Mini-posterior approach for primary total hip arthroplasty
Introduction: defining the mini-posterior approach (MPA)
Over the past half-century, total hip arthroplasty (THA) has emerged as one of the most successful orthopaedic surgeries currently performed in terms of pain relief, cost effectiveness, and clinical outcomes (1,2). As definitive treatment for end-stage arthritis of the hip, THA is also one of the most commonly performed major orthopaedic surgeries today, estimated at approximately 300,000 procedures performed per year. This number is growing in excess of 5% per year (3), and with changing US population demographics it is projected to nearly double to 574,000 per year by 2030 (4).
Generally speaking, THA can be performed with an excellent safety profile through any number of surgical approaches including anterior, anterolateral, direct lateral (DL), and posterior (among others). Historically, the posterior approach has been the most commonly utilized approach for primary THA within the community of US orthopaedic surgeons. When coupled with posterior capsular and/or external rotator muscular repair, the posterior approach has been associated with excellent outcomes following THA including a very low rate of dislocation, most commonly cited as between 0.5% and 1.0% (5,6). In addition, due in part to its extensile nature and excellent capacity for exposure of the proximal femur and acetabulum, the posterior approach has long been considered the standard-bearer to which new and/or modified surgical approaches for THA are compared.
In the past two decades, the advent of minimally invasive surgery (MIS) in orthopaedics has also manifested itself in total joint replacement via modifications of the ‘standard’ approaches for THA, including the posterior approach. The so-called ‘MPA’ has emerged in the past 10 years as a modification of its ‘standard’ predecessor. Despite this seemingly dichotomous existence of MPA versus standard posterior THA, it is actually somewhat difficult to establish a consensus definition of what constitutes a MPA.
Incision length is perhaps the simplest and most measurable way of quantifying/categorizing a surgical approach as either standard posterior or MPA. Many authors use a cutoff of <10 cm as a line of demarcation for MPA (7-9). However, others have noted that incision length is an imperfect proxy for the amount of deep surgical (e.g., muscular and capsular) dissection. They also note that the deep tissue planes are where the more important distinctions should actually be drawn as they appear to be the determinants of postoperative function, recovery, and joint stability. Accordingly, other studies have defined the MPA as muscle-sparing, often referencing the non-violation of gluteus maximus insertion, the quadratus femoris, and/or the piriformis tendons (10-13). Acknowledging the decreased direct visualization afforded by a shorter incision, some surgeons using the MPA incorporate robotic or computer navigation to ensure appropriate implant positioning (14). This carries consequences in terms of cost and operating room (OR) setup time (15).
This review will describe pertinent details of the MPA surgical technique, as well as a review of pertinent data on this technique relative to standard posterior approach as well as alternative surgical approaches for primary THA. Following this discussion, we will provide a detailed review of the authors’ preferred surgical technique.
Literature review
MPA versus standard posterior approach
Multiple well-designed studies in the past decade have directly compared the MPA versus ‘standard’ technique posterior approach THA. It is difficult to aggregate results as each author has a different definition of the MPA (as described earlier) and study setups differ. As will be seen, the outcomes of these studies are also quite variable. Varela Egocheaga et al. performed a prospective randomized study with a series of 50 primary THA patients randomized into MPA (defined as incision length <10 cm) versus standard posterior THA (9). At 1 year follow-up, the authors found that the MPA was associated with less pain, shorter hospital length of stay, and an earlier start in walking postoperatively (9). As a result of these improvements, they estimated total cost savings of the MPA at 5% per patient.
Fink et al. in 2010 performed a matched cohort study which, at maximum follow-up of six weeks, seemed to support the positive impact of the MPA (12). They found that the MPA (defined as quadratus-sparing) was associated with lower surgical blood loss, lower pain at rest, and a faster recovery (12). However, the authors found no difference in postoperative lab values including C-reactive protein (CRP) and creatine phosphokinase (CPK), used as markers of tissue invasiveness of the procedure (12). Khan et al. performed a randomized controlled trial with 100 patients using a piriformis-sparing MPA technique (13). They found a trend towards improved walking times and patient satisfaction at 6 weeks (not statistically significant), but also a tendency towards cup malposition (less anteversion and inclination, P=0.005) (13). They also reported that the surgeons participating in the study found the MPA significantly more difficult, and potentially not worth the short-term benefits imparted by the approach (13). In a study published in 2011, Goosen et al. described a learning curve associated with adoption of the MPA (mean initial incision length 7.8 cm), but noted that Harris Hip scores were higher in a cohort of patients who had undergone MPA approaches, despite longer operative times (7).
