Assessment of bone loss in anterior shoulder instability
Introduction
The pathoanatomic contributors to recurrent anterior shoulder instability involve a combination of static soft tissue attachments, dynamic muscle stabilizers and the bony congruency of the humeral head on the glenoid. All of these factors contribute to the pathoanatomy of anterior shoulder instability to varying degrees. The contributions of static capsular and ligamentous attachments in concert with the bony supports of the glenoid and humeral head must all be considered in the surgical treatment of anterior shoulder instability. The contribution of bone loss in anterior shoulder instability and its bearing on treatment options continues to evolve in the literature. The most recent treatment algorithms for anterior shoulder instability rely on accurate pre-operative assessment of bone loss on both the humeral and glenoid side, rendering precise quantitative measurements essential (1,2).
This article aims to review the differing modalities for assessing bone loss in anterior shoulder instability. We will review aspects of the patient history, physical examination, plain radiographs, computed tomography (CT) scan, magnetic resonance imaging (MRI) and arthroscopy as methods to evaluate this increasingly recognized pathology. We will also explore the emergence of the newly described interplay between bone loss on the glenoid and humeral sides with the glenoid track concept.
History
Clinical evaluation of the patient with anterior shoulder instability begins with a detailed medical history. When screening for shoulder instability, unique aspects of the patient history include age, gender, hyperlaxity, participation and return to contact sports, number of dislocations and duration of dislocation. Several of these factors have been examined in the context of failed arthroscopic Bankart repair. Balg and Boileau (3) included age, gender, hyperlaxity and participation in competitive/contact sports in the Instability Severity Index Score to predict the success of arthroscopic Bankart repair (3,4).
Milano et al. specifically correlated patient history with CT findings to identify risk factors associated with glenoid bone defects. They correlated recurrent instability, male gender, time from first dislocation and physical labor as significant risk factors associated with glenoid defects. Furthermore, they reported critical bone defects (i.e., greater than 20% glenoid bone loss) to be associated with younger age at the time of first dislocation and total number of dislocations (5). While not diagnostic of bone loss, these clinical cues should raise the suspicion for a potentially more serious injury than isolated soft tissue disruption.
Physical examination
Physical exam maneuvers in shoulder pathology are notoriously non-specific in the literature. While several physical examination techniques have been described for anterior shoulder instability, their efficacy in delineating purely soft tissue injuries from combined soft tissue and bony injuries is poorly defined. Commonly performed physical exam maneuvers used in patients with suspected anterior shoulder instability include the anterior apprehension test, relocation test, surprise test and load and shift test (6).
Lo et al., investigated the validity of the anterior apprehension, relocation and surprise test to identify anterior shoulder instability. Their findings concluded that in isolation, the surprise test was the most accurate maneuver for identifying instability (sensitivity 63.89%, specificity 98.91%) and that the three tests were best performed in combination for greatest accuracy (PPV 93.6%, NPV 71.9%) (7). However, this study did not distinguish between bony or soft tissue pathology.
Bushnell and associates sought to prospectively examine a modification of the anterior apprehension test previously suggested by Miniaci to be associated with large Hill-Sachs defects (8,9). This test referred to as the “bony apprehension test” positions the arm in 45° of abduction and 45° of external rotation; the sensation of apprehension is considered a positive test. They defined a significant bony lesion as >25% glenoid bone loss and/or an engaging Hill-Sachs lesion of at least 2 cm. Their results showed perfect sensitivity and negative predictive value with more modest specificity and positive predictive value 86% and 73%, respectively. Their findings were however limited by a small sample size, lack of blinding and an inflated rate of bony lesions (8).
While physical examination maneuvers have shown fair to good utility in diagnosing anterior shoulder instability, differentiating soft tissue vs. bony lesions on physical exam is a challenging proposition. Aside from the possibility of apprehension at lesser degrees of range of motion (i.e., abduction, external rotation), there is no clear physical exam test to accurately delineate the spectrum of disease (10).
Plain radiography
Plain radiography remains an important and inexpensive screening tool whether in the setting of acute dislocation or chronic instability. Prior to 3-dimensional imaging, many specific radiographic views of the glenohumeral joint were described for detecting humeral and glenoid bone loss. Specific views for detecting Hill-Sachs lesions include, the anteroposterior (AP) external rotation and Stryker notch view. For glenoid bone loss, the West point axillary and Bernageau profile have been commonly utilized.
