
Management of bone deficiency in revision anterior cruciate ligament reconstruction
Introduction
Reconstruction of the anterior cruciate ligament (ACL) is one of the most common sports procedures performed accounting for 120,000 cases per year (1). With the increase in the number of primary ACL reconstructions, subsequent need for revision ACL reconstruction (ACLR) is also increasing (2). A recent systematic review of Level-I and II prospective studies with a minimum duration of five years follow-up demonstrated that the ACL graft rupture rate ranged from 1.8% to 10.4%, with a pooled percentage of 5.8% (3). Other studies have shown failure rates of up to 15% in select patient populations (4). Revision surgery can be necessary due to a wide spectrum of causes, however technical errors and recurrent trauma are the leading etiologies of ACL graft rupture. Identification of the cause of failure is paramount and the management necessitates a systemic approach.
For a successful revision ACL surgery, the preoperative evaluation must include a detailed history, physical examination, and appropriate radiographic imaging. Also previous operative reports and imaging studies, including arthroscopic images, should be reviewed. Moreover, position and size of the previous bone tunnels should be critically evaluated to decide whether the patient will be treated with a single-stage or 2-stage revision. The revision of a failed ACL surgery is technically challenging and there is the potential for unexpected findings. The surgeon must have several technical tricks, tools, and innovative techniques for dealing with the obstacles of malpositioned or enlarged tunnels.
Etiology of ACL failures
The successful revision of a failed ACL surgery cannot be achieved without understanding the specific etiology and mode of failure. General causes of failure can be divided into four groups: technical errors, repeat trauma, failure of the graft incorporation, and failure to address the co-existent laxity in secondary restraints or limb malalignment, which places the graft at risk (5). The multicenter ACL revision study (MARS) group reported the distribution of these causes, as deemed by revising surgeon, as trauma (32%), technical (24%), biologic (7%) and combination of these factors (37%) (6). We should recognize that there is an inherit bias in attributing failures to trauma when evaluating one’s own patients. Revising surgeons often deemed the cause of graft failure to be multifactorial and deemed technical error to be a contributing cause 48% of the time (7).
The most common technical error is thought to be incorrect tunnel position placed outside of the native ACL footprints. Femoral tunnel malposition was rated as the most common technical failure by far (80%), followed by tibial tunnel malposition (37%) (6). It is clearly described that malpositioning of either the femoral or tibial tunnel generates excessive stress in the graft as the knee moves through its arc of motion, resulting in graft failure (8). Historically, transtibial ACLR techniques led surgeons to place femoral tunnels vertical and more anterior than the native ACL origin because the femoral tunnel position is restricted by the angulation of the tibial tunnel in the coronal plane. Vertical tunnels may restore anteroposterior stability, but produce a rotational instability with a positive pivot shift test (9). Furthermore locations and angles of tunnels are thought to correlate with tunnel enlargements because of windshield-wiper or bungee-cord motion of the graft, which may be enhanced by changing tension in the graft due to tunnel malposition (10).
Although the exact etiology of tunnel widening is still unknown, different tunnel enlargement shapes such as linear, cavitary, mushroom, and conical support the multifactorial etiology of tunnel widening (11). The degree to which mechanical (e.g., graft position, fixation method) and biologic (e.g., increased cytokine levels, synovial fluid propagation) factors contribute to tunnel widening remains unclear (12). We have noted that tibial tunnel expansion can be greater than femoral tunnel expansion, perhaps because of the effect of gravity. Several published studies report that femoral tunnel enlargements were greater with more anterior, more proximal, and more vertical femoral tunnels (13,14). Independent drilling of the tibial and femoral tunnels have been developed to overcome the limitations of the transtibial technique and improve the accuracy of tunnel placement. Recently introduced flexible reamer systems allow for accurate femoral tunnel placement within the anatomic ACL footprint without the need for knee hyperflexion, accessory portals, or a notchplasty (15). With the evolution of arthroscopic ACLR techniques, graft failure in the setting of proper tunnel positions using accessory portals, flexible reamers or double bundle reconstructions should be further investigated.
Concomitant ligament injuries, such as collateral ligament or posterolateral corner injuries can compromise ACL graft stability if left untreated (16). The malalignment of a lower extremity is an important pathologic condition regarding ACL failure. Varus malalignment can increase the stress in the ACL graft and may warrant a valgus producing osteotomy to reduce the risk of graft failure (17). Recently, excessive posterior tibial slope was reported as a risk factor for early graft failure after ACLR (18). Besides concomitant injuries, failure of fixation, tensioning and poor choice of graft may all lead to graft failure. Infection is relatively rare with the incidence of septic arthritis following ACLR reported as less than 1% (6). In the setting of graft failure without any identifiable surgical technical error or subsequent trauma, biological failure should be considered high on the differential etiologic cause list (19).
