sit-to-stand, etc. The knee is imaged simultaneously by the fluoroscopes from two directions. This procedure records the in vivo poses of the knee as a series of Two dimensional (2D) paired fluoroscopic images. The images are then automatically segmented and corrected for distortion (27). The outlines of the TKA components from the edge detection are manually reviewed and saved.
Next, a virtual replica of the DFIS is constructed. Two virtual source-intensifier pairs are created in a solid modeling program (Rhinoceros®, Robert McNeel & Associates, Seattle, WA, USA) to recreate the geometry of the real fluoroscopic system (Figure 1B). The outlines of the TKA components obtained from the DFIS are placed on their respective virtual intensifiers. Three dimensional (3D) models of the TKA tibial and femoral components are introduced into the virtual system. A local coordinate system is created for both the tibial and femoral component models. The tibial and femoral models can be manipulated independently in the virtual environment in six DOF and projected onto the virtual imaging intensifiers. If the projection outline matches the actual bony outline captured from the actual knee, the in vivo TKA position in space is reproduced by the 3D TKA models in the computer. In this manner, the knee motion can be represented by a series of 3D knee models reproduced along the motion path.
In vitro TKA biomechanics
Factors that limit high knee flexion are not well understood. While it has been implied that insufficient posterior femoral translation is a cause for limited knee flexion, resulting in early posterior impingement, retaining the PCL in a CR TKA, or substituting the PCL using a cam-spine contact mechanism, has been suggested to improve the range of motion by allowing femoral rollback. This section reviews our studies on the biomechanical efficiency of the PCL and the cam-spine mechanism in TKAs.
Kinematics of CR TKAs
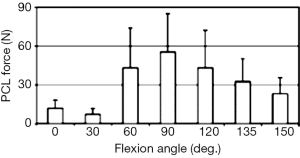
Using the above described in vitro robotic experimental setup, under a combined quadriceps and hamstring muscle load, we examined medial and lateral posterior femoral translations of the conventional CR TKA design (NexGen CR, Zimmer) and the high flexion CR TKA design (NexGen CR Flex, Zimmer) (28). The high flexion CR TKA design attempts to improve posterior tibiofemoral articular contact at high-flexion angles. The tibiofemoral kinematics of both TKAs were measured and compared with that of intact knees. Both CR TKA designs showed similar kinematics throughout the range of motion (0°–150°). Approximately 80% of the posterior femoral translation of the intact knee at 150° was restored by the TKAs. The PCL forces measured for the CR TKA components indicate that the PCL is important in the mid-flexion range but has little effect on knee kinematics at both low and deep flexion angles (Figure 2).
Next, we determined the contact areas and the peak contact locations (centroid of the contact area) of the conventional and high-flexion CR TKA designs using the robotic testing system and TekScan pressure sensors along the flexion path of the knee (17). While both TKAs showed similar kinematics throughout the range of motion, their contact behaviors were different: the peak contact point for the high flexion TKA was located more anteriorly than the conventional TKA for flexion angles greater than 90°. The tibiofemoral contact of both TKAs reached the polyethylene posterior edge at 150°. The contact on conventional TKA reached the polyethylene posterior edge approximately 15°–30° before the high-flexion TKA, but exhibited similar contact areas to high flexion TKA.
Kinematics of PS TKAs
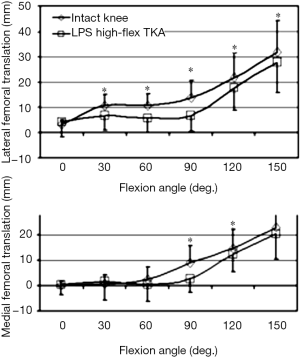
Using the robotic experimental setup, 13 cadaveric knees were studied to assess the biomechanical mechanisms that inhibit high knee flexion after fixed-bearing, high-flexion PS TKA system (LPS-Flex, Zimmer) (29). Posterior femoral translation of the femoral condyles and tibial rotation were recorded from 0°–150° of flexion under simulated physiological muscle loads (Figure 3). The data indicated that the femoral condyles of the intact knee translated posteriorly from full extension to 150°, reaching a peak of 22.9±11.3 and 31.9±12.5 mm, respectively. Following TKA, posterior femoral translation was decreased compared to the intact knee. Approximately 90% of the posterior femoral translation of the intact knee at 150° of flexion was recovered by the TKA. Internal tibial rotation was observed for all knees throughout the range of motion. The cam-spine mechanism engaged at approximately 80° and disengaged at 135°. Despite the absence of cam-spine engagement, further posterior femoral translation occurred between 135°–150°.