In 1986, Rozing et al. evaluated six radiographic views of the shoulder to determine the most useful orientation for detecting Hill-Sachs lesions. They found that the Stryker notch view obtained by aiming the tube 45° cephalad to the axillary fold with the shoulder in flexion and hand on the patients’ ear could most reliably detect a Hill-Sachs lesion (11). Alternatively, Balg and Boileau included the external rotation AP view into the Instability Severity Index Score as an indicator of significant humeral head bone loss (i.e., Hill-Sachs lesion). While likely not as sensitive as the Stryker notch view in detecting all Hill-Sachs lesions (including smaller lesions), a positive finding was assigned 2 points on their 10-point scale and was found to be significantly associated with recurrence following arthroscopic Bankart repair (3,11).
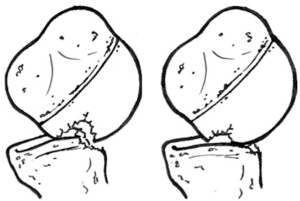
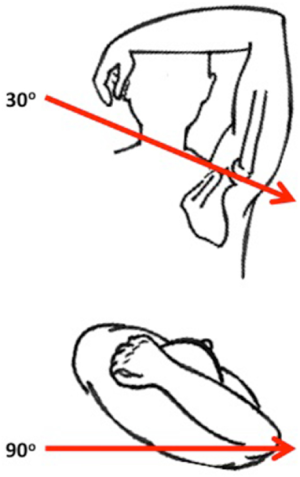
The West Point Axillary view for detecting glenoid bone loss was first described by Rokous in 1972. In this view the patient is positioned prone with the arm in 90° of abduction with the forearm hanging over the side of the bed. The X-ray tube is aimed 25° down and 25° medial into the axilla (12). Itoi tested the utility of both the traditional axillary view and West Point axillary view in quantifying glenoid bone loss. They found that there was little change in glenoid width in the presence of bone loss on a standard axillary view and a present, albeit disproportionate loss of width on the West Point view. Though they concluded that that neither view can be reliably used to quantify bone loss in the clinical setting, their findings may support the use of the West Point view as an initial screening test. An alternative view is Bernageau’s glenoid profile, taken with the patient upright with the arm abducted 135° against backboard and the beam directed 30° caudal and in the plane of the scapula (Figure 1) (13). Sommaire investigated its use for quantifying glenoid bone loss using a ratio of abnormal to normal glenoid width. They reported that patients with recurrence had a mean ratio of 5.1% vs. 4.2% in those without recurrent instability (14).
While plain radiography remains a standard of care for initial evaluation of patients with shoulder instability, in patients with suspicion of significant bone loss (e.g., multiple recurrences, failed previous surgery) cross sectional imaging [e.g., computed tomography (CT)] has supplanted plain radiography and is now considered the imaging modality of choice in this patient population.
Cross sectional and 3-dimensional imaging
Computed tomography
Glenoid bone loss
CT scan remains the most accurate advanced imaging modality for not only detecting osseous lesions in anterior instability (15,16), but also quantifying bone loss (17). Initially 2D CTs were employed using oblique en face sagittal and coronal reconstructions to compare glenoid width to the contralateral side (18), but with improvements in 3D reconstructions and lack of reliability of 2D CT scan, 3D CT has become the new gold standard (19). Several methods for quantifying glenoid bone loss have been described as either linear or surface area techniques (20,21). The two most frequently used are variations of the PICO method or percentage loss of glenoid width (22).
The PICO method was originally proposed by Baudi et al. to quantify erosive glenoid bone loss based on the percentage loss of surface area using multi-planar CT reconstructions. In this method a circle of best fit is drawn bound by the inferior margin of the healthy glenoid and then used as an overlay on the affected side to calculate the percentage loss of surface area (23). Magarelli et al. subsequently determined the intra and inter-rater reliability to be “very good” (ICC 0.98, 0.95 respectively) (24). Critics of this method quote the limitation of percentage surface area as the unit of bone loss when most treatment algorithms are based on glenoid width loss (21). Furthermore, due to the complexity of calculating surface area loss, the utility and confirmation of measurements intra-operatively using surface area methods has been elusive in the clinical scenario.