Other studies have found that the MPA and standard posterior approaches are essentially equivalent to one another. Ogonda et al. in 2005 performed a randomized controlled trial (219 hips) that showed no difference in early postoperative outcomes, defining MPA by incision length (<10 cm) (16). Chimento et al., in a similarly designed study, showed no difference in operative time, transfusion rate, length of stay, or postoperative complication rate (11). Hart et al., in cemented THA, showed no significant difference in component position between the MPA (9–10 cm incision) and standard approaches (17). Shitama et al., defining MPA as <10 cm incision, found no difference in Harris Hip scores or postoperative radiographs in a randomized, blinded study. They noted that inflammatory markers obtained in the postoperative period did not support that a shorter incision length was associated with less tissue damage or surgical invasiveness (18).
There are also several studies that have identified potential disadvantages or pitfalls of the MPA relative to the standard posterior approach. In a series of 135 patients, Woolson et al. found that despite lower BMI in an MPA cohort (incision <10 cm), there was a higher rate of wound complications and implant malposition on both the acetabular and femoral side in postoperative radiographs (19). In a randomized controlled trial (RCT) of bilateral THA [one MPA (length <8 cm) and one standard), Kim et al found that there was a higher infection rate in the MPA cohort, but no difference in clinical outcomes (20).
The comparison of standard posterior versus MPA THA was assessed in a recent systematic review and meta-analysis by Berstock et al. (10). Aggregating the studies above and several others, they found small positive trends in favor of the safety and efficacy of the MPA. Specifically, they found that the MPA was associated with a decrease in operating time (5 minutes, P<0.001) and blood loss (63 mL intraoperative, P<0.001) relative to standard posterior surgery. Postoperatively, the MPA was associated with a slightly shorter hospital stay (14 hours, P<0.001) and early improvement in Harris hip scores (1.8 points P<0.001) relative to standard posterior approach (10). Their analysis found no significant difference in the rate of dislocation or femoral fracture between the two approaches. Whether the benefits above equate to clinically significant improvement and whether they outweigh the potential for increased risk of implant malposition or postoperative complication identified by several authors above is a matter for ongoing discussion. We are unaware of any studies evaluating long-term implant survival or outcomes with the MPA versus standard posterior approach for hip arthroplasty.
MPA versus direct anterior approach (DAA)
With the recent surge in popularity of the DAA, several studies have evaluated the DAA versus the MPA (10 cm incision). Taunton et al performed a prospective RCT of 54 patients comparing the two approaches, and found that DAA patients walked without assistive devices sooner than MPA (22 days for DAA, 28 days for MPA, P=0.04) patients, but that other clinical endpoints evaluated as markers of functional recovery [discontinuance of narcotics, climbing stairs, performing activities of daily living (ADL) independently, and walking 0.5 miles] were of no significant difference for either approach (21). Zawadsky et al. reviewed a series of 150 consecutive THA [50 MPA (10–12 cm incision length)], 50 DAA in the ‘learning curve’, and 50 DAA subsequent to the ‘learning curve’ (22). They found shorter length of stay (2.9 and 2.7 versus 3.9 days) for the DAA cohorts and increased propensity to discharge to home (80% and 84% versus 56%) relative to the MPA (22). They also found a lower use of assistive devices at 6 weeks (16% and 22% for the DAA learning curve and routine groups versus 69% for the MPA cohort, P<0.001), but did not have long-term outcomes available to report (22). Nakata et al. in a consecutive series of 195 hips found improved cup position (99% of cups within the Lewinnek ‘safe zone’ versus 91% for the MPA group), and suggestion of more rapid clinical recovery (measured by use of gait or walking aids) among DAA patients. Specifically, they found that DAA patients transitioned to a cane at 12.0 days versus 15.5 in the MPA cohort (P=0.009), and had a negative Trendelenburg sign at 16.7 days, versus 24.8 days in the MPA cohort (P=0.0002) (23). Of note, they did not comment on whether this difference was of clinical significance to their patients, and they did not offer a specific definition of what made their posterior approaches “mini-posterior”.