As the tibial post in a PS TKA was designed to increase posterior femoral translation, recent retrieval studies of various PS TKA designs revealed wear and deformation on the anterior side of the tibial post. We studied the mechanisms of anterior impingement of the post with the femoral component using the robotic setup during simulated heel strike (30). Intact knee kinematics and in situ anterior cruciate ligament (ACL) forces were determined at hyperextension (−9° to 0°) and low flexion angles (0° to 30°) under the applied loads. The same knee was reconstructed using a PS TKA. The kinematics and the tibial post contact forces of the TKA were measured under the same loading conditions. Our data indicated that the ACL in the intact knee carried load and contributed to knee stability at low flexion angles and hyperextension. After TKA, substantial in situ contact forces (252.4±173 N at −9° of flexion) occurred in the tibial post, indicating anterior impingement of the post with the femoral component. Consequently, the TKA showed less posterior femoral translation compared to the intact knee after the impingement. At −9° of flexion, the medial condyle of the intact knee translated 0.1±1.1 mm whereas the medial condyle of the TKA knee translated 5.6±6.9 mm anteriorly. The lateral condyle of the intact knee translated 1.5±1.0 mm anteriorly whereas the lateral condyle of the TKA knee translated 2.1±5.8 mm anteriorly.
Comparison of kinematics of CR and PS TKAs
Both CR and PS TKAs are widely used in knee replacement surgeries. However, limited data comparing the kinematics of CR and PS TKAs with their own intact knees under identical loadings is available. We investigated the posterior femoral translation of both femoral condyles in the in intact, CR, PCL-deficient CR and PS TKA knee states (20,26). The forces through the PCL and cam-spine mechanism were also measured from 0°–120° of flexion. Both CR and PS TKAs behaved similarly to the PCL-deficient CR TKA between 0°–30° flexion. Beyond 30°, the CR TKA showed a significant increase in posterior translation of both femoral condyles. The PS TKA only showed a significant increase in posterior femoral translation after 90°. The forces in the PCL of the CR TKA and the cam-spine contact after PS TKA increased only at a flexion of ≥90°. At 120°, both arthroplasties restored approximately 80% of the posterior femoral translation of the native knee. Posterior translation of the lateral femoral condyle was greater than that observed in the medial condyle for all knees, indicating the presence of internal tibial rotation during knee flexion.
In vivo TKA biomechanics
Accurate knowledge on the in vivo biomechanics of the PCL and cam-spine mechanisms in CR or PS TKA is important for understanding the knee joint biomechanics under functional loading conditions and improving the longevity of the components. Such knowledge could provide important insight into the factors that affect knee flexion after TKA. This section reviewed our research on in vivo knee biomechanics after CR or PS TKAs.