Other methods use a ratio of intact glenoid to remaining glenoid on the affected side to quantify bone loss. These methods have been referred to as either “width loss” (20) or “linear” measurements (21). One of the first was the Griffith Index which described the comparison of the maximum glenoid width side to side as the best reference for glenoid bone loss (18). Chuang et al. proposed the glenoid index, which further took into account differences in side-to-side height in their width ratio (25). Limitations of early comparative methods included the added radiation of bilateral CT scans, their use limited to unilateral instability, and lack of consideration for the orientation of bone loss (19).
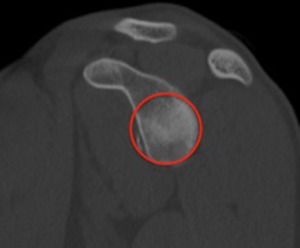
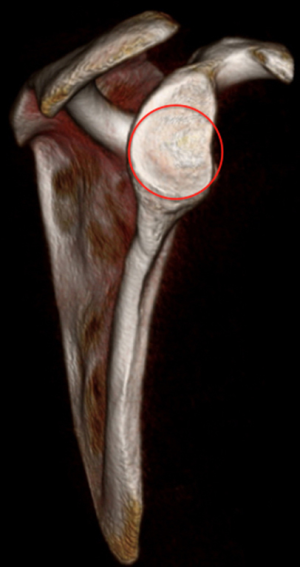
In order to alleviate the need for bilateral CT scan, newer methods of surface area loss measurements have been proposed based off of unilateral 3D CT which has been shown to be within 1.8% of the contralateral side (26). The anatomic glenoid index uses the intact inferior and posterior glenoid margin to approximate a circle of best fit (Figures 2 and 3). They confirmed that the normal glenoid was within 2.5% of the surface area of a perfect circle and was reproducible with a correlation coefficient for inter-rater reliability of 0.78 and 0.63 for small and large lesions respectively.
Humeral bone loss
The rate of Hill-Sachs lesions in recurrent anterior shoulder instability has been reported to be as high as 93% (27) and increased attention has been given to the size and location of humeral bone loss. However, because of its 3-dimensional shape (e.g., location, width, length, depth), the accurate and reproducible characterization of Hill-Sachs lesion has been more complex. Saito examined the location, orientation and size of Hill-Sachs lesions using a circle of best-fit method referenced off of a clock face starting from the center of the bicipital grove. The orientation of the lesion was designated as the mid-point between the anterior and posterior margins of the lesion, with the length defined as the distance between the two points (28). Kodali later found these measurements to be reproducible in both the sagittal and axial planes (29). Cho used a similar method for sizing but defined orientation as the angle between the deepest grove (Hill-Sachs Line) of the lesion and the long axis of the humeral shaft as the “Hill-Sachs Angle”, location was described as an angle between the bicipital groove and the groove of the Hill-Sachs lesion (30). They reported good repeatability of their measurements and concluded that larger lesions oriented more horizontal to the humeral shaft were more likely to engage the glenoid rim.
In the method of determining On vs. Off track Hill Sachs lesions, Di Giacomo and colleagues gave consideration to the bare area between the rotator cuff footprint and articular cartilage of the humeral head. This “bone bridge” plus the distance between the lateral and medial margins of the Hill Sachs lesion was coined the “Hill-Sachs Interval” and is taken into account when determining whether or not the lesion will engage (2).
Schneider et al. recently examined the inter- and intra-observer reliability of glenoid and humeral bone loss measurements on 3D CT. They reported good interobserver reliability for glenoid bone loss (90.1%) but poor interobserver reliability for humeral bone loss (72%), Intraobserver reliability was similarly better when assessing the glenoid (94% and 96% agreement), than the humerus (80% and 90% agreement). They cited a lack of standardized orientation and absence of soft tissue landmarks (i.e., rotator cuff footprint) and concluded that further studies were needed to establish more reliable and standardized methods before the measurements could be applied to treatment algorithms (17).
MRI
Despite the fact that standard MRI remains inferior to 3D CT for imaging osseous defects, it remains the imaging modality of choice for detecting soft tissue lesions of the shoulder. This often results in patients receiving two different cross sectional imaging tests in the process of their diagnostic work-up. The addition of MR to the diagnostic evaluation provides the benefit of better soft tissue detail and lack of radiation exposure at the expense of increased financial cost and patient time commitment. Owing to these potential benefits, many studies have investigated MRI as a possible substitute for 3D CT.