To our knowledge, no prospective randomized study to date has offered mid- or long-term data on clinical outcomes between the MPA and the DAA for THA.
MPA versus DL approach
Two studies have compared the MPA and the DL approaches. Schleicher et al performed a prospective analysis of 64 MPA versus 64 DL hips (24). They found no difference in perioperative metrics (surgical time, EBL, leg length discrepancy), but did find that the MPA had higher HHS and earlier rehab milestones at three and 6 months postoperatively (24). Similarly, Wenz et al. compared 124 MPA hips and 65 DL hips (25). They found that the MPA was associated with a 24% decrease in surgical time, an 18% decrease in blood loss, and fewer discharges to SNF (25).
MPA versus other MIS approaches
Meneghini et al. performed an intriguing analysis of three minimally invasive approaches for THA: MPA, a two-incision, and a mini-anterolateral (MAL) (26). Focusing on gait analysis, they found that all three cohorts in a small series improved, but that the MAL had a relative weakness in abductor function postoperatively, as evidenced by increased ground reaction forces (26). Evaluation of clinical outcomes was limited in this study (26).
Surgical technique
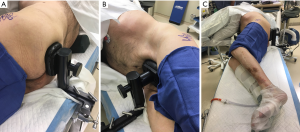
The author’s preferred technique begins with appropriate lateral positioning. Many options exist to secure patients in the lateral position, including bean bags, peg boards, and modular hip positioners. The important qualities of a hip positioner include: the ability to firmly hold the pelvis in the desired orientation, the versatility to be used for different body shapes and sizes, and the simplicity to be applied efficiently in the operating room. We use a Stulberg hip positioner to secure the patient in the lateral position, using two 6-inch posts with single pads on each post and a 2-inch or 4-inch spacer on the anterior post. The sequence of positioning begins by placing the patient on the operating table such that both Stulberg table attachments can be placed flush with the table (as opposed to angled), the anterior attachment directly anterior to the pubic symphysis, and the posterior attachment directly posterior to the sacrum. The patient is then carefully turned from the supine to the lateral position, and an axillary roll is placed approximately one hand breadth distal to the axilla. The posterior Stulberg slider is then positioned so that the posterior edge of the slider is flush with the posterior edge of the table attachment, which ensures consistent and appropriate distance of the patient from the surgeon. The patient is then moved posteriorly such that the sacrum contacts the posterior pad and held such that the pelvis is perpendicular to the floor (i.e., an imaginary line from one anterior superior iliac spine to the other is perpendicular to the floor), with the pad at or slightly above midline (Figure 1A). The anterior slider is then brought in to firmly contact the pubic symphysis to lock the pelvis into place (Figure 1B). Pillows are then placed between the arms to keep the torso level and foam is used to pad the lateral prominences at the ankle and knee. Occasionally, for larger patients, a rolled blanket is placed under the pannus for support. A final check from the foot of the table should ensure that the table is level and that the pelvis is indeed perpendicular to the floor (Figure 1C).
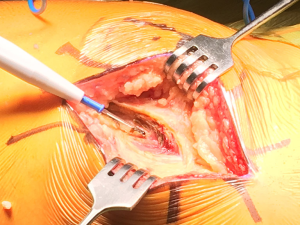
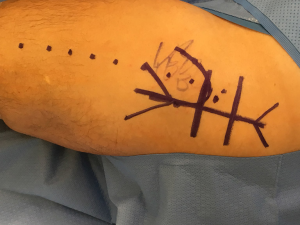
Following standard prepping and draping, we mark out the incision for the minimally invasive approach, typically centering the incision over the posterior edge of the greater trochanter and carrying the incision proximally and distally approximately 5 centimeters in each direction (Figure 2). While we are usually able to perform the procedure through this incision, patient size and body habitus occasionally necessitate extension of the incision in one or both directions. Following the incision, superficial dissection then proceeds through the subcutaneous tissue until the fascia over the greater trochanter is encountered. We find it useful to divide the fascia at the very proximal portion of the iliotibial band, and then carry the fascial incision proximally in line with the fibers of the gluteus maximus (Figure 3). This technique allows the surgeon to then split the maximus proximally in line with the natural raphe and reduce bleeding. A Charnley self-retaining retractor is then placed such that the blades are centered over the proximal greater trochanter and the closed loop is oriented distally, exposing the posterior border of the gluteus medius muscle and its insertion onto the greater trochanter.
