
Microstructural properties of the anterior cruciate ligament
Introduction
The anterior cruciate ligament (ACL) of the knee is one of the most commonly injured structures in musculoskeletal medicine (1). The ACL provides rotatory stability to the knee and functions as a restraint to anterior translation of the tibia with respect to the femur (2). The important role the ACL serves in knee stability and function explains why individuals with ACL tears can experience debilitating instability, particularly with cutting and pivoting activities, which often requires surgical reconstruction (1).
Several studies have previously analyzed the macroscopic biomechanics of the whole human ACL, however, tissue level analyses have been limited by preparation techniques and imaging technology (3-9). Previously, characterizations of ACL tissue properties have even been based on constitutive models derived from experiments using animal ligaments (10-12).
A novel imaging technique has recently been applied to the ACL called quantitative polarized light imaging (QPLI) which has enabled the simultaneous measurement of full material properties of human ACL regions compensating for these previous measurement deficits. This polarized light technique enables quantification of the microstructural and fiber alignment properties of the tissue in a dynamic setting (13,14). This review will summarize recent developments in the field related to the anatomy, histology, and material properties of the human ACL.
General anatomy of the ACL
The ACL is located in the femoral notch of the knee connecting the femur and the tibia. It functions, along with the posterior cruciate ligament, as an instantaneous center of rotation for the knee controlling knee kinematics. The ACL originates from the lateral wall fossa of the notch on the femur and attaches in the anterior aspect of the middle tibia beneath the transverse meniscal ligament. This positioning also allows the ACL to contribute to rotational stability. The average ACL length is 32 mm (range, 22–41 mm) and its width ranges from 7 to 12 mm (15).
There is significant debate in the literature regarding whether the ACL is a single construct of tissue or if it is composed of more than one bundle of tissue. Anatomic dissections have demonstrated that the ligament consists of two, three and even four distinct bundles, while other studies have described the ACL as a continuum of numerous multiple bundles or fascicles (9,16-19). From a macroscopic anatomical position, most studies agree there are two main anatomic bundles of the ACL; the anteromedial (AM) bundle and the posterolateral (PL) bundle (Figure 1) (20). These names are derived from the bony attachments of the ligament. The AM bundle becomes tight in flexion and functions mainly to resist anterior translation while the PL bundle becomes tight in extension and is a greater restraint to rotatory motion (16,21).
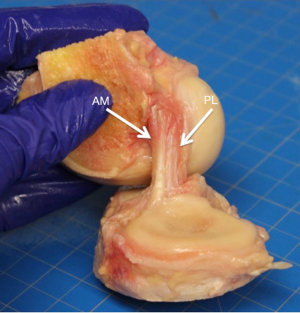
Histology of the ACL
The two bundles are oriented in a helical formation, which adds to the unique and complex ligament structure and function. This formation is believed to contribute to the ability to resist forces in multiple directions in such a large joint (16). The ACL is a dense collagenous connective tissue and is covered in a synovial membrane.
Ferretti et al. performed an anatomic and histologic analysis of 40 fetal ACLs which supported the theory that the ACL is two main bundles (20). They found that the bundles were highly cellular and separated by a tissue septum, with connective tissue that differed in density from the femur to the tibia. In a recent study by Beaulieu et al., a histologic assessment of the tissue properties of the ACL at the bony attachments, using 15 adult human cadaveric ACLs sectioned into four regions, found differences between the AM and PL bundles (22). The AM bundle had 33% more calcified fibrocartilage and 143% more uncalcified fibrocartilage. Additionally, the AM fibers attached to the femur at a greater angle compared to the PL bundle. These differences, when compared to the findings in the Ferretti et al. study, demonstrate that the ACL undergoes histologic changes with loading and age.
Petersen and Tillmann [1999] found that the major collagen composition of the ACL is Type I collagen while the loose connective tissue is Type III collagen. They also found that the AM and PL bundles were composed of different cells on histologic sections. The AM bundles, especially where it contacts the cartilage of the notch in extension, has more chondroid cells that secrete Type II collagen (23). The differences between the bundle regions on histologic analysis have also been studied with regard to biomechanics.
Biomechanics
Butler et al. performed some of the first macroscopic biomechanical analyses of the ACL and found that the AM bundle has greater failure load than the PL bundle (3,4). A recent study by Skelley et al. (24), utilizing QPLI to quantify mechanical properties and collagen organization in the ligament simultaneously, supported these findings. The AM bundle possessed greater collagen tensile strength under load compared to the PL bundle.
