Osteochondral augmentation of glenoid bone loss—distal tibia allograft
Introduction
Anterior instability of the glenohumeral joint has long been a difficult pathology for orthopaedic surgeons to manage, largely due to the combination of soft tissue and bony defects that may be present (1,2). Typically caused by an initial traumatic dislocation or subluxation, these defects can often beget recurrent instability that then leads to further deterioration of the osseous and dynamic constraints of the shoulder. Though arthroscopic soft-tissue stabilization often produces favorable outcomes, there are two categories of patients for which this is not the case: patients with significant glenohumeral bone loss and those with persistent instability following a soft-tissue stabilization procedure (3,4). Significant glenohumeral bone loss disrupts the articular arc of the shoulder, thereby decreasing its resistance to shear stress, and has been implicated in 90–100% of cases of recurrent instability following soft tissue stabilization (5). In these patients, glenohumeral bone augmentation is necessary, and both arthroscopic and open techniques with several different graft options have been described (1). In this paper, we will define significant bone loss and analyze the benefits and drawbacks of these graft options.
Prevalence and risk factors associated with shoulder instability
In patients that were followed after a primary anterior dislocation of the shoulder, those less than 23 years old reported a nearly 50% recurrence rate, regardless of treatment. In the two older age groups (23–29 and 30–40 years old), the incidence of recurrence was 25% or less (6). In addition to age, gender has been associated with differences in recurrence rate, as males are more likely to undergo repeat shoulder closed reduction (7). The presence of a glenoid bone defect is also significantly associated with recurrence of dislocation (8). The number of dislocations, younger age at first dislocation, and male gender are the most significant predictors of glenoid bone loss in anterior shoulder instability (8). Approximately 60% of shoulders with anterior instability demonstrate a concomitant glenoid defect and Hill-Sachs lesion, also known as a bipolar lesion (9). Bipolar lesions are common in patients with recurrent instability and those that participate in collision/contact sports (9).
Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder can be classified into three types: type I, displaced avulsion fracture with attached capsule; type II, a medially displaced fragment united abnormally to the glenoid rim; type III, erosion of the glenoid rim with less than 25% (type IIIA) or greater than 25% (type IIIB) deficiency (10). The majority of type I–IIIA lesions can be treated with high success rates by suturing the fracture fragment, the capsule, or both to the glenoid rim and addressing associated capsular laxity (10).
Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair include >25% glenoid bone loss, Hill-Sachs lesions qualified as large on arthroscopic examination, stretched inferior glenohumeral ligament, anterior hyperlaxity, and three or less anchors used in the repair (11). Military service members with a prior history of glenohumeral joint instability are a particularly high-risk population, with an approximately fivefold higher recurrent instability rate, regardless of the initial instability direction (12). Arthroscopic Bankart repairs are as effective as open Bankart repairs if there are no significant structural bone deficits, such as engaging Hill-Sachs or inverted-pear Bankart lesions (13). Contact athletes with bone deficiency require open Bankart repair, and any patients with significant glenoid bone loss are candidates for bone augmentation procedure (13).
What is critical bone loss?
The definition and classification of glenoid bone loss varies among recent studies of shoulder instability and requires greater specificity. The concept of significant bone loss was introduced by Burkhart and De Beer, classifying significant defects by the superior-inferior arthroscopic appearance of the glenoid as an inverted pear. The presence of an inverted pear glenoid is considered a predisposition for recurrent dislocations (13). An inverted pear glenoid occurs from a large bony Bankart lesion with significant impression (compression) defect, converting the normal pear-shaped glenoid to an inverted pear glenoid, with an approximate bone loss of 25% (14).
The major mechanisms of glenohumeral stability-glenoid concavity and ligamentous tension-are mostly affected by osseous defects and Bankart lesions, respectively (15). Bony lesions affect the biomechanics of shoulder mobility by shortening the glenoid arc length, which subsequently compromises the stability of the joint by reducing the glenoid contact surface and its concavity (13). The shortening of the glenoid arc occurs from loss of bone in the anteroinferior glenoid, creating a mismatch between the glenoid and the humerus, while the loss of bone on the glenoid rim also decreases the depth of articular conformity (16). “Critical bone loss” has been variable across the literature, ranging from 13–25%, but is an important term that requires a more thorough classification for surgical selection.
