
An innovative concept for an old therapy
Muscle weakness and decreased functional capacity are common after knee surgery. Recovery duration and success strongly depend on post-surgery rehabilitation. Although neuromuscular electrical stimulation (NMES) therapy has been used for more than 30 years for muscle rehabilitation and strengthening after orthopedic surgery, there is no consensus on the optimal application of this therapy. Several studies have investigated the NMES effectiveness for different orthopedic surgeries but the present literature remains equivocal; various approaches to NMES application have been taken and only two studies have met consistent criteria for Cochrane meta-analysis (1). In this issue of Annals of Joint, Spector et al. proposed a new approach to NMES application after orthopedic surgery that combines previous evidence-based recommendations. The authors focused on two main goals: gains in muscle strength and increases in voluntary activation. Their approach is both prospective and comprehensive, the authors suggest that the medical team and the patient participate in NMES therapy both pre- and post-surgery and that both muscle strength and voluntary activation be regularly evaluated.
The mechanisms of NMES have been previously investigated but remain incompletely understood. This may account for some reluctance of the physicians to employ NMES therapy. Indeed, NMES therapy is often considered inferior to voluntary contraction for therapy. However, NMES can result in equivalent improvements and may actually offer particular advantages. For example, NMES stimulates protein synthesis to the same extent as voluntary contraction (2). Furthermore, despite the fact that NMES is applied peripherally, central adaptations have been reported in cortical and subcortical areas due to activation of cutaneous afferents during NMES stimulation. In addition, the local muscle metabolic demand during NMES training is at least comparable, and potentially higher than during volitional muscle contraction. One important drawback of NMES is that only one muscle group is trained per session, hence it cannot improve muscle coordination as with voluntary activation. Yet, a key advantage is that NMES can be initiated soon after surgery, notably when voluntary activation is very low. Perhaps the best approach would be to combine NMES and voluntary strength training rather than employing only one, since they have complementary effects that may reduce recovery duration and improve outcomes. Indeed, the algorithm proposed is a good example of a complementary approach wherein NMES therapy is performed directly after surgery and is progressively replaced by volitional muscle contractions.
The primary point of this review is that NMES therapy should be performed at high intensity and volume. Indeed, high levels of NMES-induced contraction with a high volume of training seem to be required for significant gains in muscle strength after knee surgery (3). It is also known that a current intensity threshold must be exceeded during NMES training session for both structural (muscle mass) and functional (walking distance) changes (2). This is the rationale for regular supervision of this therapy to ensure adequate NMES-induced force during training. However, high intensity/high volume NMES therapy must be accompanied by short duration contractions to avoid muscle damage due to over training. Accordingly, a long rest period should also be incorporated in these training protocols (e.g., 30 s instead of the usual 10 to 15 s as proposed in the present review).
The concept of early NMES therapy after surgery has been introduced previously in orthopedics and is another important consideration (4). In this review, the authors suggest that NMES should be initiated during the 3 first weeks after surgery and ideally with a familiarization phase performed before the surgery. Although this would definitely increase the likelihood of improved muscle function, the clinical application may be difficult since it would require both short-term access to care after surgery and frequent visits to the physiotherapist/clinical team. Further work needs to address the clinical feasibility of this innovative algorithm.
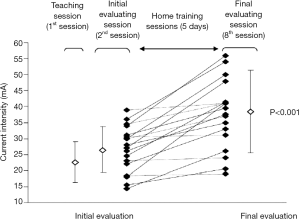
Finally, the review makes clear that it may be important to identify early responders to NMES after the first week of NMES therapy. Early responders demonstrate rapid gains in muscle strength and/or functional capacity after only a few training sessions. This is related to tolerance to NMES, that is, a relative insensitivity to the skin tingling and muscle tetanic contraction linked to high electrical current. Indeed, tolerance to NMES is individual-specific, and changes over time, mostly during the first week of training. As an example, we previously showed in chronic respiratory disease that reaching a high level of current intensity is necessary to increase muscle volume but also walking distance after 6 weeks (2). We later observed that the first week of training seems to be a good indicator of tolerance to NMES (5). As can be seen in Figure 1, ability to tolerate NMES can be easily identified after seven sessions by comparing the difference in stimulation current intensity between responders vs. non-responders. The authors of the present review additionally suggest that responders may be more likely to meet clinical criteria (i.e., visual or palpable evidence of superior patellar glide, the Fitzgerald criteria). This simple approach to identify responders to the therapy should be strongly considered by clinicians.
To conclude, NMES therapy is an interesting tool that promotes early improvement in muscle function after orthopedic surgery and so approaches to facilitate its application should be explored. A major limitation of NMES training is the absence of adequate technology for home-based, autonomous access. Devices cannot be used directly by untrained individuals due to the complexity of electrode positioning, the specificity of training parameters, and the need for follow-up. There is thus an urgent need for a consensus on specific algorithms for NMES therapy after orthopedic surgery derived from randomized controlled trials in these clinical populations. However, without cooperation of the industry to build new devices, home-based and self-application of NMES would still remain challenging.
Acknowledgments
The author is grateful to Dr. Andrew Taylor for critically editing the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Executive Editor-in-Chief, Dongquan Shi, MD, PhD (Department of Sports Medicine and Adult Reconstruction, Drum Tower Hospital, Medical School, Nanjing University, Nanjing, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.05.02). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Monaghan B, Caulfield B, O'Mathúna DP. Surface neuromuscular electrical stimulation for quadriceps strengthening pre and post total knee replacement. Cochrane Database Syst Rev 2010;CD007177 [PubMed]
- Vivodtzev I, Debigaré R, Gagnon P, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest 2012;141:716-25. [Crossref] [PubMed]
- Kittelson AJ, Stackhouse SK, Stevens-Lapsley JE. Neuromuscular electrical stimulation after total joint arthroplasty: a critical review of recent controlled studies. Eur J Phys Rehabil Med 2013;49:909-20. [PubMed]
- Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012;92:210-26. [Crossref] [PubMed]
- Vivodtzev I. Tolerance and physiological correlates of neuromuscular electrical stimulation in COPD: a pilot study. PLoS One 2014;9:e94850 [Crossref] [PubMed]
Cite this article as: Vivodtzev I. An innovative concept for an old therapy. Ann Joint 2017;2:23.