In this study, sixteen human ACLs were dissected into AM and PL bundles based on previous dissection techniques in the literature. Three samples were taken from each bundle in an ordered sequence from AM (region 1 AM bundle) to PL (region 6 PL bundle) (Figure 2). Each sample was tested in uniaxial tension, using QPLI to quantify collagen fiber alignment. After preconditioning, samples were subjected to a stress-relaxation (SR) test followed by quasi-static ramp-to-failure (RF). Peak and equilibrium stress values were computed from the SR test and modulus quantified in the toe- and linear-regions of the RF. QPLI values describing collagen orientation (AoP = angle of polarization) and strength of alignment (DoLP = degree of linear polarization) were computed for the SR test and at points corresponding to the zero, transition point, and linear region of the RF.
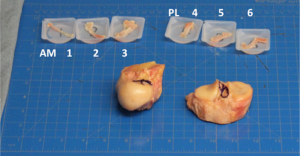
With regards to the microstructural fiber alignment, the AM bundle demonstrated greater fiber alignment during the RF than the PL bundle. The AM samples also exhibited larger peak/equilibrium stresses and less stress-relaxation during a 300-s hold compared to PL samples (25). The AM bundle demonstrated stronger and more uniform collagen fiber alignment (i.e., higher DoLP) values and less distributed AoP values compared to the PL bundle, and larger changes in alignment strength during the hold. These biomechanical and microstructural analyses led the authors to conclude that the AM bundle was composed of more aligned and stronger tissue. Essentially, the AM bundle was the “dominant” bundle.
These studies, however, did not assess the relative uniformity of tissue properties nor elucidate overall regional variation across the ligament. Such an analysis is important to demonstrate whether the ACL is truly composed of two discrete bundles with distinct properties, or whether properties vary in a gradient-like manner, suggesting a continuity of material properties across the ligament. Therefore, in a follow-up study to the Skelley et al. study, the 6 regions were further analyzed to determine if the mechanical and structural properties changed within the three AM regions and within the three PL regions. In this manner, the properties could be measured across the tissue bundles of the ligament (26). Toe- and linear-region modulus values decreased from region 1 to 6. Slopes of regression lines increased for average DoLP during RF, with significant linear variation across the ACL regions at higher strains. Standard deviation AoP values decreased during RF, indicating tighter distribution of orientation angles, with significant correlations at all points of the RF. During SR, stress values uniformly decreased, but did not show significant linear regression by region. Average DoLP and the standard deviation of AoP values changed slightly during SR, and demonstrated significant linear variation by region at both peak and equilibrium points (26).
The authors found that the microstructural and material properties evaluated appear to follow a linear gradient across the ACL, rather than varying just by bundle (26). This AM-to-PL variation provides a more accurate description of functional tissue anatomy. This also supports that on a tissue level, the ACL may not be just two discrete bundles. It may instead be a continuum of smaller tissue regions that differ throughout the ACL and the bundles are more related to anatomic attachment sites and tissue orientation.
Summary
Recent studies have focused on the anatomy, histology, microstructure, and biomechanical properties of the human ACL. Novel polarization imaging techniques have assisted in the analysis of the ACL on this microstructure level. Results have demonstrated that the AM and PL bundles exhibit significantly different properties. Most notably, the AM bundle is stronger/stiffer and has more strongly aligned collagen fibers when loaded. However, the more clinically relevant finding is that the material and microstructural human ACL properties follow a linear gradient across the ligament, rather than grouping by distinct bundle. This information provides a greater understanding of the native properties of the human ACL and serves as a guide for surgical approaches to reconstruct this ligament.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Freddie H. Fu and Jeremy M. Burnham) for the series “Trends in ACL Reconstruction” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The series “Trends in ACL Reconstruction” was commissioned by the editorial office without any funding or sponsorship. RB receives speaking fees from Arthrex. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheatham SA, Johnson D. Current concepts in ACL Injuries. Phys Sportsmed 2010;38:61-8. [Crossref] [PubMed]