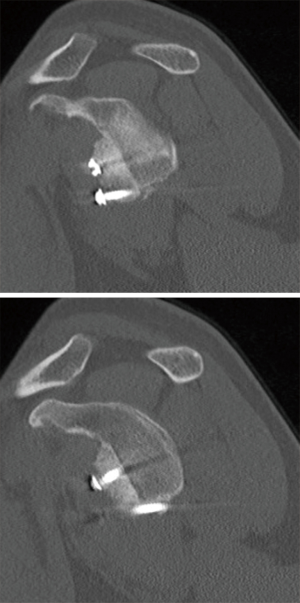
In order to properly determine the value for critical bone loss, simple physical examinations and imaging tests must be consistent. Glenoid bone loss has been noted to more readily cause subluxation events, often from a long history of shoulder instability symptoms, which may indicate loss of bony constraints of the glenohumeral joint (16,17). A physical examination should always be performed, in comparison to the contralateral shoulder, enabling a quantifiable value for direction and magnitude of laxity (16). Physical examination can determine the presence of bone loss, but the amount of bone loss is best measured by radiographic evaluation, advanced imaging techniques, and arthroscopic measurements (16). Magnetic resonance arthrography provides en face measurements of glenoid bone loss on sagittal oblique images, but the most effective determination of bone loss is taken by three-dimensional computed tomography scans, because it provides the most information of the extent and type of glenoid bone injury (17) (Figure 1).
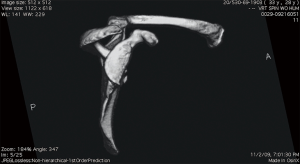
While most approaches to understanding glenoid bone loss focus on the quantifiable amount of loss, some studies find that the location of the respective lesions is also a key indicator of potential recurrent instability. Yamamoto et al. found that if a Hill-Sachs lesion lies within the glenoid track or if the medial margin of the Hill-Sachs lesion is outside of the glenoid track, there is a greater chance of overriding the rim of the glenoid (18). A study by Di Giacomo et al. addressed the necessity in evaluating the glenoid track, including the associated glenoid bone loss and the location of the Hill-Sachs lesion, as width of the glenoid track will be decreased in the presence of a glenoid bone defect (19).
Bone loss is imperative to address due to the profound effect it has on biomechanics of the shoulder, with an average limitation in external rotation of 25° per cm of imbrication (15). In a cadaveric study by Itoi et al., Bankart lesions were created and the average force required to move the humeral head the normalized distance for subluxation (7.8±1.1 mm) was measured. A force of 185 newtons (N) was required in a normal shoulder, a mere 38 N in shoulders with Bankart lesions, and 108 N in shoulders following a Bankart repair (15). This biomechanical study demonstrates the ease of subluxation from the presence of lesions and the importance of addressing these complications in order to return the shoulder to its prior organic biomechanical motion/strength.
Critical bone loss was determined to be 25% of the glenoid width by Burkhart and de Beer, as patients with significant bone loss had a 67% rate of failure compared to 4% in patients without significant bone loss. However, more recent studies show detrimental effects from even lower values of glenoid bone loss (13). In a study of 50 shoulders from football athletes, all shoulders with glenoid bone loss >13.5% (n=3) experienced recurrent instability following arthroscopic stabilization, while none of the shoulders with glenoid bone loss <13.5% (n=47) sustained a recurrent instability event (20). Though Shaha et al. utilized a critical level of 20% glenoid bone loss and found that bone loss greater than this value significantly increased failure rates (27.8% compared to 7.3%), subjective scores were based upon the critical level of 13.5% glenoid bone loss. Those with ≥13.5% bone loss had a mean WOSI score of 434, compared to 901 in those with ≤13.5% bone loss (21). Further, Shin et al. studied a critical value of 17.3% glenoid bone loss, in which the group with greater bone loss displayed worse postoperative ROWE and ASES scores relative to the group with bone loss less than 17.3% (22). Ultimately, these scores show that even in the absence of a recurrent instability event, arthroscopic stabilization on a patient with bone loss >13.5% often results in clinically unacceptable results (21).