History and physical examination
A comprehensive history and physical examination is crucial for a successful revision ACL reconstruction. The history should begin with the mechanism of primary injury, assessment of function following initial reconstruction, rehabilitation program, and the history of re-injury after the reconstruction. Asking the patient if he or she “trusts the knee” can be valuable because it is often difficult for patients to verbalize instability symptoms. The timing and symptoms associated with failure of a primary ACL reconstruction may be helpful to predict the etiology behind the failure. Early failures (<3 months) are usually related to loss of fixation and infection. Midterm failures (3–12 months) are often due to errors in surgical technique, aggressive physical therapy and unrecognized concomitant ligament injuries. Late failures (>12 months) are usually related to repeat trauma and often occur in the setting of a previously well-functioning knee. Reviewing the operative notes, imaging studies and arthroscopic images can provide important information about the previously used technique, fixation methods and implants used, complications during surgery and additional procedures performed such as meniscectomy or extraarticular reconstructions; which can compound the clinical presentation in the revision setting.
Physical examination starts with evaluating prior skin incisions, swelling and range of motion. Prone examination may allow identification of subtle flexion contractures that may not be seen in the supine position. The Lachman test is a very sensitive examination to determine the degree of anterior tibial translation and laxity compared with the normal contralateral knee (20). Knee laxity can be objectively evaluated by measuring the tibial translation with commercially available instruments (e.g., KT1000/2000 arthrometer, MEDmetric, San Diego). Anterior-posterior tibial translation difference of more than 3 mm is commonly used criterion to quantify failure of ACL reconstruction (21). The degree of rotatory instability can be elucidated by pivot shift test, but it is often difficult to perform this clinical maneuver in the office (22). Concomitant ligamentous injuries such as posterolateral and posteromedial instability can be evaluated with the Dial test and Slocum’s test, respectively (23,24). New devices to measure rotation are on the horizon but not universally available at this point (25). The posterior drawer test and sag sign are helpful to assess posterior cruciate ligament insufficiency (26). Through a comprehensive knee examination, the alignment of the entire extremity and gait pattern should be noted because ACL deficient patients may exhibit abnormal rotational or varus/valgus thrusts with ambulation (27). This can give insight in subtle modes of graft failure that might require intervention at the time of revision surgery.
Finally, if there is any concern of infection at the operation site, then systemic inflammatory markers, including white blood cell count, C-reactive protein and erythrocyte sedimentation rate should be obtained. If any of these laboratories are concerning, cell count and microbiological examination of synovial fluid aspirate should be studied rapidly.
Imaging
The preoperative radiographic evaluation of the failed ACL reconstruction is critical to determine not only the potential cause for failure and concomitant pathology, but also the current state of the tunnels. Previous tunnel positions, enlargement/widening of tunnels and hardware type can be assessed on plain anterior-posterior (AP) and lateral views. Weight-bearing posteroanterior (PA) radiographs are helpful to evaluate the presence of joint space narrowing and intercondylar notch configuration. Should the clinical exam be concerning for collateral ligament involvement, then varus and valgus stress radiographs are particularly useful to identify and objectively quantify the collateral or corner injuries (28,29). A full-length standing AP view is also recommended if any varus or valgus malalignment is appreciated on clinical exam. Magnetic resonance imaging is a valuable tool to determine the position of tunnels and status of the graft; however, is not able to verify whether the clinically injured or elongated graft is functional. Therefore, some authors suggest the use of MR-arthrography to improve the diagnostic power of this modality (30). Moreover, MRI is not suitable for the assessment of bone graft incorporation. Although metal induced artifacts may complicate its effectiveness, MRI is a useful to evaluate meniscal, chondral, and concomitant ligament injuries. Excessive tunnel osteolysis may compromise rigid fixation of the new graft during revision procedures. If there is any concern about tunnel osteolysis, it is helpful to obtain CT scan preoperatively. CT scans with/without 3D reconstructions may provide further information regarding the amount of lysis as plain films may underestimate the problem. Recent studies demonstrated CT scans are the most reliable imaging modality for evaluation of ACL bone tunnels when compared to MRI and radiographs, with superior intra- and inter-observer reliability (31,32). Furthermore, the degree of osteolysis is crucial in the determination of whether a 2-stage repair with bone grafting is required.