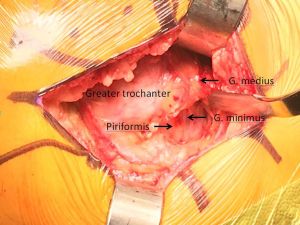
The deeper dissection now involves bluntly finding the natural gap between the undersurface of the gluteus medius muscle and the short external rotators posteriorly and retracting the gluteus medius anteriorly with an angled Hohmann retractor, exposing the piriformis tendon and short external rotators (Figure 4). An “L” shaped incision is then made parallel to and just cephalad to the piriformis tendon, keeping the piriformis, a portion of the gluteus minimus, and posterior capsule together as one tissue sleeve (Figure 5A and B). The distal extent of this capsular incision is then minimized to limit the tissue damage to the short external rotators. Often the quadratus femoris muscle is able to be spared completely, with variable stripping of the more proximal muscles. Once adequate exposure is obtained, the hip is dislocated posteriorly by placing the hip in flexion, adduction, and internal rotation. It is very important to minimize torque on the femoral shaft during this maneuver, as excessive force can result in fracture. Often we use a bone hook placed under the femoral neck to help lift the femoral head out of the acetabulum. Very rarely, if the femoral head cannot be safely dislocated, the femoral neck can be cut in situ with a wedge cut to mobilize the proximal femur.
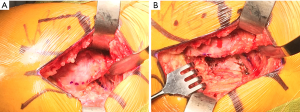
Following dislocation, a Hohmann retractor is placed along the medial calcar to protect the soft tissues (Figure 6) as the femoral neck cut is made perpendicular to the long axis of the neck, from the piriformis fossa laterally to approximately one finger breadth proximal to the lesser trochanter medially, although the precise amount of femoral neck excised is typically dependent on preoperative planning. Once the femoral head is removed, we begin to expose the acetabulum by placing the foot on a padded, elevated mayo stand such that the hip is flexed, adducted, and internally rotated, effectively moving the greater trochanter into a non-obstructive position and relaxing the musculoligamentous structures of the proximal femur to promote mobilization.
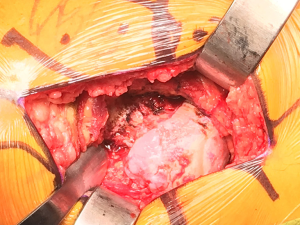
Appropriate acetabular exposure is then accomplished with three retractors (Figure 7). The first retractor is an angled Hohmann placed just over the anterior wall of the acetabulum, carefully positioned directly on the bone to minimize risk to the anterior neurovascular structures, retracting the femur anteriorly. In difficult cases, the femur can be further mobilized by dividing portions of the inferior capsule and the reflected head of the rectus femoris muscle. Rarely, the femoral insertion of the gluteus maximus tendon can be incised and tagged for later repair in difficult exposures. The second retractor is a Dorr deep posterior capsule and sciatic nerve protection retractor (Innomed). The blunt tip of the retractor is placed just distal to the transverse acetabular ligament with the fin placed over the posterior wall, retracting the posterior capsule and soft tissue. Lastly, a second angled Hohmann or superior acetabular retractor is placed deep to the gluteus medius, with the sharp tip resting on the ilium above the superior lip of the acetabulum. At this point, the labrum and soft tissue are removed circumferentially from the lip of the acetabulum and the pulvinar is removed from the cotyloid notch, exposing the medial wall of the acetabulum. Occasionally a medial osteophyte must be removed from the floor of the acetabulum to expose the medial wall if the notch has been completely overgrown.