Addressing concerns of glenoid bone loss begins with determining the shape of the glenoid. In a study of 53 patients with anterior shoulder instability, Lo et al. found 38 showed evidence of bone loss anteriorly, with the mean amount and percentage of bone loss significantly greater in the inverted pear glenoid group (mean, 8.6 mm, 36%; range, 6–12 mm, 25–45%) than the non-inverted pear glenoid group (mean, 1.5 mm, 6.2%; range, 0–3 mm, 0–12.5%) (14). Treating patients with significant glenoid bone loss using isolated soft-tissue repair without bone grafting increases the chance of repair failure due to the increased demand of resistance at the soft tissue repair interface (19). The critical value for bone loss has yet to become conclusive; while previously, values less than 15% to 20% (5 to 7 mm of bone) were thought to be successfully treated with soft-tissue stabilization alone, recent literature now suggests open repair or bone augmentation procedures should be considered in patients displaying as little as 13.5% of bone loss, as arthroscopic Bankart repair fails to yield favorable outcomes biomechanically or subjectively in this cohort (17,21,23). We recommend bony augmentation in the setting of ≥20% glenoid bone loss.
The Latarjet procedure—risks and failure rate
The Latarjet procedure, first described by Dr. Michel Latarjet in 1954, has proven to be a useful treatment method for patients with anteroinferior instability, specifically those with critical glenohumeral bone loss or in those that have failed soft tissue stabilization procedures (3,4).
A systematic review by Bhatia et al. encompassing ten different papers on Latarjet results reported good to excellent results for at least 90% of patients and a rate of recurrent anterior shoulder instability of 0% to 8% after a mean follow-up ranging from 6 months to 14.3 years postoperatively (24). Instability usually required revision surgery and was defined as dislocation, subluxation, or positive apprehension on physical exam; subjective apprehension was not considered recurrent instability. It is worth noting that despite the efficacious results of this procedure, multiple studies reported radiographic changes following Latarjet surgery. Allain et al. found glenohumeral osteoarthritis at the time of final follow-up in more than half of the 58 shoulders they retrospectively studied, though most of these cases showed grade 1 changes (24). Hovelius et al. reported an overall satisfaction rate of 98% in 118 patients, however, a follow-up radiographic study of these patients found moderate to severe dislocation arthropathy in 14% (25). These radiographic changes were almost always asymptomatic (24).
Malunion and nonunion have been credited as a cause of postoperative recurrent instability. Lafosse et al. reported four cases of nonunion (4.1%), Schmid et al. reported one case of malunion (2.0%), and Burkhart et al. reported one case of asymptomatic fibrous nonunion that did not require revision (1.0%) (24). Further, Shah et al. studied CT scans of 29 shoulders specifically to assess for incorporation of the graft, and found union of the graft in 21 shoulders (72%) at a mean of 6.5 months postoperatively. Of the eight shoulders without osseous union, 3 (10.3%) showed glenohumeral instability and required revision surgery; the remaining 5 were asymptomatic and did not affect patient outcomes (26). Hovelius et al. also studied radiographs to assess for graft incorporation, and found that 246 (83%) showed osseous union, 34 (13%) showed fibrous union, 14 (5%) showed malunion, and 3 (1%) were unable to be visualized (25).
Osteolysis of the graft is also an important concern with Latarjet procedure and may be a cause of pain, stiffness, subtle instability, or dislocation. Di Giacomo et al. performed CT analysis on 26 patients that were followed prospectively after a Latarjet procedure in order to determine the extent and location of osteolysis of the coracoid graft (27). At a mean of 17.5±6.5 months after surgery, 59.5% of the graft underwent osteolysis on average, with the superficial portion of the proximal coracoid being most affected, and the distal region, especially in the deep portion, least involved. However, 92.3% of the patients were satisfied after surgery and no failures were reported, making it difficult to determine if any correlation exists between radiologic osteolysis of the graft and clinical failure (27). In a later study, Di Giacomo and colleagues compared the rate of osteolysis between patients with significant glenoid bone loss (>15%) and those without it. They found significantly less osteolysis of the graft in the patients with significant bone loss (39.6% vs. 65.1%), suggesting that lack of mechanical stimuli may contribute to bone resorption and that bone grafting is less essential without significant bone loss (28).