Tian and colleagues sought to validate fat suppressed 3D volumetric interpolated breath-hold examination MR sequences (3D VIBE MR) as a substitute to 2D CT to quantify glenoid bone loss. Using a best fit circle method (31) they compared 3D VIBE MR to 2D CT in 56 patients concluding that 3D VIBE MR was highly consistent with 2DCT (r=0.921, P<0.001) for measuring glenoid bone loss (32). Friedman et al. later reported only moderate agreement between the two modalities when comparing MR to 2D CT based on glenoid width measurements (33). However, a significant limitation of both studies may be the fact that the widely accepted gold standard for measuring glenoid bone loss is 3D CT reconstructions, and not 2D multiple slice CT scan (19).
Gyftopoulos used 2D MR to assess bone loss in anterior shoulder instability. Using Sugaya’s circle method (31) and standard width measurements for the Hill-Sachs lesion, they applied their MR measurements to Di Giacomo’s method for determining on versus off track Hill-Sachs lesions (2). They concluded that 2D MR was a suitable modality for measuring both humeral and glenoid bone loss, and argued that it may be more accurate than CT for determining the Hill-Sachs interval due to the improved soft tissue visualization of the rotator cuff foot print (34).
3D MRI has been investigated in two recent studies. A cadaveric study by Yanke et al. equated the use of 1.5 and 3-T MRI to that of 3D CT scan in 6 specimens (35). Gyftopoulos et al. retrospectively compared 3D MR to intraoperative bare-spot measurements for glenoid bone loss reporting no difference in overall average measurements (36). However their study was limited by a small sample size, and did not include humeral head bone loss measurements or 3D CT comparison.
Notwithstanding the efforts to validate MRI as an alternate imaging modality, 3D CT remains the gold standard in quantifying bone loss in shoulder instability and until further improvements are made, many patients will still require both diagnostic tests prior to appropriate treatment (37).
Arthroscopy
Arthroscopy allows direct visualization and inspection of the bony and soft tissue lesions associated with anterior shoulder instability. Several methods have been described including dynamic visualization, qualitative observation and quantitative arthroscopic measurements. Much of this literature originates from the work of Burkhart and colleagues. They initially classified lesions dichotomously into inverted pear versus non-inverted pear shaped glenoids and engaging versus non-engaging Hill-Sachs lesions (38).
Glenoid bone loss was considered significant when the amount of bone loss was severe enough to alter the normal “pear shaped” anatomy of the native glenoid. When the bone loss of the anterior-inferior aspect of the glenoid was severe enough so that the inferior aspect of the glenoid was narrower than the superior aspect of the glenoid, the lesions was termed an “inverted pear” shaped glenoid (38).
Similarly, Hill-Sachs lesions were classified into engaging and non-engaging Hill-Sachs lesions according to their interaction during dynamic arthroscopy. A Hill-Sachs lesion was only considered engaging when it “engaged” or locked over the anteroinferior glenoid rim in an athletic position of glenohumeral flexion, abduction and external rotation (Figure 4) (38,39). They considered the presence of an engaging Hill-Sachs lesion as significant humeral bone loss. Both an inverted pear glenoid and/or an engaging Hill-Sachs lesion have been demonstrated to be negative prognostic factors for success of arthroscopic Bankart repair (38). This has since been considered by several authors as the gold standard for classifying Hill-Sachs lesions as engaging or non-engaging (30). However, other authors have questioned the utility of this dynamic test and debate remains as to whether or not this test should be performed pre or post soft tissue Bankart repair (2,40).
Unfortunately, these dynamic observations must be performed arthroscopically and therefore cannot be utilized pre-operatively for surgical decision-making. To account for this, a more objective quantitative pre-operative method that incorporated both the humeral and glenoid side into the decision-making algorithm was developed and based on the glenoid track concept.
Glenoid track concept
Yamamoto et al. (39) first proposed the “glenoid track” concept using cadaveric 3D CT scans. Conceptually, the glenoid track represents the contact area of the glenoid on the humeral head as the arm is brought through a physiologic range of motion. As the arm moves into an athletic position (i.e., 60° of glenohumeral abduction) the glenoid track migrates from inferomedial to superolateral on the humeral head. The glenoid track was defined as spanning from the medial margin of the rotator cuff footprint, to a point 18.4±2.5 mm, or 84% of the width of the native glenoid medially onto the articular surface of the humerus.