Single versus 2-stage revision ACLR: evolving technical considerations
In the setting of massive osteolysis, which is most often described as greater than 14 mm of tunnel widening; tunnel convergence that would compromise future graft fixation; or a relatively poor biologic milieu for tendon-bone healing, serious consideration should be given to staging the revision ACLR until the above-listed factors have been addressed. In general, tunnel placement can be defined as anatomic, indicating the ACL tunnels are within its anatomic footprint; non-anatomic, indicating the ACL tunnels are completely outside the footprint; or overlapping, where there is partial placement of the tunnel within the native anatomic footprint. The latter many times being the most difficult to remedy in a revision situation. With the advent of newer surgical techniques, including independent femoral tunnel drilling, revision ACL reconstruction algorithms have needed to evolve given previous non-anatomic trans-tibial approaches routinely allowed completely native, divergent femoral tunnels to be drilled at the time of revision surgery; yet, more recent anatomically placed femoral tunnels potentially compromise this ability to perform a single stage revision, regardless of if osteolysis is present or not. The largest volume of tunnel osteolysis routinely occurs in the femur more often than the tibia; however, the etiology for its development is not concrete (33). While mechanical and biologic causes likely both contribute to the development of the radiographic finding, there has been no literature to date that correlates the presence of widening tunnels with the subjective or objective finding of graft failure (33-37). Regardless of the etiology, it is an entity that must be recognized preoperatively in the revision setting so that the success of the revision surgery is optimized.
Graft selection
Allograft tissue for primary ACLR has not only shown higher rates of failure in the young active population, but also higher rates of failure in the revision setting (36,38-41). While hybrid constructs with low dose irradiated tissue have shown dramatically higher failure rates, non-irradiated hybrid allograft autograft constructs at the time of primary ACLR have recently shown promising results and have similar failure rates as do autograft constructs (42-45). When possible, the senior author prefers to use autograft tissue for intra-articular revision cruciate ligament reconstructions. This could include tripling a semitendinosus autograft to make a 5 stranded graft if the tendon length allows or even prepping out the contralateral limb to obtain hamstring or bone-patellar tendon-bone (BPTB) autografts as well, with supporting literature not only showing that MRI evidence exists for hamstring tendon regrowth but also with little to no gross donor site morbidity with very limited changes in knee flexion or extension strength differences compared to preoperatively (46-49). Quadriceps tendon soft tissue graft, with or without bone plug, is also an option and can be a useful graft given its relatively large depth at harvest with reported satisfactory outcomes in the literature (50). While literature does exist for the re-harvesting of BPTB autograft in the ipsilateral leg at the time of revision surgery or in harvesting a quadriceps tendon with bone block, the senior author recommends prudence in doing so to limit a stress riser with the resultant devastating complication of a patellar fracture post-operatively (51).
Tunnel osteolysis
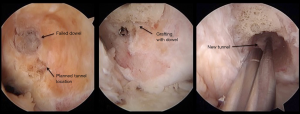
As previously mentioned, in the setting of prior anatomic or non-anatomic tunnel placement, greater than 14 mm of tunnel osteolysis is a general set point for determining whether to stage the revision procedure or not (52). These grossly expansile, non-uniform lesions can make graft hardware fixation and bone tendon healing difficult to optimize. Previous studies have provided a classification schema as to the shape of the osteolytic defect (53). The senior author recommends obtaining a CT scan to quantify the amount of osteolysis present in multiple orthogonal views given CT provides better definition than plain radiography (Figure 1).
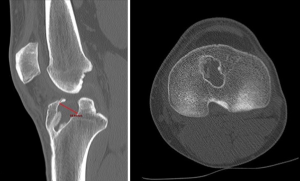
Should a 2-stage procedure be required secondary to tunnel widening for anatomic and overlapping tunnels, then all previous metallic hardware should be removed. A guide wire can be placed in the same location as the previous femoral tunnel or by using routine standard tip or elbow aiming guide for the tibial tunnel. The tibial tunnel angle can be measured preoperatively on sagittal CT scan, which can be used as a rough reference point intra-operatively as well for setting the tibial guidewire placement. If the hardware was absorbable, then the guide wire can be drilled through it if not removed. Reaming over the wire allows visualization of the tunnel. In the setting of gross osteolysis, which relegates the surgeon to providing a 2-stage procedure with bone grafting as a first stage, it is vital to debride all soft tissue and remnant graft material out of the tunnel. Often the previous tunnels can be identified by probing to localize the defect. A curette can be used to remove soft tissue and then a tunnel dilator, usually much smaller than the planned reaming, can be inserted into the defect to center a guide pin for further reaming. Serial reamers can be used, starting a size smaller than anticipated after sizing, to sequentially ream the prior tunnel and gain adequate circumferential bony bed/walls. Standard femoral and tibial ACL set reamers can be used to a point, but for the more massive cavitary defects, using intramedullary reamers from a trauma or total joint arthroplasty set can be helpful given their increasing diameter sizes larger than the standard ACL set allows. When determining the appropriate bone graft, there are multiple choices, to include: allograft chips, allograft bone dowels, or allograft femoral head; commercially available bone substitutes; or autogenous bone graft by iliac crest bone graft. For the reproducibility of bone fill, the senior author routinely uses allograft cylindrical bone dowels, which come prepackaged as different lengths, 15–30 mm, and diameters, ranging from 10–20 mm. These can be inserted by arthroscopic assisted techniques and can even be stacked for adequate length (54) (Figure 2). Routinely these bone dowels are partially cannulated, are drilled to completion so that they easily slide over a 3/32nd wire or beath pin and are inserted with the use of a cannulated bone tamp.