Despite the largely positive results following Latarjet procedure, complications have been reported that must be considered and explained to the patient before proceeding with surgery. Quantifying the rate of these complications is difficult, as they are inconsistently reported in the literature. In comparing several studies that included prospective, retrospective, and systematic reviews, the complication rate ranged from 3.8% to 25.0% (4,24-26). The most commonly reported complications were neurologic injury, infection, hardware failure, and hematomas (4,24-26,29). Less common complications included frozen shoulder and delayed wound healing (24,26).
Nerve injuries were reported in three separate studies, with rates ranging from 3.1% to 20.6% (4,26,29). The most commonly affected nerve was the axillary nerve, however, there were also reports of musculocutaneous, suprascapular, and radial nerve involvement. While the vast majority of these injuries resolved spontaneously within 6 months, those affecting the axillary nerve were more likely to persist as sensory disturbances at final follow-up, with one patient also experiencing residual weakness (26) and another still experiencing neuralgia (4).
Infection is a risk with any surgery, and Latarjet is no exception. In the four studies that reported infections, the rates ranged from 1.3% to 6.3% (4,26). Most of these infections were superficial and were treated successfully with oral antibiotics, however, there were reports of deeper infections that required irrigation and debridement with intravenous antibiotics (4).
Hardware-related complications were rare, occurring 0.5–3.1% of the time (4,24,25) and included screw loosening, osteolysis around screws, and coracoid graft fracture. While many of these issues were asymptomatic, Hovelius et al. reported two revision surgeries due to screw-graft problems (25). Eleven hematomas were reported in 4 of the studies, only 1 of which required intervention (4,24,25).
Certain patient characteristics seemed to increase the risk of complications. Both Gartsman et al. and Shah et al. noted that increased age was associated with a higher risk for complications (4,26). Shah et al. investigated these risk factors further, and found three variables that were significantly associated with higher rates of complications following Latarjet procedure: Workers’ Compensation claim, the use of cannulated screws, and increased age (26). Patients filing a Worker’s Compensation claim and those that received 4.0 or 4.5 mm cannulated screws were 12 times more likely to suffer a complication (P=0.0260), while the likelihood of a complication increased 7.5% with every 1-year increase in age (P=0.0188). The power of this study was not sufficient to demonstrate significance for smoking, prior surgery, prior open surgery, or gender as risk factors for complications (26).
The Latarjet procedure is an effective surgery both as a primary option in patients with glenoid bone loss or as a revision surgery in patients with recurrent instability following failed soft tissue stabilization procedures. However, the risks of complications, nonunion, and arthritic progression, as well as the additional morbidity from autograft harvest, must be considered.
Translational evidence for distal tibia allograft (DTA)
Although the Latarjet procedure has provided patients with favorable clinical results and a low risk of recurrent instability, reported complications and functional deficits have led physicians to explore other graft options for their glenoid bone augmentation. These have included other autografts, such as the iliac crest or lateral coracoid, as well as osteochondral allografts including the lateral distal tibia, medial tibial plateau, coracoid, radial head, and distal radius (1,2,5). It has been hypothesized that with a more flush and anatomic fit, a graft will best normalize articular contact pressures, provide better articular surface to prevent instability, and cause less glenohumeral osteoarthritis (1,2). Multiple studies have investigated the translational benefits of these alternative grafts, and the DTA has demonstrated great promise as a potential option.