Di Giacomo later used Yamamoto’s findings to classify Hill-Sachs lesions based on their relationship to the glenoid. He proposed that if the Hill-Sachs lesion was contained within this contact area, or “glenoid track”, the Hill-Sachs lesions was considered on-track, and was therefore non-engaging. In contrast, if the Hill-Sachs lesion was large enough so that it was outside or medial to the “glenoid track”, the Hill-Sachs lesions was considered off-track, and should be considered when pre-operatively planning the surgical reconstruction (e.g., remplissage, humeral head allograft, Latarjet reconstruction) (2).
Importantly, the glenoid track concept was able to account for both humeral and glenoid bone loss. As the glenoid width decreased in size from anterior-inferior glenoid bone loss, the size of the glenoid track decreased. This results in smaller Hill-Sachs lesions engaging the glenoid rim in the presence of significant glenoid bone loss. Similarly, as the Hill-Sachs defect extended further medial, it became more likely to engage a deficient glenoid rim (39).
Using this classification Di Giacomo proposed a treatment algorithm based on the whether or not the Hill-Sachs lesion was on/off track. Lesions with more than 25% glenoid bone loss were deemed to require a Latarjet procedure, while it was recommended that “Off-track lesions” receive a Remplissage (Figure 5). They developed both radiographic (CT) and static arthroscopic methods to measure the size of the lesions, and predict their expected interaction (38).
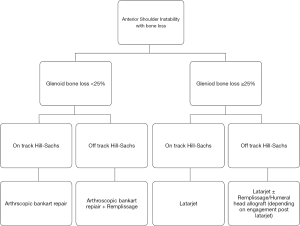
When measuring these lesions, whether on CT or arthroscopy the anatomic landmarks remain the same. The Hill-Sachs interval, the distance from the medial rotator cuff footprint to the medial edge of the lesion is measured using an arthroscopic probe or on the posterior CT view of the humeral head using calibrated imaging software. The glenoid track is measured in reference to the widest diameter of the glenoid (i.e., the glenoid bare spot) arthroscopically, or using a circle of best-fit method based on the en face 3D CT view (2). The main utility of this approach is that it allows for both pre-operative and intraoperative classification.
In a proof of concept study, Shaha et al. retrospectively applied to glenoid track concept to the pre-operative MRIs of 57 patients who underwent arthroscopic Bankart repair. Of “off-track” lesions, 75% suffered from recurrent instability vs. only 8% of “on track” lesions. Further, a subgroup analysis of patients with bipolar bone loss showed that the concept correctly predicted post-operative stability 90% of the time (41). The authors concluded that the glenoid track concept was a better predictor than glenoid bone loss alone for post-operative stability. However, the study was limited by the fact that it only included eight “off-track” lesions in the analysis.
Furthermore, Schneider et al. investigated the reliability and reproducibility of the glenoid track concept and treatment algorithm using 3D CT scan. They found the interobserver reliability of the on vs. off track classification to be only 72% and the agreement for treatment based on the algorithm to be 65%. Both were graded as having poor interobserver reliability and they concluded that the method was not reliable enough to be used for treatment decisions (17).
The concept of the glenoid track theory and proposed treatment algorithm is an appealing proposition to surgeons to aid in clinical decision-making. However, at present, further evaluation of the method and prospective studies are required to establish its validity.