Kinematics of CR TKAs
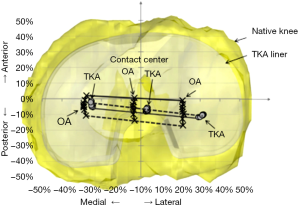
Using the above described combined MRI and DFIS technique, we evaluated the in vivo kinematics of the knee with medial compartment OA before and after a CR TKA during weight-bearing knee flexion, and compared the results to those of normal knees (31-33). Similar internal/external tibial rotation was seen in the OA and normal knees. However, the OA knees had less overall posterior femoral translation relative to the tibia between 0°–105° flexion and more varus knee rotation between 0°–45° flexion compared to the normal knees. Additionally, in the OA knees the femur was located more medially than in the normal knees, particularly between 30°–60° flexion. After CR TKA, the knee kinematics were not restored to normal. The overall internal tibial rotation and posterior femoral translation between 0°–105° knee flexion were dramatically reduced. Additionally, CR TKA introduced an abnormal anterior femoral translation during early knee flexion, and the femur was located lateral to the tibia throughout weight-bearing flexion. We also found that the CR TKA resulted in more posterior contact positions on the tibial surface and a reduced range of motion in the medial and lateral compartments (Figure 4). The distances between medial and lateral contact locations in the CR TKA knees were statistically larger than the OA knees. The articular contact centers have shifted from the medial side of the tibial plateau preoperatively to the lateral side after operation. In analysis of the function of the PCL in the CR TKA, we found that the PCL bundles of the OA knees were overstretched during late knee flexion and orientated more medially throughout flexion compared with normal knees. After CR TKA, PCL bundles were further overstretched during late flexion and changed from medially directed in normal and OA knees to almost sagittally directed, which may compromise its function in controlling knee rotation.
We investigated the in vivo six DOF knee kinematics and tibiofemoral contact location after TKA using a conventional and a high flexion CR component (15 NexGen CR, 11 NexGen CR-Flex) (18). Each patient performed a single-leg lunge and imaged by the DFIS. Data were analyzed at hyperextension, 0°–90° using 15° increments, and at maximum flexion. The average maximum weight-bearing flexions were similar between the CR patients (110.1°±13.4°) and the CR-Flex patients (108.2°±13.2°) and no significant differences in kinematics between the two groups were found. However, at high flexion, the tibiofemoral articulating surfaces were more conforming in the CR Flex design than the CR design, suggesting that the use of the high flexion component improved the tibiofemoral contact environment at high flexion in patients who could achieve high flexion.
In vivo kinematics of PS TKAs
We analyzed the posterior femoral translation, internal tibial rotation and cam-post engagement in 24 knees with a PS TKA (LPS Flex, Zimmer) while performing a weight-bearing, single leg lunge from full extension to maximum flexion (34). The cam-post engagement was determined when the surface model of the femoral cam overlapped with that of the tibial post. The mean maximum flexion angle for all the subjects was 112.5°±13.1°. The mean flexion angle where cam–post engagement was observed was 91.1°±10.9°. The femur moved anteriorly from 0°–30° and posteriorly through the remaining flexion range. The internal tibial rotation increased approximately 6° from full extension to 90° of flexion and decreased slightly with further flexion. Both the medial and lateral contact point moved posteriorly from 0°–30°, remained relatively constant from 30°–90°, and then moved further posterior from 90° to maximum flexion. The in vivo cam-post engagement corresponded to increased posterior translation and reduced internal tibial rotation at high flexion of the PS TKA.
Next, we investigated biomechanics of PS TKA patients during weight-bearing flexion >130° (6), including six DOF kinematics, tibiofemoral contact, and cam-post contact. The patients achieved average weight-bearing flexion of 139.5°±4.5°. Posterior femoral translation and internal tibial rotation increased gradually beyond 90° flexion, and a sharp increase in varus rotation was noted at maximum flexion. Initial cam-post engagement was observed at 100.3°±6.7° flexion. Five knees had cam-post disengagement before maximum flexion. Lateral femoral condylar lift-off was found in five out of seven knees at maximum flexion, and medial condylar lift-off was found in one knee.
We measured the in vivo anterior tibial post contact area between the femoral component box and anterior aspect of the tibial post at full knee extension in 11 OA patients after TKA (NexGen LPS, Zimmer) (35). Anterior tibial post contact (Figure 5), ranging between 0.5 and 80.9 mm2, was detected in 63% of the healthy patients (7 out of the 11 patients) at weight-bearing full extension of the knee. The patients with anterior tibial post contact had significantly higher hyperextension angles (−8.4°±4.3°) than those without contact (1.4°±7.28°). A statistically significant difference was also detected in the femoral component flexion with respect to the femoral shaft between the patients with anterior post contact (2.7°±2.7°) and without anterior post contact (−1.3°±2.2°). These data indicated that anterior post contact did occur in hyperextension within posterior stabilizing TKA patients.