DeHaan et al. studied bilateral shoulders in 17 cadaveric specimens to compare the radius of curvature of the native glenoid to several potential grafts in order to assess congruence for anterior glenoid augmentation (2). The inferior coracoid, used in Latarjet procedure, was the only autograft that conformed to the glenoid’s radius of curvature, matching the superior-inferior measurement 59% of the time and the anteroposterior measurement 94% of the time. Neither the lateral coracoid nor the iliac crest had any measurements that were within 5 mm of the glenoid’s radius of curvature (2). Of the osteochondral allografts, the DTA produced the best results, as 94% of specimens measured within the interquartile range of the glenoid radius of curvature. The medial tibial plateau measured within the interquartile range with 68% of specimens, while only 12% of the specimens from the scaphoid fossa of the distal radius fell within the interquartile range. None of the specimens from the radial head or the lunate fossa of the distal radius were measured within the interquartile range of the glenoid (2). Based on these results, the authors concluded that the autograft of the inferior coracoid and the osteochondral allograft of the lateral distal tibia were ideal sites for glenoid augmentation due to their similar radii of curvature. Of additional importance, this study also found that the radius of curvature of each anatomic site was independent of age, sex, height, or weight, meaning that these relative anatomic relationships are preserved throughout different patient populations (2).
To specifically compare the Latarjet inferior coracoid graft and the DTA, Bhatia et al. studied eight cadaveric shoulders and compared glenohumeral contact areas, contact pressures, and peak forces between an intact glenoid, a glenoid with a 30% anterior defect, a glenoid after augmentation with Latarjet coracoid graft, and a glenoid after augmentation with DTA (1). After dissecting the shoulders of all soft tissue, these variables were measured in three static positions of humeral abduction with a 440-N compressive load: 30°, 60°, and 60° of abduction with 90° of external rotation (ABER). Although the DTAs were unable to normalize glenohumeral contact areas and contact pressures at all humeral positions, significant improvements over the Latarjet coracoid graft were demonstrated (1). DTAs showed significantly higher glenohumeral contact areas than Latarjet at 60° of abduction and at the ABER position. They also demonstrated significantly lower glenohumeral peak forces than Latarjet reconstruction in the ABER position. In fact, the peak forces in the ABER position following Latarjet surgery were found to be more similar to the 30% defect glenoid than the intact glenoid, which may propagate chondral injuries, elevate joint reactive forces, and contribute to the signs of glenohumeral osteoarthritis detailed in the previous section (1). It should be noted, however, that this study did not incorporate the modified Latarjet orientation (Latarjet-INF position) that has been reported to improve contact pressure profiles, because fixation difficulties have prevented it from becoming widely used in the authors’ practice. It should also be emphasized that the soft tissues were dissected in this study, eliminating the sling effect from the conjoined tendon in Latarjet procedures.
Beyond these biomechanical advantages, DTAs are also advantageous in that they provide osteochondral restoration of the articular surface defect, avoid donor-site morbidity, and are not as limited in size as coracoid grafts for reconstructing larger areas of bone loss. DTAs are also dense, weightbearing corticocancellous bone, allowing for screw fixation with less worry of fracture as was reported in Latarjet procedures (30). These studies were instrumental in proving the potential efficacy of DTA in anterior glenoid augmentation, however, more studies with clinical follow-up and radiological evidence were necessary in order to prove the clinical benefit of this procedure.
Clinical and radiographic evidence for DTA
Surgeons that have adopted allografts in lieu of the Latarjet coracoid autograft, though limited by sample size, have reported encouraging results in terms of radiologic results and clinical outcomes. Several different groups have demonstrated, at the very least, comparable results to the coracoid autograft using allografts, including the DTA.
