
Open Latarjet: tried, tested and true
Introduction
Traumatic anterior shoulder instability is a common injury for the contact athlete, with high rates of recurrence in some athletic populations (1-4). Anatomical Bankart repair procedures have been successful, however high recurrence rates have been shown in certain patient groups (5,6). Open coracoid transfer for treatment of anterior shoulder instability was first described in 1954 by Latarjet (7) and Trillat (8) and in 1958 by Helfet (9), who named the procedure after Rowley Bristow who had taught him. The Latarjet and Bristow procedures subsequently became commonly used and interchangeable for coracoid transfer, in spite of the modifications to the original descriptions. It is commonly accepted though that a Bristow procedure involves the coracoid tip being transferred to the anterior glenoid with the conjoint tendon facing anterior, whereas a Latarjet involves the entire coracoid being transferred and rotated so that the conjoint tendon is directed inferior.
A biomechanical study demonstrated that whilst both procedures conferred stability to the glenoid, the Latarjet provided more stability in the setting of glenoid deficiency compared to the Bristow (10).
A review of the Latarjet procedure compared to anatomical Bankart repair found that the advantages of the Latarjet included a lower recurrence and dislocation rate, whilst loss of external rotation was less with Latarjet of 11.5° compared to 20.9° with Bankart repair (11).
Principles
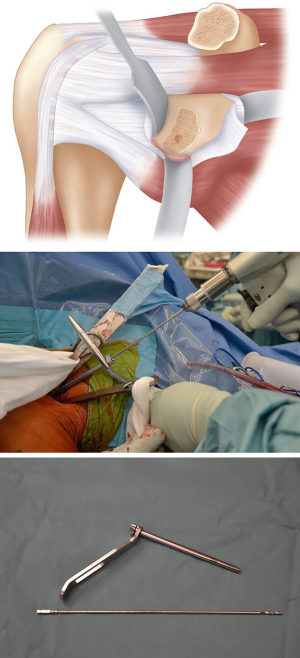
The coracoid transfer procedures have been modified since the original descriptions, leading to the current techniques being quite different to those originally described (12-14). However, the current principles of the Latarjet procedure remain unchanged, with the “triple blocking effect” described by Patte (Figure 1), improving glenohumeral stability by: the “bony effect” to increase the anterior-posterior diameter of the glenoid, the “sling effect” of the conjoint tendon on the inferior capsule and subscapularis muscle in the position of dislocation (abduction and external rotation) and finally the repair of the capsule and inferior glenohumeral ligament to the coracoacromial ligament (CAL) to recreate the capsulolabral reconstruction (16).
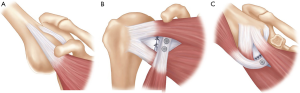
The anatomical effects of the Latarjet have been quantified, which show that in cadaveric specimens the conjoint tendon and subscapularis muscle works through a “belt-suspension-stabilization” mechanism with the arm in abduction and external rotation. The conjoint tendon prevents the inferior subscapularis from superior migration, keeping it against the anteroinferior aspect of the humeral head. The capsulolabral repair to the CAL provides stability in the anterior and inferior directions, closing a “vulnerable gap” that the conjoint tendon and subscapularis do not address. They also found that the stabilizing effect of Latarjet is eliminated with a deficient subscapularis tendon (17).
Indications
The specific indication for the Latarjet procedure differs amongst surgeons, however it is generally accepted that it is indicated in patients with anterior glenohumeral instability that are unlikely to have a successful outcome from either an arthroscopic or open anatomical Bankart repair. Contraindications for the Latarjet procedure include subscapularis tear and anterior articular glenoid fracture of >30%, which should be treated with either primary fixation or iliac crest bone grafting (18).
The Instability Severity Index Score was developed to guide treatment choice between arthroscopic Bankart repair compared to Latarjet procedure and included risk factors such as age, hyperlaxity, sport participation, sport type—contact compared to non-contact as well as glenoid bone loss and Hill-Sachs lesions (19). Although the principles are widely utilized, a follow-up study was not able to predict recurrent dislocation following arthroscopic Bankart repair up to 2 years post operatively (20).