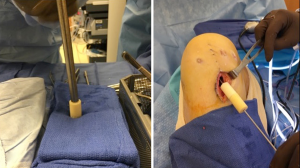
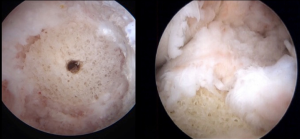
Some investigators have suggested upsizing the graft by 1 mm so that a more press fit purchase can be gained; however, we have found that the allograft bone dowels can be brittle at times and reaming line to line can aid in their safe delivery and impaction without compromising stability (55) (Figure 3).
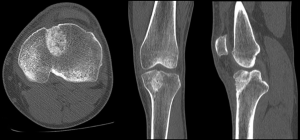
We routinely obtain a CT scan at approximately 4 months to assist in surgical planning and the revision ligament reconstruction is not entertained until satisfactory bone consolidation is present, roughly between 4–6 months (Figure 4). A recent prospective study assessing autogenous bone graft incorporation in the tibia by CT scan prior to revision ACL reconstruction showed higher occupying ratios, union ratios, and bone mineral density scores at 24 weeks than at the 12 week point postoperatively (56).
Overlapping ACL tunnels and convergence
Tunnel convergence with the expansion or overlapping of newly drilled tunnels into previous tunnels, either expectedly or unexpectedly, can present the surgeon with a unique intraoperative decision given the existence of convergence can compromise graft/implant fixation. This can occur in the setting of anatomically placed tunnels, but it more often seen in overlapping tunnels given the pre-existing tunnel’s aperture and trajectory is to be partially included in the newly drilled tunnel. Upon it’s recognition, staging the immediate revision ACL reconstruction and proceeding with bone grafting with a return to the operating room (OR) for definitive revision ACL reconstruction once graft incorporation is confirmed is always an option. However, without significant tunnel osteolysis and with an acceptable aperture on the femoral or tibial side, then there are different techniques that can be used to help ameliorate the situation of tunnel convergence or overlap. Prior to drilling the new tunnel, adjustments can be made to provide divergent tunnels (funnel technique), which maintain the same anatomic aperture. On the femoral side, using a 2-incision outside-in drilling technique can provide a new divergent angle for the tunnel but keep the existing femoral aperture. Changing the guide angle or start point on the proximal tibia can also allow divergence of the tunnels in the hopes of only communicating at the tibial articular aperture. Sometimes maintaining the previous titanium hardware in place can be used as a void filler and when a stainless steel reamer is used to create the new femoral or tibial tunnel, a new tunnel adjacent to the previous tunnel can be created given titanium is softer than stainless steel (57).
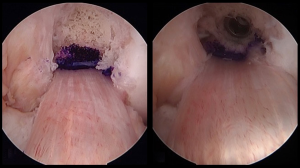
Should this be recognized after the new tunnel has been drilled, then an additional option includes stacking screws, which can be used in the setting of poor bone quality or if previous aperture fixation was used. The old screw can be removed and replaced after the new tunnel is drilled, with a new screw placed directly adjacent to the old screw to gain satisfactory aperture graft fixation. The senior author’s preference is to use an allograft bone dowel, similar to a 2-stage approach as described above for massive osteolysis. The previous tunnel can be reamed to debride remnant graft and to freshen its edges and then a cannulated allograft bone dowel is impacted into the previous tunnel. A guidewire can then be placed in standard fashion, as would be performed as a primary ACL reconstruction, and over-reamed per routine. The reamed dowel serves as a new wall for stability for the newly placed revision ACL graft (Figure 5).
Should satisfactory bone tunnels be present after reaming, then standard fixation can be placed. Should a significant amount of tibial tunnel convergence be unavoidable, despite attempts made at new drilling angles or start sites or the above listed techniques, recent laboratory biomechanical data provides guidance as to the best techniques for graft fixation in a single stage setting (58). These would include filling the tunnel with a press fit bone plug or a bioabsorbable screw and not a dilatation technique or leaving the previous tunnel empty. As always, the same single stage bone graft techniques as described in the above paragraph can be utilized where the previous tunnel can be reamed, filled with an allograft dowel plug and new tunnel drilled directly adjacent to it with the bone dowel serving as a stabilizing wall to the newly placed revision ACL graft. Standard fixation can be used as the primary fixation; however, regardless of the fixation used, additional backup fixation should be used as well given the above listed study’s findings (Figure 6).