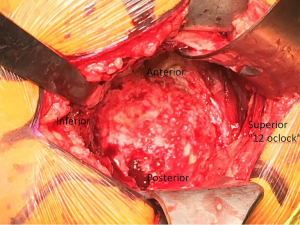
Reaming is then commenced, with the first reamer intended to remove medial osteophyte and contact the medial wall of the acetabulum. Care should be taken to avoid reaming through the medial wall, as iatrogenic medial wall defects can increase the risk of cup protrusion into the pelvis. We then increase the size of the reamers in a stepwise fashion until appropriate bony contact is made circumferentially and bleeding bone is encountered. Once again care is taken to avoid overreamming that can result in iatrogenic anterior or posterior wall defects. Once the acetabulum has been sufficiently reamed, the acetabular shell is impacted into place, with appropriate position of the cup dictated by bony anatomy, orientation of the transverse ligament, and standard principles of cup placement. When the socket is fully seated, screws are used for adjunctive fixation if thought to be appropriate, and any remaining peripheral osteophytes are removed from the acetabulum. A trial liner is then placed.
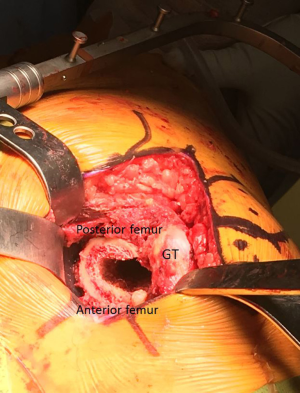
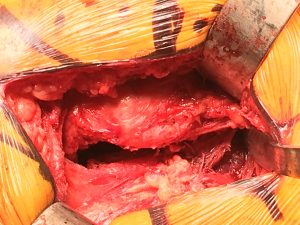
Attention is then turned to the femoral component. With an assistant standing on the anterior side of the patient holding the lower extremity in flexion, adduction, and internal rotation, proximal femoral exposure is facilitated with two retractors (Figure 8). The first is a double-footed retractor placed against the medial calcar to retract the soft tissues and also aid in elevating the femur from the wound. The second retractor is a double-angled Hohmann retractor placed deep to the gluteus medius, with the sharp tip on the ileum, used to retract the gluteus medius laterally, thereby fully exposing the piriformis fossa. A chisel osteotome is then used to remove any remaining lateral femoral neck, facilitating appropriate broach placement and avoidance of varus broaching. Once axial and rotational stability is achieved with the appropriate broach size, different trials are used to achieve appropriate length and offset. At this point, the overall construct is fully assessed, including stability, component fixation, and range of motion. When all aspects of the construct are felt to be appropriate, the trial components are removed and final components are then placed. Once more the final construct is assessed, and when deemed to be satisfactory, a layered closure is then performed, beginning with a primary repair of the posterior capsule (Figure 9) and external rotators and progressing through the deep fascia, subcutaneous tissue, and skin.
Conclusions
THA can be performed safely and with excellent patient outcomes through a wide variety of surgical approaches. A relatively new phenomenon, the so-called MPA is typically defined as incorporating a standard posterior muscular interval with more limited muscular dissection and/or incision length <10 cm. While multiple early studies have evaluated the MPA relative to other approaches including the ‘standard’ posterior approach and DAA, there is no consensus as to a single preferred approach for THA. To date, we are unaware of any studies evaluating long-term outcomes of the MPA in comparison to other surgical approaches. Currently available literature supports that the MPA can be a safe and effective means of performing THA, though it has not been shown to have clinically superior outcomes. The authors’ preferred surgical technique is reviewed in detail.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.06.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elmallah RK, Chughtai M, Khlopas A, et al. Determining Cost-Effectiveness of Total Hip and Knee Arthroplasty Using the Short Form-6D Utility Measure. J Arthroplasty 2017;32:351-4. [Crossref] [PubMed]
- Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res 1993;191-201. [PubMed]
- Kurtz SM, Ong KL, Lau E, et al. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624-30. [Crossref] [PubMed]
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [PubMed]
- Ho KW, Whitwell GS, Young SK. Reducing the rate of early primary hip dislocation by combining a change in surgical technique and an increase in femoral head diameter to 36 mm. Arch Orthop Trauma Surg 2012;132:1031-6. [Crossref] [PubMed]