Factors affecting high flexion
We evaluated the in vivo heights and anterior-posterior (AP) translations of the medial and lateral femoral condyles of 11 CR TKA patients before and after surgery using two flexion axes: surgical transepicondylar axis (sTEA) and geometric center axis (GCA) (36). Each patient performed a weight-bearing, single leg lunge. We also measured the pre- and postoperative length changes of the superficial medial (sMCL) and lateral collateral ligaments (LCL) (37). Each ligament was divided into three equal portions: anterior, middle and posterior portions. The relationship between the ligament length changes caused by flexion of the knee after TKAs were quantitatively analyzed.
In general, following TKA, the knees were well-balanced at 0°–90°. However, the medial and lateral femoral condyle heights were not equal at mid-flexion (15°–45°, medial condyle lower than lateral by 2.4 mm at least, P<0.01). While the femoral condyle heights increased from the preoperative values (>2 mm increase on average, P<0.05), they were similar to the intact knees except that the medial sTEA was lower than the intact medial condyle between 0°–90°. Both condyles were significantly higher (>2 mm, P<0.01) than the healthy knees at >90° of flexion. Anterior femoral translation of the TKA knee was more pronounced at mid-flexion, whereas limited posterior translation was found at deep flexion. The sMCL showed significant length increases only at low flexion (0°–30°) after TKA; the LCL showed decreases in length at full extension, but increases with further flexion after TKA. The amount of increases of the maximum flexion angle after TKA was negatively correlated with the increases of the elongations of the anterior portion (P=0.010, r=0.733) (Figure 6A), middle portion (P=0.049, r=0.604) of the sMCL and the anterior portion (P=0.010, r=0.733) of the LCL.
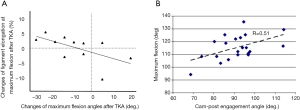
In addition, we investigated the relationship between the timing of cam-post engagement and the maximum flexion angle of 24 patients with a PS TKA during in vivo weight-bearing flexion (34). The data indicated that initial cam-post engagement was mildly correlated with the maximum flexion angle of the knee (r=0.51, P=0.019) (Figure 6B). A later cam-post engagement may correlate to greater flexions. If the factors that affect cam-post engagement timing can be established, proper manipulation of those factors may improve the function of the knee after PS TKA.
Summary
Postoperative knee kinematics are critical to the clinical success of the TKA knees. Previous literature has reported paradoxical anterior translation and reduced range of posterior femoral condyle translations of TKA knees (3,5-7,9,11,18,38-41). Most of the contemporary TKAs, on average, did not result in maximum knee flexion angles beyond 120° (42,43). Therefore, improved understanding of the knee kinematics after TKA is critical for improvement of the TKA design and surgical implants. Our lab has over 20 years of experience on analyzing both in vitro and in vivo TKA kinematics. This article reviewed the major results of our studies on CR and PS TKAs.
In vitro studies
The data of our in vitro studies indicated that most of the posterior femoral translation and tibial rotation could be restored following TKAs using the CR or PS prosthesis. However, these conditions alone may not be sufficient to fully produce the amount of knee flexion that is observed in the intact knee. The clinical outcome after TKA may be affected by other factors, such as preoperative range of motion, flexion space balancing, posterior tibiofemoral articular contact stability, quadriceps contraction, and patient motivation (44). The data did show that the PCL is an important structure in CR TKAs in the mid-range of knee flexion and proper balancing is imperative to the kinematics success of the implant. The cam-spine engagement is valuable in restoring posterior femoral translation in the PS TKAs after engagement. The anterior tibial post impingement in a PS TKA was shown to function as a substitute for the ACL during hyperextension, contributing to anterior stability. However, anterior post impingement could result in additional polyethylene wear and tibial post failure. Transmitted impingement forces might also cause backside wear and component loosening. Understanding the advantages and disadvantages of the tibial post function may help to further improve component design and surgical techniques and thus enhance knee stability and component longevity after TKA.