Bone loss >25% with an “inverted pear” glenoid or an engaging Hill-Sachs were proposed as indications for a Latarjet by Burkhart and De Beer due to the recurrence rate of 67% with arthroscopic procedures in these patients (21). A follow-up study by the authors showed a 4.9% recurrence rate with the Latarjet procedure (21). A biomechanical study found that with a Hill-Sachs defect of 25%, joint stiffness could be restored to near intact level with the Latarjet (22).
Contact athletes have also been shown to have higher rates of bone loss as well as high rates of failure with both open and arthroscopic anatomical Bankart repair (6,23-25). The Latarjet procedure has also been shown to provide improved stability in contact athletes (21,26,27).
We recommend the Latarjet procedure in patients who have anterior shoulder instability with a glenoid bone defect, an engaging Hill-Sachs lesion, a contact athlete, failed Bankart repair—either open or arthroscopic, or any other patient deemed to be at high risk for failure with a soft tissue Bankart repair. Therefore, the decision for a Latarjet is based on a combination of factors.
Clinical assessment
A standard clinical examination of the shoulder recording range of motion, cuff integrity and positive biceps provocation tests are recorded. Instability testing follows the standard procedure described by Gerber which includes anterior apprehension with a relocation test, anterior draw and sulcus sign (28). The Gagey hyper passive abduction test (29) can be useful to detect inferior glenohumeral ligament distension, especially if there is asymmetry between the shoulders. A sulcus sign and increased external rotation greater than 90 degrees can be used to assess for “constitutional hyperlaxity” which if present leads to a higher split in the subscapularis muscle to provide more stability (30).
Radiological assessment
X-ray series should include AP shoulder views in internal rotation, neutral and external rotation. Glenoid profile views should be performed, with our preference being the Bernageau view (31,32), however other authors have found that the West Point view, a variation on the axillary lateral, to be able to accurately assess bone loss (33,34). If there is concern quantifying the bone loss, a three-dimensional CT scan can assist this giving accurate assessment of bone loss. If concern exists for associated rotator cuff pathology or in revision cases, a MRI or CT arthrogram can give valuable information.
Technique
Position
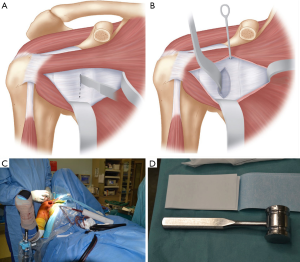
A beach chair position is used, which can be aided by a TENET/T-Max table attachment and Spider Arm limb positioner (Smith and Nephew, Andover, MA, USA). The scapula should be supported on the table and flat, with a towel underneath to aid stability and allow sliding of the scapula on the table, which can help joint exposure. The border of the lateral acromion being level with the lateral aspect of the table is a good guide; however we have often moved the acromion lateral to facilitate arthroscopy when indicated. The arm must be draped and free to move in abduction, external and internal rotation to aid the operation (Figure 2).
Incision and dissection
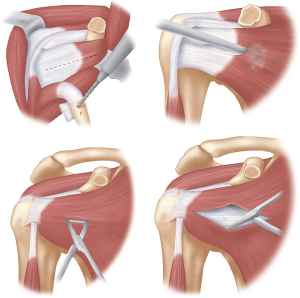
The coracoid can be marked on the skin and an incision starting at the tip of the coracoid and extended inferiorly towards the axillary fold of 4–5 cm is most commonly used, however the incision can be smaller or larger depending on the patient’s body habitus. The deltopectoral interval is opened with the cephalic vein taken laterally with the deltoid, and a self-retainer can be inserted to aid retraction. Medial branches of the cephalic vein in the appearance of a Mercedes-Benz symbol are often encountered and should be carefully ligated with sutures to prevent post-operative haematoma formation. The arm is moved into an abducted, externally rotated position and a Hohmann retractor is placed over the top of the coracoid.
Coracoid exposure and osteotomy
With the arm in abduction and external rotation the CAL is exposed, and sharply incised at least 1 cm from the coracoid attachment (Figure 3). This exposes the coracohumeral ligament beneath which must be released, with care not to damage the underlying subscapularis muscle.