Sayegh et al. collectively studied allografts from the iliac crest, femoral head, distal tibia, glenoid, and humeral head in a systematic review of eight studies including 70 shoulders with recurrent anterior instability after a mean follow-up of 44.5 months (range, 32–90 months) (31). The results were largely positive, with 93.4% satisfied and a mean final Rowe score of 90.6 (mean improvement of 57.5). Only 9.8% of patients continued to have pain in the shoulder, 7.1% continued to experience instability (dislocation, subluxation, or apprehension), and 2.9% suffered recurrence of glenohumeral dislocation. Bony integration of the graft was achieved in 100% of shoulders without any signs of graft resorption at long-term follow-up (31). These were excellent results for allografts in general, and the results for DTA specifically are even more encouraging. Provencher and colleagues would later report on 27 of their patients following DTA augmentation of the anterior glenoid with an average follow-up of 45 months (range, 30–66 months) and showed significant improvement in ASES score, WOSI index, and SANE score (32). There were no significant differences in range of motion (ROM) between the affected and nonaffected shoulders in any direction, and there were no signs of apprehension or cases of recurrent instability in any patients at final follow-up. CT data showed an allograft healing rate of 89% (range, 80–100%), average allograft angle of 14.9 (range, 6.6–29.3), and average allograft lysis of 3% (range, 0–25%). Of note, grafts with lesser allograft angles showed superior healing, demonstrating that optimal allograft placement results in superior bony incorporation with the native glenoid (32). These studies showed that allografts, and specifically DTA, can provide excellent clinical outcomes with a stable joint and minimal graft resorption/lysis when used to augment bony defects of the shoulder. Further investigation is needed to prove its efficacy in larger populations and for longer term follow-up.
Conclusions
In this review, we have demonstrated the biomechanical, radiographic, and clinical efficacy of DTA augmentation for treating anterior shoulder instability in the setting of significant glenoid bone loss. While the Latarjet procedure has been the most common management for these patients and has produced satisfactory results, the non-anatomic and non-cartilaginous nature of this repair has led to concerns over the early development of glenohumeral osteoarthritis (5). The DTA, in contrast, provides a more congruent fit with the glenoid’s radius of curvature, maximizing the glenoid arc articulation and increasing the shoulder’s resistance to shear stress. The cartilaginous surface of the DTA restores the osteochondral joint surface, decreasing glenohumeral contact pressure, contact area, and peak forces, and potentially slowing the arthritic progression that has been reported following Latarjet procedures. The DTA is also a dense, corticocancellous bone that can repair larger glenoid defects and allows for excellent screw-fixation and graft incorporation (30). Further, the DTA shows lower rates of osteolysis than the Latarjet, and allografting avoids any harvest site morbidity that may be associated with autografts. While there are other allografts available, few have comparable congruency with the glenoid, and the DTA carries a lower risk of contamination than the more centrally located coracoid allograft (30). Though limited by sample size, outcomes from multiple centers have demonstrated excellent results both radiographically and clinically.
Ultimately, principles of surgical management are guided by the extent of osseous injuries to the glenoid, the surgeon’s personal experience with specific reconstructive techniques, and patient-specific factors such as professional and athletic demands (1) (Table 1).
Pearls and pitfalls associated with surgical technique (Figures 2-5)
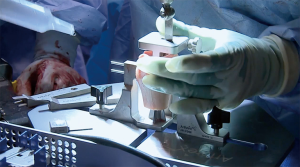
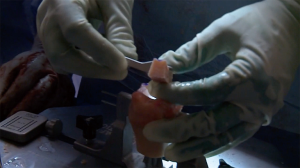
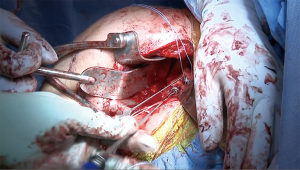