Graft fixation techniques
To optimize the healing process and success rates of revision ACL reconstructions, secure graft fixation is paramount. Should new femoral and/or tibial tunnels be obtained without gross convergence and in the setting of no gross osteolysis, then routine primary graft fixation methods can be employed. However, should the surgeon have any question with regard to the stability of the graft/bone/fixation interface, then additional fixation types can be utilized to secure the graft and back-up the repair. Extended sized cortical buttons as suspensory fixation or tying the graft sutures over a post screw can be a useful technique used during outside-in 2 incision techniques or should slight cortical posterior wall blow out be suspected on the femoral side. As mentioned above, double-stacked screws can also provide satisfactory fixation, more routinely used on the femoral side. For the tibial side, routinely cavitary osteolysis and the relatively poor bone quality of proximal tibial metaphyseal bone provide the difficulty in obtaining satisfactory fixation. In addition to routine screw or screw and sheath constructs, staples, screw and spiked washer constructs or even extended sized cortical buttons can be used to gain graft fixation. In fact, extracortical fixation specifically is required to resist the load to failure for graft fixation when undersized screws are placed for aperture fixation, which can occur in implant to tunnel size mismatches secondary to tunnel widening, convergence or osteolysis (58). Double graft fixation in the revision ACL reconstruction setting is routinely recommended and the surgeon should have a low threshold to consider it should there be any concern of graft stability for incorporation.
Additional considerations
When assessing any failed cruciate ligament reconstruction for potential revision surgery, close attention to the postoperative course from the index procedure, the mechanism of original injury, the mode of most recent failure, surgical technique used, and any baseline underlying mechanical axis or joint line abnormalities should be critically reviewed and evaluated. Causes of early and late failure need to be defined. Failure to do so could jeopardize the outcome of the future revision ACL reconstruction. Recently, there has been interest and focus on the anterolateral ligament (ALL) and its contribution to rotational stability to the knee. While clinical and biomechanical studies have shown the benefit of its reconstruction at the time of ACL reconstruction and its influence on the pivot shift test, other studies have shown concern with over-constraining the knee and its lack of functional influence until excessive anterior tibial subluxation is gained, only seen in ACL deficient knees (59-62). In the setting of a referral revision practice, it is vital to review the patients’ clinical exam for subtle differences in the posterolateral corner (PLC) either from the acute injury or from chronic attenuation with associated varus malalignment, the presence of a varus thrust, or excessive tibial slope. All of which can predispose the graft to excessive loads and failure (63-65). Consideration should be given to the need for PLC reconstructions or high tibial osteotomy, in these situations, respectively, as a single stage procedure presuming the patient can maintain postoperative precautions and therapy compliance. Any concern for the lack thereof, then high consideration should be given to staging the procedures.
Clinical outcomes
In the setting of revision ACLR, it is imperative to discuss with the patient realistic expectations with regard to timelines to recovery, likelihood of return to sport and sporting level, associated intra-articular pathology, as well as graft re-rupture rates. Clinical findings show that rates of return to sporting activities, Marx activity levels and other patient reported outcomes are lower in the revision setting compared to baseline and in comparison to the index ACLR (66-68). Revision graft re-rupture rates are reported as 3–4 times higher than primary ACLR, with a systematic review reporting a 13.7% rate of graft failure in revision cases (69). In addition to this finding, patients undergoing their third or more revision ACL reconstruction were 4.7 times more likely to undergo additional surgeries and were 25.8 times more likely to suffer a subsequent ACL graft re-rupture (38). At the time of revision surgery, the importance of graft choice cannot be overstated with the selection of autograft resulting in patients being 2.78 times less likely to sustain a graft re-rupture in comparison to allograft use and with autograft predicting better scores on patient reported outcome measures at the 2-year follow up (38). As stated above, should no further autograft choices be viable from the injured limb, then it is the senior author’s recommendation that contralateral limb harvest should be highly considered for autograft use as the sole graft choice; however, recent data from the MARS cohort suggests that hybrid grafts in the revision setting are still better than only allograft tissue when used in the reconstructive process (38).