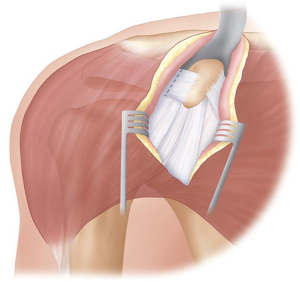
The superior and medial side of the coracoid now must be exposed, with the arm adducted and internally rotated to aid this. Superior soft tissue is released with electrocautery, with care to leave the coracoclavicular ligaments intact. The pectoralis minor is then released medially, being careful not to extend the release past the distal tip of the coracoid, to avoid damaging the blood supply to the coracoid. A periosteal elevator can then be used to remove any soft tissue from the under surface of the coracoid.
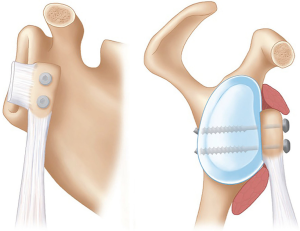
A 90° oscillating saw is used from a medial to lateral position to perform the osteotomy. It should be started at the base of the coracoid, at the juncture of the horizontal and vertical parts (Figure 4). It is vital that the osteotomy is perpendicular to the coracoid so that it does not extend into the glenoid articular surface. Leverage with a curved osteotome from the medial side can complete the osteotomy on the lateral side. This should enable a coracoid graft of approximately 26 mm to be harvested (35).
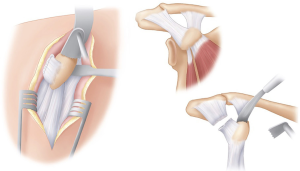
The arm is then repositioned back into abduction, externally rotation with the remaining coracohumeral ligament released. Positioning the arm back into neutral and grasping the coracoid with a pair of toothed bone holding forceps, it can be delivered out of the wound via gentle longitudinal traction. The arm is then positioned in adduction and external rotation and the conjoint tendon is released along its lateral border distally.
With the coracoid positioned out of the wound and the skin protected by a small surgical swab and a broad osteotome, the inferior part can be prepared. Soft tissue must be removed with care taken to leave the CAL intact. Any soft tissue that can potentially fold back under can get between the coracoid and the glenoid which may lead to a non-union. The oscillating saw is used to decorticate the inferior surface and expose a broad cancellous base. Two drill holes are then made within the coracoid; a sharp Steinmann pin can be used to mark the sites prior to drilling. The drill holes should be positioned on the central axis about 1 cm apart, with care to leave enough bone distally to decrease fracture risk (Figure 5). We use a 3.2 mm drill, and have changed to using 4.5 mm partially threaded cannulated screws (DePuy Synthes, West Chester, PA, USA), due to 4.5 mm malleolar screws being no longer available. The drill holes are cleared with electrocautery, especially on the superior surface to allow for easy identification of the holes later during fixation. A suture is then placed and tied through one of the suture holes which allows for easy retrieval later, and the coracoid is returned to the wound underneath the pectoralis major.
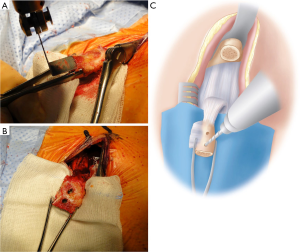
Glenoid exposure
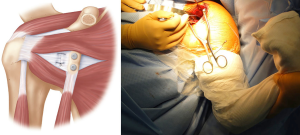
The arm is adducted and externally rotated exposing the subscapularis muscle, with careful dissection to identify the superior and inferior borders. A horizontal split is made in the subscapularis, generally at the junction of the upper two-thirds and lower third. This split is moved superiorly to the midpoint of the muscle for patients with signs of generalised ligamentous laxity. The split can be made with curved Mayo scissors, leaving the capsule intact. With the capsule exposed by the open limbs of the scissors, a small swab with a suture limb attached is inserted between subscapularis and the capsule medially, into the subscapularis fossa. We have found that by adding a suture to the small swab allows it to be located easier at the end of the operation. A medial retractor, either a Hohmann or Bankart retractor, is placed over the swab exposing the capsule medial to the joint line. An inferior Bennett retractor is then used, before the subscapularis split is extended laterally to the lesser tuberosity (Figure 6).