The author’s preferred surgical technique has been detailed in previous reports (5,30), however, there are several elements of the procedure that should be emphasized. It is important that the surgeon utilize the instability type incision, which is more medial than the standard deltopectoral incision, starting near the tip of the coracoid and extending directly inferiorly to the superior axillary fold. Special care should be taken to avoid excess medial retraction on the conjoined tendon, so as to protect the musculocutaneous nerve. We recommend a subscapularis-splitting approach, but it is important not to split the subscapularis medial to the coracoid, as this can injure the nerve to the subscapularis. If the surgeon is unable to bluntly separate the subscapularis from the capsule, they may incise the subscapularis along the capsule to expose the joint. Any labral tissue is elevated and dissected medially, with caution taken to protect the axillary nerve, because the labrum will be repaired to the anterior aspect of the allograft. Exposure of the capsule should be carried out meticulously, with attention given to preserving sufficient tissue for capsular repair to the DTA after it is secured in place. This capsular repair is typically carried out with screws and washers into the DTA, but suture anchors may instead be placed in the native glenoid bone superior and inferior to the graft. Flush and congruent placement of the graft on the anterior glenoid is necessary for sufficient bone-to-bone contact to allow for graft incorporation and healing; this may require several back-and-forth trials with intervening microadjustments to the graft, recipient site surface, or both. During closure, the subscapularis split should be repaired with non-absorbable suture (5).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Albert Lin and Jason J. Shin) for the series “Trends in Anterior Shoulder Instability” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.10.08). The series “Trends in Anterior Shoulder Instability” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhatia S, Van Thiel GS, Gupta D, et al. Comparison of glenohumeral contact pressures and contact areas after glenoid reconstruction with latarjet or distal tibial osteochondral allografts. Am J Sports Med 2013;41:1900-8. [Crossref] [PubMed]
- Dehaan A, Munch J, Durkan M, et al. Reconstruction of a bony bankart lesion: best fit based on radius of curvature. Am J Sports Med 2013;41:1140-5. [Crossref] [PubMed]
- Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007;23:1033-41. [Crossref] [PubMed]
- Gartsman GM, Waggenspack WN Jr, O'Connor DP, et al. Immediate and early complications of the open Latarjet procedure: a retrospective review of a large consecutive case series. J Shoulder Elbow Surg 2017;26:68-72. [Crossref] [PubMed]
- Frank RM, Romeo AA, Provencher MT. Glenoid Reconstruction With Distal Tibia Allograft for Recurrent Anterior Shoulder Instability. Orthopedics 2017;40:e199-205. [Crossref] [PubMed]
- Hovelius L, Eriksson K, Fredin H, et al. Recurrences after initial dislocation of the shoulder. Results of a prospective study of treatment. J Bone Joint Surg Am 1983;65:343-9. [Crossref] [PubMed]
- Leroux T, Ogilvie-Harris D, Veillette C, et al. The epidemiology of primary anterior shoulder dislocations in patients aged 10 to 16 years. Am J Sports Med 2015;43:2111-7. [Crossref] [PubMed]
- Milano G, Grasso A, Russo A, et al. Analysis of risk factors for glenoid bone defect in anterior shoulder instability. Am J Sports Med 2011;39:1870-6. [Crossref] [PubMed]
- Nakagawa S, Ozaki R, Take Y, et al. Relationship Between Glenoid Defects and Hill-Sachs Lesions in Shoulders With Traumatic Anterior Instability. Am J Sports Med 2015;43:2763-73. [Crossref] [PubMed]
- Bigliani LU, Newton PM, Steinmann SP, et al. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 1998;26:41-5. [Crossref] [PubMed]
- Boileau P, Villalba M, Hery JY, et al. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am 2006;88:1755-63. [PubMed]
- Cameron KL, Mountcastle SB, Nelson BJ, et al. History of shoulder instability and subsequent injury during four years of follow-up: a survival analysis. J Bone Joint Surg Am 2013;95:439-45. [Crossref] [PubMed]
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs: significance of the inverted-pear glenoid and the humeral engaging Hill-Sachs lesion. Arthroscopy 2000;16:677-94. [Crossref] [PubMed]