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Freddie H. Fu and Jeremy M. Burnham) for the series “Trends in ACL Reconstruction” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.06.12). The series “Trends in ACL Reconstruction” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 2014;42:2363-70. [Crossref] [PubMed]
- Leroux T, Wasserstein D, Dwyer T, et al. The epidemiology of revision anterior cruciate ligament reconstruction in Ontario, Canada. Am J Sports Med 2014;42:2666-72. [Crossref] [PubMed]
- Wright RW, Magnussen RA, Dunn WR, et al. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am 2011;93:1159-65. [Crossref] [PubMed]
- Allen MM, Pareek A, Krych AJ, et al. Are Female Soccer Players at an Increased Risk of Second Anterior Cruciate Ligament Injury Compared With Their Athletic Peers? Am J Sports Med 2016;44:2492-8. [Crossref] [PubMed]
- Harner CD, Giffin JR, Dunteman RC, et al. Evaluation and treatment of recurrent instability after anterior cruciate ligament reconstruction. Instr Course Lect 2001;50:463-74. [PubMed]
- Wright RW, Huston LJ, Spindler KP, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med 2010;38:1979-86. [Crossref] [PubMed]
- Chen JL, Allen CR, Stephens TE, et al. Differences in mechanisms of failure, intraoperative findings, and surgical characteristics between single- and multiple-revision ACL reconstructions: a MARS cohort study. Am J Sports Med 2013;41:1571-8. [Crossref] [PubMed]
- Dargel J, Gotter M, Mader K, et al. Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies Trauma Limb Reconstr 2007;2:1-12. [Crossref] [PubMed]
- Woo SL, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg Am 2002;84-A:907-14. [Crossref] [PubMed]
- Segawa H, Omori G, Tomita S, et al. Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc 2001;9:206-10. [Crossref] [PubMed]
- Klein JP, Lintner DM, Downs D, et al. The incidence and significance of femoral tunnel widening after quadrupled hamstring anterior cruciate ligament reconstruction using femoral cross pin fixation. Arthroscopy 2003;19:470-6. [Crossref] [PubMed]
- Maak TG, Voos JE, Wickiewicz TL, et al. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 2010;18:695-706. [Crossref] [PubMed]
- Xu Y, Ao Y, Wang J, et al. Relation of tunnel enlargement and tunnel placement after single-bundle anterior cruciate ligament reconstruction. Arthroscopy 2011;27:923-32. [Crossref] [PubMed]
- Ko YW, Rhee SJ, Kim IW, et al. The Correlation of Tunnel Position, Orientation and Tunnel Enlargement in Outside-in Single-Bundle Anterior Cruciate Ligament Reconstruction. Knee Surg Relat Res 2015;27:247-54. [Crossref] [PubMed]
- Rasmussen JF, Lavery KP, Dhawan A. Anatomic Anterior Cruciate Ligament Reconstruction With a Flexible Reamer System and 70° Arthroscope. Arthrosc Tech 2013;2:e319-22. [Crossref] [PubMed]
- Getelman MH, Friedman MJ. Revision anterior cruciate ligament reconstruction surgery. J Am Acad Orthop Surg 1999;7:189-98. [Crossref] [PubMed]
- Noyes FR, Barber-Westin SD, Hewett TE. High tibial osteotomy and ligament reconstruction for varus angulated anterior cruciate ligament-deficient knees. Am J Sports Med 2000;28:282-96. [PubMed]
- Christensen JJ, Krych AJ, Engasser WM, et al. Lateral Tibial Posterior Slope Is Increased in Patients With Early Graft Failure After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2015;43:2510-4. [Crossref] [PubMed]
- Kamath GV, Redfern JC, Greis PE, et al. Revision anterior cruciate ligament reconstruction. Am J Sports Med 2011;39:199-217. [Crossref] [PubMed]
- Cooperman JM, Riddle DL, Rothstein JM. Reliability and validity of judgments of the integrity of the anterior cruciate ligament of the knee using the Lachman's test. Phys Ther 1990;70:225-33. [Crossref] [PubMed]
- van Eck CF, Loopik M, van den Bekerom MP, et al. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of instrumented knee laxity tests. Knee Surg Sports Traumatol Arthrosc 2013;21:1989-97. [Crossref] [PubMed]
- Lopomo N, Signorelli C, Rahnemai-Azar AA, et al. Analysis of the influence of anaesthesia on the clinical and quantitative assessment of the pivot shift: a multicenter international study. Knee Surg Sports Traumatol Arthrosc 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Slocum DB, Larson RL. Rotatory instability of the knee: its pathogenesis and a clinical test to demonstrate its presence. 1968. Clin Orthop Relat Res 2007;5-13; discussion 3-4. [Crossref] [PubMed]