The capsule should now be well exposed and the joint line should either be clear on visualization or palpation. A vertical capsulotomy is performed at the level of the joint line, with an angled blade handle making this step easier. The capsulotomy is extended medially at the superior margin to ensure ease of exposure. A joint retractor is then introduced through the capsulotomy to retract the humeral head posteriorly and to expose the anterior glenoid, with our preference being a small Fukuda (DePuy Synthes, West Chester, PA, USA) (Figure 7); however a Trillat retractor (Axone Medical, Lyon, France) can also be used.
Exposure is then improved by placing a 4 mm Steinmann pin superiorly into the anterior scapular neck, with a Hohmann placed inferiorly between the capsule and labrum for inferior exposure. A Bankart retractor is then placed medially, which can be secured to a mallet using a drape tape to free up the assistant.
Glenoid preparation
The whole anterior glenoid should be visualized and the labrum resected from inferior to superior. We have found that resection is easier if it is started at the level of the joint inferiorly (5 o’clock position for right side), extended medially for 2 cm, then superiorly for 2–3 cm, and then turning the incision back laterally to the joint line at the 2 o’clock position (Figure 8). This adequately exposes the anterior glenoid to allow for its preparation. A curved osteotome is used to perform an osteotomy of the anterior glenoid to create a flat surface of cancellous bone for the graft to sit on. In patients without glenoid bone loss, osteotomy is performed to create a bone defect to allow for graft seating, which often necessitates removal of 10–20% of anterior glenoid.
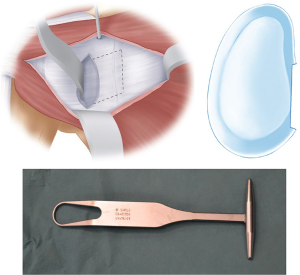
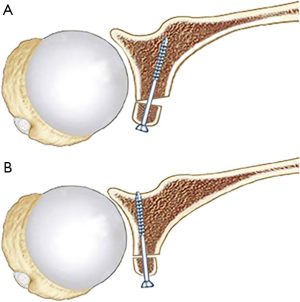
After the anterior glenoid is flat with cancellous bone exposed, the inferior drill hole is created. This drill hole is positioned between 4–5 o’clock in the right shoulder and medial enough to avoid any overhang of the graft, with the coracoid ideally positioned 1–2 mm medial to the articular margin. Positioning the screw hole too inferiorly may result in glenohumeral dislocation above the graft. A previous study on coracoid graft dimensions found that positioning of the drill hole 7 mm medial to the articular surface avoids the graft overhanging the articular surface (35). The drill also must be directed parallel to the articular surface rather than medial to allow compression of the graft, reduce lateral overhang and position the screw away from the spinoglenoid notch and the suprascapular nerve (27). We have found this step much easier since converting to a long drill bit and long drill guide, allowing better angulation and leverage of the drill compared to the previous standard short drill bit (36) (Figure 9).
Coracoid fixation and closure
The coracoid is retrieved from the wound and a guidewire for the cannulated screw is placed through the inferior hole to act as a joystick to guide the graft into the glenoid drill hole. A 34 mm length, 4.5 mm partially threaded cannulated screw is inserted, with care taken to avoid over tightening and causing a potential coracoid graft fracture by using a “two finger” technique. Ideally, the graft should be positioned flush with the glenoid bone, which is usually 1–2 mm medial to the articular surface (Figure 10). The superior screw hole is then drilled aiming to be perpendicular to the articular surface, measured and second screw is inserted (Figure 11). A final check of the coracoid graft position is then undertaken, with any malrotation corrected with removal and replacement of screws after rotation of the graft. Small overhang may be addressed by removal of bone with a bone rongeur or high-speed burr. Care should be taken not to remove too much graft with the burr as this can increase the risk of fracture and avulsion of the coracoid tip.
The CAL stump is then identified and an absorbable suture placed in the inferior portion. The intra-articular retractor is then removed and the shoulder positioned in external rotation. The suture is then passed into the inferior capsule and repaired. Another suture is passed through the superior CAL stump and superior capsule which is repaired with the arm in the same position. This ensures that the capsular repair is stable for early post-operative range of motion (Figure 12).
The swab with the suture attached is then removed from the subscapularis fossa, and all retractors are removed. Haemostasis is confirmed and after irrigation the wound is closed. We do not close the subscapularis and do not routinely use a drain.