These data may serve as an aid in the development of a rationale for additional improvement in surgical techniques and prosthesis designs, so that normal knee function may be restored. However, it should be noted that the clinical relevance of the in vitro data should be verified using in vivo observations.
In vivo studies
The data on in vivo TKA kinematics can help understand the biomechanical functions of the knee with end-stage knee OA after contemporary CR or PS TKAs. Our studies indicated that the CR or PS TKA could result in significant changes in kinematics of the knees in both anteroposterior and mediolateral directions.
The current CR TKA systems and surgical techniques may not adequately re-establish normal biomechanics of PCL bundles and the collateral ligament bundles after surgery. Our data suggest that a well-balanced knee intraoperatively might not necessarily result in mid- and deep-flexion balance during functional weight-bearing motion. This implies mid-flexion instability and deep flexion tightness of the knee. The results indicated that the length increases of the collateral ligaments at maximum flexion after TKA were associated with the decreases of the maximum flexion of the knee. Collateral ligament management should be optimized at higher knee flexion angles in order to optimize maximum flexion after TKAs.
In PS TKAs, while excessive anterior tibial post contact may cause polyethylene wear and potentially lead to failure, we found that the tibial post may also act as a substitute for the ACL at low flexion, thus providing stability to the joint after posterior stabilizing TKAs. Posterior engagement of the cam-post did help improve posterior femoral translation, but disengagement was also noticed in high flexion. Timing of the initial posterior engagement of the cam-spine mechanism was associated with the maximal flexion of the knee.
Conclusions
These data provide insights into the kinematic characters of contemporary TKAs, such as mid-range instability, deep knee flexion and may be useful for improvement of future prosthesis designs and surgical techniques for the treatment of knees with end-stage OA. Future longitudinal studies should determine the influence of the changed in vivo knee kinematics on the longevity of polyethylene liner and long-term clinical outcomes of the TKA.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2016.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Archibeck MJ, White RE Jr. What's new in adult reconstructive knee surgery. J Bone Joint Surg Am 2006;88:1677-86. [Crossref] [PubMed]
- Harris WH, Sledge CB. Total hip and total knee replacement (2). N Engl J Med 1990;323:801-7. [Crossref] [PubMed]
- Watanabe T, Ishizuki M, Muneta T, et al. Knee kinematics in anterior cruciate ligament-substituting arthroplasty with or without the posterior cruciate ligament. J Arthroplasty 2013;28:548-52. [Crossref] [PubMed]
- Cho SH, Cho HL, Lee SH, et al. Posterior femoral translation in medial pivot total knee arthroplasty of posterior cruciate ligament retaining type. J Orthop 2013;10:74-8. [Crossref] [PubMed]
- Daniilidis K, Skwara A, Vieth V, et al. Highly conforming polyethylene inlays reduce the in vivo variability of knee joint kinematics after total knee arthroplasty. Knee 2012;19:260-5. [Crossref] [PubMed]
- Moynihan AL, Varadarajan KM, Hanson GR, et al. In vivo knee kinematics during high flexion after a posterior-substituting total knee arthroplasty. Int Orthop 2010;34:497-503. [Crossref] [PubMed]
- Omori G, Onda N, Shimura M, et al. The effect of geometry of the tibial polyethylene insert on the tibiofemoral contact kinematics in Advance Medial Pivot total knee arthroplasty. J Orthop Sci 2009;14:754-60. [Crossref] [PubMed]