- Kim JG, Lee YS, Kim YJ, et al. Correlation between the rotational degree of the dial test and arthroscopic and physical findings in posterolateral rotatory instability. Knee Surg Sports Traumatol Arthrosc 2010;18:123-9. [Crossref] [PubMed]
- Lorbach O, Wilmes P, Theisen D, et al. Reliability testing of a new device to measure tibial rotation. Knee Surg Sports Traumatol Arthrosc 2009;17:920-6. [Crossref] [PubMed]
- Rubinstein RA Jr, Shelbourne KD, McCarroll JR, et al. The accuracy of the clinical examination in the setting of posterior cruciate ligament injuries. Am J Sports Med 1994;22:550-7. [Crossref] [PubMed]
- George MS, Dunn WR, Spindler KP. Current concepts review: revision anterior cruciate ligament reconstruction. Am J Sports Med 2006;34:2026-37. [Crossref] [PubMed]
- James EW, Williams BT, LaPrade RF. Stress Radiography for the Diagnosis of Knee Ligament Injuries: A Systematic Review. Clin Orthop Relat Res 2014;472:2644-57. [Crossref] [PubMed]
- Gwathmey FW Jr, Tompkins MA, Gaskin CM, et al. Can stress radiography of the knee help characterize posterolateral corner injury? Clin Orthop Relat Res 2012;470:768-73. [Crossref] [PubMed]
- Gnannt R, Chhabra A, Theodoropoulos JS, et al. MR imaging of the postoperative knee. J Magn Reson Imaging 2011;34:1007-21. [Crossref] [PubMed]
- Marchant MH Jr, Willimon SC, Vinson E, et al. Comparison of plain radiography, computed tomography, and magnetic resonance imaging in the evaluation of bone tunnel widening after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2010;18:1059-64. [Crossref] [PubMed]
- Meuffels DE, Potters JW, Koning AHJ, et al. Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: Reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta Orthop 2011;82:699-703. [Crossref] [PubMed]
- Buelow JU, Siebold R, Ellermann A. A prospective evaluation of tunnel enlargement in anterior cruciate ligament reconstruction with hamstrings: extracortical versus anatomical fixation. Knee Surg Sports Traumatol Arthrosc 2002;10:80-5. [Crossref] [PubMed]
- Aglietti P, Zaccherotti G, Simeone AJ, et al. Anatomic versus non-anatomic tibial fixation in anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc 1998;6:S43-48. [Crossref] [PubMed]
- Clatworthy MG, Annear P, Bulow JU, et al. Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc 1999;7:138-45. [Crossref] [PubMed]
- Fahey M, Indelicato PA. Bone tunnel enlargement after anterior cruciate ligament replacement. Am J Sports Med 1994;22:410-4. [Crossref] [PubMed]
- Fink C, Zapp M, Benedetto KP, et al. Tibial tunnel enlargement following anterior cruciate ligament reconstruction with patellar tendon autograft. Arthroscopy 2001;17:138-43. [Crossref] [PubMed]
- MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med 2014;42:2301-10. [Crossref] [PubMed]
- Jackson DW, Windler GE, Simon TM. Intraarticular reaction associated with the use of freeze-dried, ethylene oxide-sterilized bone-patella tendon-bone allografts in the reconstruction of the anterior cruciate ligament. Am J Sports Med 1990;18:1-10. [Crossref] [PubMed]
- Roberts TS, Drez D Jr, McCarthy W, et al. Anterior cruciate ligament reconstruction using freeze-dried, ethylene oxide-sterilized, bone-patellar tendon-bone allografts. Two year results in thirty-six patients. Am J Sports Med 1991;19:35-41. [Crossref] [PubMed]
- Kane PW, Wascher J, Dodson CC, et al. Anterior cruciate ligament reconstruction with bone-patellar tendon-bone autograft versus allograft in skeletally mature patients aged 25 years or younger. Knee Surg Sports Traumatol Arthrosc 2016;24:3627-33. [Crossref] [PubMed]
- Burrus MT, Werner BC, Crow AJ, et al. Increased Failure Rates After Anterior Cruciate Ligament Reconstruction With Soft-Tissue Autograft-Allograft Hybrid Grafts. Arthroscopy 2015;31:2342-51. [Crossref] [PubMed]
- Wei J, Yang HB, Qin JB, et al. A meta-analysis of anterior cruciate ligament reconstruction with autograft compared with nonirradiated allograft. Knee 2015;22:372-9. [Crossref] [PubMed]
- Lamblin CJ, Waterman BR, Lubowitz JH. Anterior cruciate ligament reconstruction with autografts compared with non-irradiated, non-chemically treated allografts. Arthroscopy 2013;29:1113-22. [Crossref] [PubMed]
- Leo BM, Krill M, Barksdale L, et al. Failure Rate and Clinical Outcomes of Anterior Cruciate Ligament Reconstruction Using Autograft Hamstring Versus a Hybrid Graft. Arthroscopy 2016;32:2357-63. [Crossref] [PubMed]
- Janssen RP, van der Velden MJ, Pasmans HL, et al. Regeneration of hamstring tendons after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2013;21:898-905. [Crossref] [PubMed]