Postoperative management
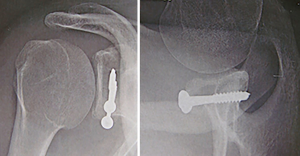
A simple broad arm sling is worn for the first 2 weeks. A self-directed active assisted full range of motion program is commenced on the first post-operative day. After 2 weeks, activities of daily living are allowed. Patients should avoid athletic exercises, such as running, cycling and swimming, for 6 to 8 weeks and should avoid upper limb strengthening for 3 months. After 3 months, if the graft has united radiographically, and the clinical assessment confirms a stable shoulder, the patient can progressively return to strengthening and contact sports (Figure 13).
Complications
The most commonly reported complications include infection, haematoma formation, neurological injury, vascular complications and coracoid graft complications.
The reported rate of infection is low, whilst one author reported an infection rate of 6% (37), most authors have shown an infection rate of <1% (11,21,38). These infections are usually treated successfully with irrigation and lavage in the operating theatre and a course of targeted antibiotics.
Vascular injuries that have been reported include axillary artery pseudoaneurysms, presenting from 6 months to 15 years after the primary procedure, and one intraoperative laceration to the axillary artery requiring vascular surgical intervention. The neurological injuries reported in the same systematic review included temporary palsies of the ulnar, median, radial and suprascapular nerve with permanent palsies noted with the musculocutaneous and axillary nerves as well as the brachial plexus at the trunk level (38).
Coracoid graft fracture is reported to occur in 1.5% of cases with evidence of lysis in 3.2% of patients. Positioning of the graft is also vital, with lateral overhang of the graft being associated with development of arthritis whereas medial placement of graft is associated with increased risk of dislocation (39,40). Non-union of the coracoid occurs in up to 9.4% (38), however this technique has a pseudarthrosis rate of 1.5% at 20 years follow up (39).
Overall the rate of reoperation following a Latarjet is around 5–7%. Infection, haematoma as well as screw removal and glenoid bony rim fractures are the most common indications for reoperation (11,38).
Results
The rate of instability after a Latarjet is low, with 2.9–5.0% rate of dislocation and 5.8% rate of subluxations being reported in literature (38). Of these episodes of repeat instabilities, the reoperation rate has been reported to be 3.4% (11).
Mizuno et al. (39) recently published long-term follow up of the described technique at a mean of 20 years. They found recurrent instability in 5.9% of patients, 2.9% with dislocation and 2.9% with subluxation, most after a new traumatic event. The patients with repeat dislocation were noted to have a medially placed coracoid graft. Patient satisfaction and functional scores were high, with 93.4% returning to sport, Rowe scores improving from 37.9 to 89.6, and 95.6% of patients satisfied or very satisfied.
The rate of radiographic arthritis after a long-term follow-up was also assessed. Patients who had preoperative evidence of arthritis had a 50% chance of progression radiographically, whereas 20% developed arthritis if not present preoperatively. They found that if the coracoid graft had lateral overhang over the glenoid that this was strongly associated with the development and progression of arthritis. Other long-term studies have found the rate of arthritis in patients to range from 35–71% (41-45), however the rate of moderate to severe arthritis in recurrent shoulder instability treated non-operatively has been reported to be 39% at 25 years follow-up (46).
Loss of external rotation has also been shown to be less in Latarjet procedures compared to anatomical Bankart repairs. In one study, external rotation loss was found to be 11.5° in Latarjet procedures compared to 20.9° with anatomical Bankart repairs (11). When comparing the management of subscapularis during glenoid exposure, horizontal split in subscapularis results in a 10.5° loss of external rotation compared to 13.3° in studies in which a vertical split of subscapularis was performed (14).
Discussion
The Latarjet procedure is a safe and reliable procedure for patients with traumatic anterior shoulder instability. In patients with glenoid bone loss and contact athletes the failure rate is lower than an anatomical Bankart repair.
These are some technical tips to reduce complications when performing the procedure.
Coracoid related complications:
- Coracoid exposure and osteotomy at the base should yield a graft of approximately 2.5 cm, which should decrease the risk of fracture and allow for a large coracoid surface area to aide union.
- Coracoid fracture risk is decreased by ensuring that: the longest coracoid graft possible is harvested, using a partially threaded cancellous screw to allow for smaller drill hole in coracoid, and using a “2-finger” screw tightening technique to avoid over tightening the screws.