- Lubowitz JH. Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc 2014;22:1190-5. [Crossref] [PubMed]
- Butler DL, Guan Y, Kay MD, et al. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech 1992;25:511-8. [Crossref] [PubMed]
- Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech 1986;19:425-32. [Crossref] [PubMed]
- Dargel J, Gotter M, Mader K, et al. Biomechanics of the anterior cruciate ligament and implica-tions for surgical reconstruction. Strategies Trauma Limb Reconstr 2007;2:1-12. [Crossref] [PubMed]
- Kato Y, Ingham SJM, Maeyama A, et al. Biomechanics of the human triple-bundle anterior cruciate ligament. Arthroscopy 2012;28:247-54. [Crossref] [PubMed]
- Mae T, Shino K, Miyama T, et al. Single- versus two-femoral socket anterior cruciate ligament reconstruction technique: Biomechanical analysis using a robotic simulator. Arthroscopy 2001;17:708-16. [Crossref] [PubMed]
- Noyes FR, DeLucas JL, Torvik PJ. Biomechanics of anterior cruciate ligament failure: an analy-sis of strain-rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg Am 1974;56:236-53. [Crossref] [PubMed]
- Sapega AA, Moyer RA, Schneck C, et al. Testing for isometry during reconstruction of the ante-rior cruciate ligament. Anatomical and biomechanical considerations. J Bone Joint Surg Am 1990;72:259-67. [Crossref] [PubMed]
- De Vita R, Slaughter WS. A structural constitutive model for the strain rate-dependent behavior of anterior cruciate ligaments. Int J Solids Struct 2006;43:1561-70. [Crossref]
- Danto MI, Woo SL. The mechanical properties of skeletally mature rabbit anterior cruciate ligament and patellar tendon over a range of strain rates. J Orthop Res 1993;11:58-67. [Crossref] [PubMed]
- Pioletti DP, Rakotomanana L, Leyvraz PF. Strain rate effect on the mechanical behavior of the anterior cruciate ligament–bone complex. Medical Engineering & Physics 1999;21:95-100. [Crossref] [PubMed]
- York T, Kahan L, Lake SP, et al. Real-time high-resolution measurement of collagen alignment in dynamically loaded soft tissue. J Biomed Opt 2014;19:066011 [Crossref] [PubMed]
- York T, Powell SB, Gao S, et al. Bioinspired Polarization Imaging Sensors: From Circuits and Optics to Signal Processing Algorithms and Biomedical Applications. Proceedings of the IEEE 2014;102:1450-69. [Crossref] [PubMed]
- Amis AA, Dawkins GP. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br 1991;73:260-7. [PubMed]
- Gobbi A, Mahajan V, Karnatzikos G, et al. Single- versus double-bundle ACL reconstruction: is there any difference in stability and function at 3-year followup? Clin Orthop Relat Res 2012;470:824-34. [Crossref] [PubMed]
- Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res 1983;19-25. [PubMed]
- Norwood LA, Cross MJ. Anterior cruciate ligament: functional anatomy of its bundles in rotato-ry instabilities. Am J Sports Med 1979;7:23-6. [Crossref] [PubMed]
- Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg Am 1985;67:257-62. [Crossref] [PubMed]
- Ferretti M, Levicoff EA, Macpherson TA, et al. The fetal anterior cruciate ligament: an anatomic and histologic study. Arthroscopy 2007;23:278-83. [Crossref] [PubMed]
- Fu FH. Double-Bundle ACL Reconstruction. Orthopedics 2011;34:281-3. [Crossref] [PubMed]
- Beaulieu ML, Carey GE, Schlecht SH, et al. On the heterogeneity of the femoral enthesis of the human ACL: microscopic anatomy and clinical implications. J Exp Orthop 2016;3:14. [Crossref] [PubMed]
- Petersen W, Tillmann B. Structure and vascularization of the cruciate ligaments of the human knee joint. Anat Embryol 1999;200:325-34. [Crossref] [PubMed]
- Skelley NW, Castile RM, York TE, et al. Differences in the microstructural properties of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Am J Sports Med 2015;43:928-36. [Crossref] [PubMed]
- Castile RM, Skelley NW, Babaei B, et al. Microstructural properties and mechanics vary be-tween bundles of the human anterior cruciate ligament during stress-relaxation. J Biomech 2016;49:87-93. [Crossref] [PubMed]
- Skelley NW, Castile RM, Cannon PC, et al. Regional Variation in the Mechanical and Micro-structural Properties of the Human Anterior Cruciate Ligament. Am J Sports Med 2016;44:2892-9. [Crossref] [PubMed]
Cite this article as: Skelley NW, Lake SP, Brophy RH. Microstructural properties of the anterior cruciate ligament. Ann Joint 2017;2:19.