Summary
As imaging modalities have evolved, so has the methodology for assessing and quantifying bone loss in anterior shoulder instability. A better understanding of the dynamic interaction between the glenoid and humeral head in the native and pathologic shoulder have lead surgeons towards a customized approach to treating specific patterns of anterior instability and bone loss. Continued research in this area to validate advancing imaging methods and treatment algorithms show promise to improve patient outcomes and decrease post-operative recurrence rates.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Albert Lin and Jason J. Shin) for the series “Trends in Anterior Shoulder Instability” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.10.09). The series “Trends in Anterior Shoulder Instability” was commissioned by the editorial office without any funding or sponsorship. IKYL reports personal fees from Arthrex, grants and personal fees from Smith and Nephew, personal fees from Wolsters Kluwer, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007;23:1033-41. [Crossref] [PubMed]
- Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from “engaging/non-engaging” lesion to “on-track/off-track” lesion. Arthroscopy 2014;30:90-8. [Crossref] [PubMed]
- Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br 2007;89:1470-7. [Crossref] [PubMed]
- Olds M, Ellis R, Donaldson K, et al. Risk factors which predispose first-time traumatic anterior shoulder dislocations to recurrent instability in adults: a systematic review and meta-analysis. Br J Sports Med 2015;49:913-22. [Crossref] [PubMed]
- Milano G, Grasso A, Russo A, et al. Analysis of risk factors for glenoid bone defect in anterior shoulder instability. Am J Sports Med 2011;39:1870-6. [Crossref] [PubMed]
- Gil JA, DeFroda S, Owens BD. Current Concepts in the Diagnosis and Management of Traumatic, Anterior Glenohumeral Subluxations. Orthop J Sports Med 2017;5:2325967117694338 [Crossref] [PubMed]
- Lo IK, Nonweiler B, Woolfrey M, et al. An evaluation of the apprehension, relocation, and surprise tests for anterior shoulder instability. Am J Sports Med 2004;32:301-7. [Crossref] [PubMed]
- Bushnell BD, Creighton RA, Herring MM. The bony apprehension test for instability of the shoulder: a prospective pilot analysis. Arthroscopy 2008;24:974-82. [Crossref] [PubMed]
- Miniaci A, Gish MW. Management of anterior glenohumeral instability associated with large Hill-Sachs defects. Tech Shoulder Elbow Surg 2004;5:170-5. [Crossref]
- Piasecki DP, Verma NN, Romeo AA, et al. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg 2009;17:482-93. [Crossref] [PubMed]
- Rozing PM, de Bakker HM, Obermann WR. Radiographic views in recurrent anterior shoulder dislocation. Comparison of six methods for identification of typical lesions. Acta Orthop Scand 1986;57:328-30. [Crossref] [PubMed]
- Rokous JR, Feagin JA, Abbott HG. Modified axillary roentgenogram. A useful adjunct in the diagnosis of recurrent instability of the shoulder. Clin Orthop Relat Res 1972;84-6. [PubMed]
- Bernageau J, Patte D, Debeyre J, et al. Value of the glenoid profil in recurrent luxations of the shoulder. Rev Chir Orthop Reparatrice Appar Mot 1976;62:142-7. [PubMed]
- Sommaire C, Penz C, Clavert P, et al. Recurrence after arthroscopic Bankart repair: Is quantitative radiological analysis of bone loss of any predictive value? Orthop Traumatol Surg Res 2012;98:514-9. [Crossref] [PubMed]
- Sanders TG, Zlatkin M, Montgomery J. Imaging of glenohumeral instability. Seminars in roentgenology 2010;45:160-79. [Crossref] [PubMed]
- Bishop JY, Jones GL, Rerko MA, et al. 3-D CT is the most reliable imaging modality when quantifying glenoid bone loss. Clin Orthop Relat Res 2013;471:1251-6. [Crossref] [PubMed]
- Schneider AK, Hoy GA, Ek ET, et al. Interobserver and intraobserver variability of glenoid track measurements. J Shoulder Elbow Surg 2017;26:573-9. [Crossref] [PubMed]
- Griffith JF, Antonio GE, Tong CW, et al. Anterior shoulder dislocation: quantification of glenoid bone loss with CT. AJR Am J Roentgenol 2003;180:1423-30. [Crossref] [PubMed]
- Bois AJ, Fening SD, Polster J, et al. Quantifying glenoid bone loss in anterior shoulder instability: reliability and accuracy of 2-dimensional and 3-dimensional computed tomography measurement techniques. Am J Sports Med 2012;40:2569-77. [Crossref] [PubMed]