- Coracoid non-union risk can be decreased by:
- Ensuring a large coracoid graft is harvested and a flat cancellous surface prepared on the inferior surface;
- Preparing the anterior glenoid surface by excising overlying soft tissue and then performing an osteotomy with a sharp osteotome back to flat cancellous bone;
- Using two partially threaded screws to ensure compression and rigid fixation of the graft;
- Placing screws parallel to the articular surface, therefore orthogonal to the prepared anterior glenoid, to allow for maximal compression between the coracoid and glenoid surfaces.
Degenerative joint disease:
- Ensuring there is no lateral overhang of the graft can decrease risk of developing degenerative joint disease.
- A drill hole 7 mm medial to the articular surface will usually position the graft accurately and avoidance of the use of washers for the screws decreases the chance of impingement onto the humeral head.
- Visualization and palpation with Mayo scissors of the graft after fixation is vital, with lateral overhang necessitating either repositioning of the graft or debridement of the overhanging coracoid with a high speed burr or bone rongeur.
- Placing screws parallel to the articular surface is important to decrease the rate of lateral overhang, which can occur with over hanging screw heads (Figure 11).
Infection and haematoma:
- Infection rates are low with this procedure, but we feel that it is increased if a haematoma develops so meticulous haemostasis is important.
- We recommend ligation of the medial branches of the cephalic vein, the Mercedes-Benz vessels, with sutures. In our experience, by ligating these branches the rate of bleeding is low and the need for a drain is also low.
- Resting of the arm in a sling for the first 2 weeks has also led to a low rate of haematoma formation.
Stiffness:
- We have not found this to be a major issue with our technique, as the subscapularis split protects the muscle and the repair of the CAL to the capsule in full external rotation has not limited range of motion. We allow early self-directed range of motion exercises to avoid stiffness.
Recurrent instability:
- The rate of instability and dislocation is low, with accurate positioning of the graft vital. A coracoid positioned too inferior allows the humeral head to dislocate above the graft whilst positioning the graft too medially has a higher re-dislocation rate. Patients with voluntary dislocations and uncontrolled epilepsy also have much higher re-dislocation rates (47) and this procedure should not be used for those patients. Our preference for surgical treatment of the failed Latarjet is conversion to a modified Eden-Hybinette iliac crest bone graft procedure (48), however arthroscopic techniques have also been described (49).
We feel that this technique has been tested and is reproducible in the management of anterior shoulder instability, particularly in patients with glenoid bone loss, large Hill-Sachs lesions or in the contact athlete. Following the described surgical technique can avoid most complications. However like most surgical procedures, correct patient selection is important.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Albert Lin and Jason J. Shin) for the series “Trends in Anterior Shoulder Instability” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.10.01). The series “Trends in Anterior Shoulder Instability” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hodhody G, Mackenzie TA, Funk L. Shoulder injuries in adolescent rugby players. Shoulder Elbow 2016;8:159-66. [Crossref] [PubMed]
- Owens BD, Agel J, Mountcastle SB, et al. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med 2009;37:1750-4. [Crossref] [PubMed]
- Headey J, Brooks JH, Kemp SP. The epidemiology of shoulder injuries in English professional rugby union. Am J Sports Med 2007;35:1537-43. [Crossref] [PubMed]
- Whitehouse T, Orr R, Fitzgerald E, et al. The Epidemiology of Injuries in Australian Professional Rugby Union 2014 Super Rugby Competition. Orthop J Sports Med 2016;4:2325967116634075 [Crossref] [PubMed]