- Scott RD. The evolving incidence and reasons for re-operation after fixed-bearing PCL retaining total knee arthroplasty. J Bone Joint Surg Br 2012;94:134-6. [Crossref] [PubMed]
- Sharkey PF, Hozack WJ, Rothman RH, et al. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res 2002;7-13. [Crossref] [PubMed]
- Naudie DD, Ammeen DJ, Engh GA, et al. Wear and osteolysis around total knee arthroplasty. J Am Acad Orthop Surg 2007;15:53-64. [Crossref] [PubMed]
- Harman MK, Banks SA, Hodge WA. Polyethylene damage and knee kinematics after total knee arthroplasty. Clin Orthop Relat Res 2001;383-93. [Crossref] [PubMed]
- Li G, Most E, Otterberg E, et al. Biomechanics of posterior-substituting total knee arthroplasty: an in vitro study. Clin Orthop Relat Res 2002;214-25. [Crossref] [PubMed]
- Pijls BG, Nelissen RG. The era of phased introduction of new implants. Bone Joint Res 2016;5:215-7. [Crossref] [PubMed]
- Matsuzaki T, Matsumoto T, Muratsu H, et al. Kinematic factors affecting postoperative knee flexion after cruciate-retaining total knee arthroplasty. Int Orthop 2013;37:803-8. [Crossref] [PubMed]
- Baier C, Springorum HR, Götz J, et al. Comparing navigation-based in vivo knee kinematics pre- and postoperatively between a cruciate-retaining and a cruciate-substituting implant. Int Orthop 2013;37:407-14. [Crossref] [PubMed]
- Kitagawa A, Tsumura N, Chin T, et al. In vivo comparison of knee kinematics before and after high-flexion posterior cruciate-retaining total knee arthroplasty. J Arthroplasty 2010;25:964-9. [Crossref] [PubMed]
- Most E, Sultan PG, Park SE, et al. Tibiofemoral contact behavior is improved in high-flexion cruciate retaining TKA. Clin Orthop Relat Res 2006;59-64. [Crossref] [PubMed]
- Suggs JF, Kwon YM, Durbhakula SM, et al. In vivo flexion and kinematics of the knee after TKA: comparison of a conventional and a high flexion cruciate-retaining TKA design. Knee Surg Sports Traumatol Arthrosc 2009;17:150-6. [Crossref] [PubMed]
- Lewandowski PJ, Askew MJ, Lin DF, et al. Kinematics of posterior cruciate ligament-retaining and -sacrificing mobile bearing total knee arthroplasties. An in vitro comparison of the New Jersey LCS meniscal bearing and rotating platform prostheses. J Arthroplasty 1997;12:777-84. [Crossref] [PubMed]
- Li G, Zayontz S, Most E, et al. Cruciate-retaining and cruciate-substituting total knee arthroplasty: an in vitro comparison of the kinematics under muscle loads. J Arthroplasty 2001;16:150-6. [Crossref] [PubMed]
- Skinner HB, Barrack RL, Cook SD, et al. Joint position sense in total knee arthroplasty. J Orthop Res 1984;1:276-83. [Crossref] [PubMed]
- Hilding MB, Lanshammar H, Ryd L. Knee joint loading and tibial component loosening. RSA and gait analysis in 45 osteoarthritic patients before and after TKA. J Bone Joint Surg Br 1996;78:66-73. [PubMed]
- Li G, Suggs J, Hanson G, et al. Three-dimensional tibiofemoral articular contact kinematics of a cruciate-retaining total knee arthroplasty. J Bone Joint Surg Am 2006;88:395-402. [Crossref] [PubMed]
- Banks SA, Harman MK, Bellemans J, et al. Making sense of knee arthroplasty kinematics: news you can use. J Bone Joint Surg Am 2003;85-A:64-72. [PubMed]
- Li G, Wuerz TH, DeFrate LE. Feasibility of using orthogonal fluoroscopic images to measure in vivo joint kinematics. J Biomech Eng 2004;126:314-8. [Crossref] [PubMed]
- Most E, Zayontz S, Li G, et al. Femoral rollback after cruciate-retaining and stabilizing total knee arthroplasty. Clin Orthop Relat Res 2003;101-13. [Crossref] [PubMed]
- Bingham J, Li G. An optimized image matching method for determining in-vivo TKA kinematics with a dual-orthogonal fluoroscopic imaging system. J Biomech Eng 2006;128:588-95. [Crossref] [PubMed]