- Lo IK, Parten PM, Burkhart SS. The inverted pear glenoid: an indicator of significant glenoid bone loss. Arthroscopy 2004;20:169-74. [Crossref] [PubMed]
- Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am 2000;82:35-46. [Crossref] [PubMed]
- Piasecki DP, Verma NN, Romeo AA, et al. Glenoid bone deficiency in recurrent anterior shoulder instability: diagnosis and management. J Am Acad Orthop Surg 2009;17:482-93. [Crossref] [PubMed]
- Provencher MT, Bhatia S, Ghodadra NS, et al. Recurrent shoulder instability: current concepts for evaluation and management of glenoid bone loss. J Bone Joint Surg Am 2010;92:133-51. [Crossref] [PubMed]
- Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-56. [Crossref] [PubMed]
- Di Giacomo G, Itoi E, Burkhart SS. Evolving concept of bipolar bone loss and the Hill-Sachs lesion: from "engaging/non-engaging" lesion to "on-track/off-track" lesion. Arthroscopy 2014;30:90-8. [Crossref] [PubMed]
- Dickens JF, Owens BD, Cameron KL, et al. The Effect of Subcritical Bone Loss and Exposure on Recurrent Instability After Arthroscopic Bankart Repair in Intercollegiate American Football. Am J Sports Med 2017;45:1769-75. [Crossref] [PubMed]
- Shaha JS, Cook JB, Song DJ, et al. Redefining "Critical" Bone Loss in Shoulder Instability: Functional Outcomes Worsen With "Subcritical" Bone Loss. Am J Sports Med 2015;43:1719-25. [Crossref] [PubMed]
- Shin SJ, Kim RG, Jeon YS, et al. Critical Value of Anterior Glenoid Bone Loss That Leads to Recurrent Glenohumeral Instability After Arthroscopic Bankart Repair. Am J Sports Med 2017;45:1975-81. [Crossref] [PubMed]
- Garcia GH, Taylor SA, Fabricant PD, et al. Shoulder Instability Management: A Survey of the American Shoulder and Elbow Surgeons. Am J Orthop (Belle Mead NJ) 2016;45:E91-7. [PubMed]
- Bhatia S, Frank RM, Ghodadra NS, et al. The outcomes and surgical techniques of the latarjet procedure. Arthroscopy 2014;30:227-35. [Crossref] [PubMed]
- Hovelius L, Sandstrom B, Olofsson A, et al. The effect of capsular repair, bone block healing, and position on the results of the Bristow-Latarjet procedure (study III): long-term follow-up in 319 shoulders. J Shoulder Elbow Surg 2012;21:647-60. [Crossref] [PubMed]
- Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am 2012;94:495-501. [Crossref] [PubMed]
- Di Giacomo G, Costantini A, de Gasperis N, et al. Coracoid graft osteolysis after the Latarjet procedure for anteroinferior shoulder instability: a computed tomography scan study of twenty-six patients. J Shoulder Elbow Surg 2011;20:989-95. [Crossref] [PubMed]
- Di Giacomo G, de Gasperis N, Costantini A, et al. Does the presence of glenoid bone loss influence coracoid bone graft osteolysis after the Latarjet procedure? A computed tomography scan study in 2 groups of patients with and without glenoid bone loss. J Shoulder Elbow Surg 2014;23:514-8. [Crossref] [PubMed]
- Delaney RA, Freehill MT, Janfaza DR, et al. 2014 Neer Award Paper: neuromonitoring the Latarjet procedure. J Shoulder Elbow Surg 2014;23:1473-80. [Crossref] [PubMed]
- Provencher MT, Ghodadra N, LeClere L, et al. Anatomic osteochondral glenoid reconstruction for recurrent glenohumeral instability with glenoid deficiency using a distal tibia allograft. Arthroscopy 2009;25:446-52. [Crossref] [PubMed]
- Sayegh ET, Mascarenhas R, Chalmers PN, et al. Allograft reconstruction for glenoid bone loss in glenohumeral instability: a systematic review. Arthroscopy 2014;30:1642-9. [Crossref] [PubMed]
- Provencher MT, Frank RM, Golijanin P, et al. Distal Tibia Allograft Glenoid Reconstruction in Recurrent Anterior Shoulder Instability: Clinical and Radiographic Outcomes. Arthroscopy 2017;33:891-7. [Crossref] [PubMed]
- Provencher MT, LeClere LE, Ghodadra N, et al. Postsurgical glenohumeral anchor arthropathy treated with a fresh distal tibia allograft to the glenoid and a fresh allograft to the humeral head. J Shoulder Elbow Surg 2010;19:e6-11. [Crossref] [PubMed]
Cite this article as: Murphy CP, Sanchez A, Kennedy MI, Frank RM, Provencher MT. Osteochondral augmentation of glenoid bone loss—distal tibia allograft. Ann Joint 2017;2:66.