- Zimmermann SM, Scheyerer MJ, Farshad M, et al. Long-Term Restoration of Anterior Shoulder Stability: A Retrospective Analysis of Arthroscopic Bankart Repair Versus Open Latarjet Procedure. J Bone Joint Surg Am 2016;98:1954-61. [Crossref] [PubMed]
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94. [Crossref] [PubMed]
- Latarjet M. Treatment of recurrent dislocation of the shoulder. Lyon Chir 1954;49:994-7. [PubMed]
- Trillat A. Treatment of recurrent dislocation of the shoulder; technical considerations. Lyon Chir 1954;49:986-93. [PubMed]
- Helfet AJ. Coracoid transplantation for recurring dislocation of the shoulder. J Bone Joint Surg Br 1958;40-B:198-202. [PubMed]
- Giles JW, Degen RM, Johnson JA, et al. The Bristow and Latarjet Procedures: Why These Techniques Should Not Be Considered Synonymous. J Bone Joint Surg Am 2014;96:1340-8. [Crossref] [PubMed]
- An VV, Sivakumar BS, Phan K, et al. A systematic review and meta-analysis of clinical and patient-reported outcomes following two procedures for recurrent traumatic anterior instability of the shoulder: Latarjet procedure vs. Bankart repair. J Shoulder Elbow Surg 2016;25:853-63. [Crossref] [PubMed]
- Randelli P, Cucchi D, Butt U. History of shoulder instability surgery. Knee Surg Sports Traumatol Arthrosc 2016;24:305-29. [Crossref] [PubMed]
- van der Linde JA, van Wijngaarden R, Somford MP, et al. The Bristow–Latarjet procedure, a historical note on a technique in comeback. Knee Surg Sports Traumatol Arthrosc 2016;24:470-8. [Crossref] [PubMed]
- Cowling PD, Akhtar MA, Liow RY. What is a Bristow-Latarjet procedure? A review of the described operative techniques and outcomes. Bone Joint J 2016;98-B:1208-14. [Crossref] [PubMed]
- Young AA, Walch G. Shoulder Instability: A Comprehensive Approach. Chapter 16: Open bony augmentation of glenoid bone loss—the latarjet and variants—surgical technique. Amsterdam: Elsevier, 2012:197-208.
- Patte D, Debeyre J. Luxations récidivantes de l'épaule. Tech Chir Orthop 1980;44265:44-52.
- Wellmann M, de Ferrari H, Smith T, et al. Biomechanical investigation of the stabilization principle of the Latarjet procedure. Arch Orthop Trauma Surg 2012;132:377-86. [Crossref] [PubMed]
- Edwards TB, Walch G. The Latarjet Procedure for Recurrent Anterior Shoulder Instability: Rationale and Technique. Oper Tech Sports Med 2002;10:25-32. [Crossref]
- Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br 2007;89:1470-7. [Crossref] [PubMed]
- Bouliane M, Saliken D, Beaupre LA, et al. Evaluation of the Instability Severity Index Score and the Western Ontario Shoulder Instability Index as predictors of failure following arthroscopic Bankart repair. Bone Joint J 2014;96-B:1688-92. [Crossref] [PubMed]
- Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007;23:1033-41. [Crossref] [PubMed]
- Degen RM, Giles JW, Johnson JA, et al. Remplissage Versus Latarjet for Engaging Hill-Sachs Defects Without Substantial Glenoid Bone Loss: A Biomechanical Comparison. Clin Orthop Relat Res 2014;472:2363-71. [Crossref] [PubMed]
- Cho NS, Hwang JC, Rhee YG. Arthroscopic Stabilization in Anterior Shoulder Instability: Collision Athletes Versus Noncollision Athletes. Arthroscopy 2006;22:947-53. [Crossref] [PubMed]
- Roberts SN, Taylor DE, Brown JN, et al. Open and arthroscopic techniques for the treatment of traumatic anterior shoulder instability in Australian rules football players. J Shoulder Elbow Surg 1999;8:403-9. [Crossref] [PubMed]
- Petrera M, Tsuji M, Theodoropoulos J. Arthroscopic Bankart Repair With Suture Anchors in Collision Versus Non-collision Athletes (SS-10). Arthroscopy 2011;27:e34 [Crossref]
- Neyton L, Young A, Dawidziak B, et al. Surgical treatment of anterior instability in rugby union players: clinical and radiographic results of the Latarjet-Patte procedure with minimum 5-year follow-up. J Shoulder Elbow Surg 2012;21:1721-7. [Crossref] [PubMed]
- Joshi MA, Young AA, Balestro JC, et al. The Latarjet-Patte procedure for recurrent anterior shoulder instability in contact athletes. Clin Sports Med 2013;32:731-9. [Crossref] [PubMed]