- Most E, Li G, Sultan PG, et al. Kinematic analysis of conventional and high-flexion cruciate-retaining total knee arthroplasties: an in vitro investigation. J Arthroplasty 2005;20:529-35. [Crossref] [PubMed]
- Li G, Most E, Sultan PG, et al. Knee kinematics with a high-flexion posterior stabilized total knee prosthesis: an in vitro robotic experimental investigation. J Bone Joint Surg Am 2004;86-A:1721-9. [PubMed]
- Li G, Papannagari R, Most E, et al. Anterior tibial post impingement in a posterior stabilized total knee arthroplasty. J Orthop Res 2005;23:536-41. [Crossref] [PubMed]
- Li C, Hosseini A, Tsai TY, et al. Articular contact kinematics of the knee before and after a cruciate retaining total knee arthroplasty. J Orthop Res 2015;33:349-58. [Crossref] [PubMed]
- Yue B, Varadarajan KM, Moynihan AL, et al. Kinematics of medial osteoarthritic knees before and after posterior cruciate ligament retaining total knee arthroplasty. J Orthop Res 2011;29:40-6. [Crossref] [PubMed]
- Yue B, Varadarajan KM, Rubash HE, et al. In vivo function of posterior cruciate ligament before and after posterior cruciate ligament-retaining total knee arthroplasty. Int Orthop 2012;36:1387-92. [Crossref] [PubMed]
- Suggs JF, Hanson GR, Park SE, et al. Patient function after a posterior stabilizing total knee arthroplasty: cam-post engagement and knee kinematics. Knee Surg Sports Traumatol Arthrosc 2008;16:290-6. [Crossref] [PubMed]
- Hanson GR, Suggs JF, Kwon YM, et al. In vivo anterior tibial post contact after posterior stabilizing total knee arthroplasty. J Orthop Res 2007;25:1447-53. [Crossref] [PubMed]
- Dimitriou D, Tsai TY, Park KK, et al. Weight-bearing condyle motion of the knee before and after cruciate-retaining TKA: In-vivo surgical transepicondylar axis and geometric center axis analyses. J Biomech 2016;49:1891-8. [Crossref] [PubMed]
- Park KK, Hosseini A, Tsai TY, et al. Elongation of the collateral ligaments after cruciate retaining total knee arthroplasty and the maximum flexion of the knee. J Biomech 2015;48:418-24. [Crossref] [PubMed]
- Mikashima Y, Tomatsu T, Horikoshi M, et al. In vivo deep-flexion kinematics in patients with posterior-cruciate retaining and anterior-cruciate substituting total knee arthroplasty. Clin Biomech (Bristol, Avon) 2010;25:83-7. [Crossref] [PubMed]
- Yoshiya S, Matsui N, Komistek RD, et al. In vivo kinematic comparison of posterior cruciate-retaining and posterior stabilized total knee arthroplasties under passive and weight-bearing conditions. J Arthroplasty 2005;20:777-83. [Crossref] [PubMed]
- Dennis DA, Komistek RD, Mahfouz MR, et al. Multicenter determination of in vivo kinematics after total knee arthroplasty. Clin Orthop Relat Res 2003;37-57. [Crossref] [PubMed]
- Chouteau J, Lerat JL, Testa R, et al. Kinematics of a cementless mobile bearing posterior cruciate ligament-retaining total knee arthroplasty. Knee 2009;16:223-7. [Crossref] [PubMed]
- Sumino T, Gadikota HR, Varadarajan KM, et al. Do high flexion posterior stabilised total knee arthroplasty designs increase knee flexion? A meta analysis. Int Orthop 2011;35:1309-19. [Crossref] [PubMed]
- Sumino T, Rubash HE, Li G. Does cruciate-retaining total knee arthroplasty enhance knee flexion in Western and East Asian patient populations? A meta-analysis. Knee 2013;20:376-83. [Crossref] [PubMed]
- Sultan PG, Most E, Schule S, et al. Optimizing flexion after total knee arthroplasty: advances in prosthetic design. Clin Orthop Relat Res 2003;167-73. [Crossref] [PubMed]
Cite this article as: Li G, Kernkamp WA, Rubash HE. In vitro and in vivo kinematics of total knee arthroplasty—a review of the research at the Orthopaedic Bioengineering Laboratory of the Massachusetts General Hospital (MGH). Ann Joint 2016;1:20.