- Saliken DJ, Bornes TD, Bouliane MJ, et al. Imaging methods for quantifying glenoid and Hill-Sachs bone loss in traumatic instability of the shoulder: a scoping review. BMC Musculoskelet Disord 2015;16:164. [Crossref] [PubMed]
- Bois AJ, Walker RE, Kodali P, et al. Imaging instability in the athlete: the right modality for the right diagnosis. Clin Sports Med 2013;32:653-84. [Crossref] [PubMed]
- Gottschalk LJ, Bois AJ, Shelby MA, et al. Mean Glenoid Defect Size and Location Associated With Anterior Shoulder Instability: A Systematic Review. Orthop J Sports Med 2017;5:2325967116676269 [Crossref] [PubMed]
- Baudi P, Righi P, Bolognesi D, et al. How to identify and calculate glenoid bone deficit. Chir Organi Mov 2005;90:145-52. [PubMed]
- Magarelli N, Milano G, Sergio P, et al. Intra-observer and interobserver reliability of the ‘Pico’computed tomography method for quantification of glenoid bone defect in anterior shoulder instability. Skeletal Radiol 2009;38:1071-5. [Crossref] [PubMed]
- Chuang TY, Adams CR, Burkhart SS. Use of preoperative three-dimensional computed tomography to quantify glenoid bone loss in shoulder instability. Arthroscopy 2008;24:376-82. [Crossref] [PubMed]
- Jeske HC, Oberthaler M, Klingensmith M, et al. Normal glenoid rim anatomy and the reliability of shoulder instability measurements based on intrasite correlation. Surg Radiol Anat 2009;31:623-5. [Crossref] [PubMed]
- Yiannakopoulos CK, Mataragas E, Antonogiannakis E. A comparison of the spectrum of intra-articular lesions in acute and chronic anterior shoulder instability. Arthroscopy 2007;23:985-90. [Crossref] [PubMed]
- Saito H, Itoi E, Minagawa H, et al. Location of the Hill-Sachs lesion in shoulders with recurrent anterior dislocation. Arch Orthop Trauma Surg 2009;129:1327-34. [Crossref] [PubMed]
- Kodali P, Jones MH, Polster J, et al. Accuracy of measurement of Hill-Sachs lesions with computed tomography. J Shoulder Elbow Surg 2011;20:1328-34. [Crossref] [PubMed]
- Cho SH, Cho NS, Rhee YG. Preoperative analysis of the Hill-Sachs lesion in anterior shoulder instability: how to predict engagement of the lesion. Am J Sports Med 2011;39:2389-95. [Crossref] [PubMed]
- Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am 2003;85-A:878-84. [Crossref] [PubMed]
- Tian CY, Shang Y, Zheng ZZ. Glenoid bone lesions: comparison between 3D VIBE images in MR arthrography and nonarthrographic MSCT. J Magn Reson Imaging 2012;36:231-6. [Crossref] [PubMed]
- Friedman LG, Ulloa SA, Braun DT, et al. Glenoid Bone Loss Measurement in Recurrent Shoulder Dislocation: Assessment of Measurement Agreement Between CT and MRI. Orthop J Sports Med 2014;2:2325967114549541 [Crossref] [PubMed]
- Gyftopoulos S, Beltran LS, Bookman J, et al. MRI evaluation of bipolar bone loss using the on-track off-track method: A feasibility study. AJR Am J Roentgenol 2015;205:848-52. [Crossref] [PubMed]
- Yanke AB, Shin JJ, Pearson I, et al. Three-Dimensional Magnetic Resonance Imaging Quantification of Glenoid Bone Loss Is Equivalent to 3-Dimensional Computed Tomography Quantification: Cadaveric Study. Arthroscopy 2017;33:709-15. [Crossref] [PubMed]
- Gyftopoulos S, Beltran LS, Yemin A, et al. Use of 3D MR reconstructions in the evaluation of glenoid bone loss: a clinical study. Skeletal Radiol 2014;43:213-8. [Crossref] [PubMed]
- Rerko MA, Pan X, Donaldson C, et al. Comparison of various imaging techniques to quantify glenoid bone loss in shoulder instability. J Shoulder Elbow Surg 2013;22:528-34. [Crossref] [PubMed]
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94. [Crossref] [PubMed]
- Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-56. [Crossref] [PubMed]
- Provencher M, Abrams J, Boileau P, et al. editors. Challenging problems in shoulder instability: How to get it right the first time and what to do if you don’t. ICL 282. Presented at the 2013 annual meeting of the American Academy of Orthopaedic Surgeons, Chicago, IL, 2013.
- Shaha JS, Cook JB, Rowles DJ, et al. Clinical Validation of the Glenoid Track Concept in Anterior Glenohumeral Instability. J Bone Joint Surg Am 2016;98:1918-23. [Crossref] [PubMed]
Cite this article as: Kwong CA, Gusnowski EM, Tam KKW, Lo IKY. Assessment of bone loss in anterior shoulder instability. Ann Joint 2017;2:63.