- Gerber C, Ganz R. Clinical assessment of instability of the shoulder. With special reference to anterior and posterior drawer tests. J Bone Joint Surg Br 1984;66:551-6. [PubMed]
- Gagey OJ, Gagey N. The hyperabduction test. J Bone Joint Surg Br 2001;83:69-74. [Crossref] [PubMed]
- Young AA, Maia R, Berhouet J, et al. Open Latarjet procedure for management of bone loss in anterior instability of the glenohumeral joint. J Shoulder Elbow Surg 2011;20:S61-9. [Crossref] [PubMed]
- Bernageau J, Patte D, Debeyre J, et al. Value of the glenoid profil in recurrent luxations of the shoulder. Rev Chir Orthop Reparatrice Appar Mot 1976;62:142-7. [PubMed]
- Ikemoto RY, Nascimento LG, Bueno RS. The Technique to Calculate Glenoid Bone Loss With the Bernageau Profile View: Is it Possible? Tech Shoulder Elb Surg 2010;11:37-40. [Crossref]
- Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am 2010;92:133-51. [Crossref] [PubMed]
- Itoi E, Lee SB, Amrami KK, et al. Quantitative assessment of classic anteroinferior bony Bankart lesions by radiography and computed tomography. Am J Sports Med 2003;31:112-8. [Crossref] [PubMed]
- Young AA, Baba M, Neyton L, et al. Coracoid graft dimensions after harvesting for the open Latarjet procedure. J Shoulder Elbow Surg 2013;22:485-8. [Crossref] [PubMed]
- Meyer DC, Moor BK, Gerber C, et al. Accurate coracoid graft placement through use of a drill guide for the Latarjet procedure. J Shoulder Elbow Surg 2013;22:701-8. [Crossref] [PubMed]
- Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am 2012;94:495-501. [Crossref] [PubMed]
- Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg 2013;22:286-92. [Crossref] [PubMed]
- Mizuno N, Denard PJ, Raiss P, et al. Long-term results of the Latarjet procedure for anterior instability of the shoulder. J Shoulder Elbow Surg 2014;23:1691-9. [Crossref] [PubMed]
- Hovelius L, Sandström B, Olofsson A, et al. The effect of capsular repair, bone block healing, and position on the results of the Bristow-Latarjet procedure (study III): long-term follow-up in 319 shoulders. J Shoulder Elbow Surg 2012;21:647-60. [Crossref] [PubMed]
- Singer GC, Kirkland PM, Emery RJ. Coracoid transposition for recurrent anterior instability of the shoulder. A 20-year follow-up study. J Bone Joint Surg Br 1995;77:73-6. [PubMed]
- Hovelius L, Sandström B, Sundgren K, et al. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I--clinical results. J Shoulder Elbow Surg 2004;13:509-16. [Crossref] [PubMed]
- Hovelius LK, Sandström BC, Rösmark DL, et al. Long-term results with the Bankart and Bristow-Latarjet procedures: recurrent shoulder instability and arthropathy. J Shoulder Elbow Surg 2001;10:445-52. [Crossref] [PubMed]
- Hovelius L, Sandström B, Saebö M. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study II-the evolution of dislocation arthropathy. J Shoulder Elbow Surg 2006;15:279-89. [Crossref] [PubMed]
- Allain J, Goutallier D, Glorion C. Long-term results of the Latarjet procedure for the treatment of anterior instability of the shoulder. J Bone Joint Surg Am 1998;80:841-52. [Crossref] [PubMed]
- Hovelius L, Saeboe M. Neer Award 2008: Arthropathy after primary anterior shoulder dislocation--223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg 2009;18:339-47. [Crossref] [PubMed]
- Raiss P, Lin A, Mizuno N, et al. Results of the Latarjet procedure for recurrent anterior dislocation of the shoulder in patients with epilepsy. J Bone Joint Surg Br 2012;94:1260-4. [Crossref] [PubMed]
- Lunn JV, Castellano-Rosa J, Walch G. Recurrent anterior dislocation after the Latarjet procedure: outcome after revision using a modified Eden-Hybinette operation. J Shoulder Elbow Surg 2008;17:744-50. [Crossref] [PubMed]
- Castagna A, Garofalo R, Melito G, et al. The role of arthroscopy in the revision of failed Latarjet procedures. Musculoskelet Surg 2010;94:S47-55. [Crossref] [PubMed]
Cite this article as: Mattern O, Young A, Walch G. Open Latarjet: tried, tested and true. Ann Joint 2017;2:65.