- Kumar V, Sharma S, James J, et al. Total hip replacement through a posterior approach using a 22 mm diameter femoral head: the role of the transverse acetabular ligament and capsular repair in reducing the rate of dislocation. Bone Joint J 2014;96-B:1202-6. [Crossref] [PubMed]
- Goosen JH, Kollen BJ, Castelein RM, et al. Minimally invasive versus classic procedures in total hip arthroplasty: a double-blind randomized controlled trial. Clin Orthop Relat Res 2011;469:200-8. [Crossref] [PubMed]
- Kiyama T, Naito M, Shitama H, et al. Comparison of skin blood flow between mini- and standard-incision approaches during total hip arthroplasty. J Arthroplasty 2008;23:1045-9. [Crossref] [PubMed]
- Varela Egocheaga JR, Suarez-Suarez MA, Fernandez-Villan M, et al. An Sist Sanit Navar 2010;33:133-3. [Minimally invasive posterior approach in total hip arthroplasty Prospective randomised trial]. [Crossref] [PubMed]
- Berstock JR, Blom AW, Beswick AD. A systematic review and meta-analysis of the standard versus mini-incision posterior approach to total hip arthroplasty. J Arthroplasty 2014;29:1970-82. [Crossref] [PubMed]
- Chimento GF, Pavone V, Sharrock N, et al. Minimally invasive total hip arthroplasty: a prospective randomized study. J Arthroplasty 2005;20:139-44. [Crossref] [PubMed]
- Fink B, Mittelstaedt A, Schulz MS, et al. Comparison of a minimally invasive posterior approach and the standard posterior approach for total hip arthroplasty A prospective and comparative study. J Orthop Surg Res 2010;5:46. [Crossref] [PubMed]
- Khan RJ, Maor D, Hofmann M, et al. A comparison of a less invasive piriformis-sparing approach versus the standard posterior approach to the hip: A randomised controlled trial. J Bone Joint Surg Br 2012;94:43-50. [Crossref] [PubMed]
- Sugano N, Takao M, Sakai T, et al. Comparison of mini-incision total hip arthroplasty through an anterior approach and a posterior approach using navigation. Orthop Clin North Am 2009;40:365-70. [Crossref] [PubMed]
- Beckmann J, Stengel D, Tingart M, et al. Navigated cup implantation in hip arthroplasty. Acta Orthop 2009;80:538-44. [Crossref] [PubMed]
- Ogonda L, Wilson R, Archbold P, et al. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg Am 2005;87:701-10. [PubMed]
- Hart R, Stipcak V, Janecek M, et al. Component position following total hip arthroplasty through a miniinvasive posterolateral approach. Acta Orthop Belg 2005;71:60-4. [PubMed]
- Shitama T, Kiyama T, Naito M, et al. Which is more invasive-mini versus standard incisions in total hip arthroplasty? Int Orthop 2009;33:1543-7. [Crossref] [PubMed]
- Woolson ST, Mow CS, Syquia JF, et al. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg Am 2004;86-A:1353-8. [Crossref] [PubMed]
- Kim YH. Comparison of primary total hip arthroplasties performed with a minimally invasive technique or a standard technique: a prospective and randomized study. J Arthroplasty 2006;21:1092-8. [Crossref] [PubMed]
- Taunton MJ, Mason JB, Odum SM, et al. Direct anterior total hip arthroplasty yields more rapid voluntary cessation of all walking aids: a prospective, randomized clinical trial. J Arthroplasty 2014;29:169-72. [Crossref] [PubMed]
- Zawadsky MW, Paulus MC, Murray PJ, et al. Early outcome comparison between the direct anterior approach and the mini-incision posterior approach for primary total hip arthroplasty: 150 consecutive cases. J Arthroplasty 2014;29:1256-60. [Crossref] [PubMed]
- Nakata K, Nishikawa M, Yamamoto K, et al. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty 2009;24:698-704. [Crossref] [PubMed]
- Schleicher I, Haas H, Adams TS, et al. Minimal-invasive posterior approach for total hip arthroplasty versus standard lateral approach. Acta Orthop Belg 2011;77:480-7. [PubMed]
- Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics 2002;25:1031-43. [PubMed]
- Meneghini RM, Smits SA, Swinford RR, et al. A randomized, prospective study of 3 minimally invasive surgical approaches in total hip arthroplasty: comprehensive gait analysis. J Arthroplasty 2008;23:68-73. [Crossref] [PubMed]
Cite this article as: Henderson RA, Good RP, Levicoff EA. Mini-posterior approach for primary total hip arthroplasty. Ann Joint 2017;2:32.