- Wilk KE, Andrews JR, Clancy WG. Quadriceps muscular strength after removal of the central third patellar tendon for contralateral anterior cruciate ligament reconstruction surgery: a case study. J Orthop Sports Phys Ther 1993;18:692-7. [Crossref] [PubMed]
- McRae S, Leiter J, McCormack R, et al. Ipsilateral versus contralateral hamstring grafts in anterior cruciate ligament reconstruction: a prospective randomized trial. Am J Sports Med 2013;41:2492-9. [Crossref] [PubMed]
- Shelbourne KD, Beck MB, Gray T. Anterior cruciate ligament reconstruction with contralateral autogenous patellar tendon graft: evaluation of donor site strength and subjective results. Am J Sports Med 2015;43:648-53. [Crossref] [PubMed]
- Forkel P, Petersen W. Anatomic reconstruction of the anterior cruciate ligament with the autologous quadriceps tendon: Primary and revision surgery. Oper Orthop Traumatol 2014;26:30-42. [Crossref] [PubMed]
- O'Shea JJ, Shelbourne KD. Anterior cruciate ligament reconstruction with a reharvested bone-patellar tendon-bone graft. Am J Sports Med 2002;30:208-13. [PubMed]
- Mayr R, Rosenberger R, Agraharam D, et al. Revision anterior cruciate ligament reconstruction: an update. Arch Orthop Trauma Surg 2012;132:1299-313. [Crossref] [PubMed]
- Peyrache MD, Djian P, Christel P, et al. Tibial tunnel enlargement after anterior cruciate ligament reconstruction by autogenous bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc 1996;4:2-8. [Crossref] [PubMed]
- Werner BC, Gilmore CJ, Hamann JC, et al. Revision Anterior Cruciate Ligament Reconstruction: Results of a Single-stage Approach Using Allograft Dowel Bone Grafting for Femoral Defects. J Am Acad Orthop Surg 2016;24:581-7. [Crossref] [PubMed]
- Said HG, Baloch K, Green M. A new technique for femoral and tibial tunnel bone grafting using the OATS harvesters in revision anterior cruciate ligament reconstruction. Arthroscopy 2006;22:796.e1-3. [Crossref] [PubMed]
- Uchida R, Toritsuka Y, Mae T, et al. Healing of tibial bone tunnels after bone grafting for staged revision anterior cruciate ligament surgery: A prospective computed tomography analysis. Knee 2016;23:830-6. [Crossref] [PubMed]
- Miller MD. Revision cruciate ligament surgery with retention of femoral interference screws. Arthroscopy 1998;14:111-4. [Crossref] [PubMed]
- Schliemann B, Treder M, Schulze M, et al. Influence of Different Tibial Fixation Techniques on Initial Stability in Single-Stage Anterior Cruciate Ligament Revision With Confluent Tibial Tunnels: A Biomechanical Laboratory Study. Arthroscopy 2016;32:78-89. [Crossref] [PubMed]
- Sonnery-Cottet B, Thaunat M, Freychet B, et al. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique With a Minimum 2-Year Follow-up. Am J Sports Med 2015;43:1598-605. [Crossref] [PubMed]
- Bonanzinga T, Signorelli C, Grassi A, et al. Kinematics of ACL and anterolateral ligament. Part I: Combined lesion. Knee Surg Sports Traumatol Arthrosc 2017;25:1055-61. [Crossref] [PubMed]
- Schon JM, Moatshe G, Brady AW, et al. Anatomic Anterolateral Ligament Reconstruction of the Knee Leads to Overconstraint at Any Fixation Angle. Am J Sports Med 2016;44:2546-56. [Crossref] [PubMed]
- Thein R, Boorman-Padgett J, Stone K, et al. Biomechanical Assessment of the Anterolateral Ligament of the Knee: A Secondary Restraint in Simulated Tests of the Pivot Shift and of Anterior Stability. J Bone Joint Surg Am 2016;98:937-43. [Crossref] [PubMed]
- van de Pol GJ, Arnold MP, Verdonschot N, et al. Varus alignment leads to increased forces in the anterior cruciate ligament. Am J Sports Med 2009;37:481-7. [Crossref] [PubMed]
- Gadikota HR, Seon JK, Wu JL, et al. The effect of isolated popliteus tendon complex injury on graft force in anterior cruciate ligament reconstructed knees. Int Orthop 2011;35:1403-8. [Crossref] [PubMed]
- Crawford MD, Diehl LH, Amendola A. Surgical Management and Treatment of the Anterior Cruciate Ligament-Deficient Knee with Malalignment. Clin Sports Med 2017;36:119-33. [Crossref] [PubMed]
- Mars Group. The Development and Early to Midterm Findings of the Multicenter Revision Anterior Cruciate Ligament Study. J Knee Surg 2016;29:528-32. [Crossref] [PubMed]
- Wright R, Spindler K, Huston L, et al. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg 2011;24:289-94. [Crossref] [PubMed]
- Lefevre N, Klouche S, Mirouse G, et al. Return to Sport After Primary and Revision Anterior Cruciate Ligament Reconstruction: A Prospective Comparative Study of 552 Patients From the FAST Cohort. Am J Sports Med 2017;45:34-41. [Crossref] [PubMed]
- Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am 2012;94:531-6. [Crossref] [PubMed]
Cite this article as: Laidlaw MS, Buyukdogan K, Werner BC, Miller MD. Management of bone deficiency in revision anterior cruciate ligament reconstruction. Ann Joint 2017;2:38.


