
Overuse injuries of the knee
Introduction
When looking at sport related injuries, the knee is one of the most commonly involved joints seen in the pediatric population. The National High School Sports-Related Injury Surveillance System was started in 2005 and surveys hundreds of high school athletic trainers across the country with the main goal of providing accurate epidemiologic data for sports related injuries (1). In 2015–2016 it estimated that out of nearly 1.4 million high school athletic injuries, 207,582 or 14.9%, involved the knee. Only head/face and ankle injuries were found to be more frequently injured. However, when looking at the impact of such events, knee injuries have been found to be the most severe. Using the same database Darrow et al. (2) specifically looked at sports injuries that resulted in the loss of >21 days of sports participation. Over the two-year study period they estimated that high school athletes sustained 446,715 severe injuries. Knee injuries accounted for the highest percentage of these injuries (29%) as well as the largest proportion of injuries that ultimately required surgery (53.9%).
Traumatic knee injuries such as fractures or anterior cruciate ligament (ACL) ruptures garner a large amount of attention in the literature and ultimately have more clinically significant consequences, however, growth-related overuse injuries are much more common and can also have a profound impact on the physical well being of a child or adolescent. The primary goal of this article is to provide an updated review of the recent literature on a few of the most common overuse injuries including patellofemoral pain, Osgood-Schlatter disease (OSD), juvenile osteochondritis dissecans (JOCD), and discoid meniscus.
Epidemiology of knee overuse injuries
Establishing accurate epidemiologic data for overuse injuries is challenging for several reasons. As mentioned earlier, most large database studies report only on traumatic injuries (3,4). This lack of data likely results from the fact that the typical gradual presentation and characteristics of overuse injuries is difficult to capture in an epidemiologic study. Another issue is the actual use of the phrase “overuse injury” and what it actually refers to. Roos and Marshall (5) looked at 35 articles dealing with “overuse injury” and determined that the phrase was used in one of three contexts: to describe a mechanism of injury, to categorize a diagnosis and was used interchangeably with the phrase “non-contact”. In the end they recommended that the term “overuse injury” be referred to as a mechanism of gradual onset that has an underlying pathogenesis of repetitive microtrauma.
With this in mind there is some recent data that does quantify theses injuries. One study from Denmark prospectively looked at a cohort of 1,259 children aged 6 to 12 years (6). They found that overuse injuries were 2.5 times more prevalent than traumatic injuries and that specifically knee injuries comprised of 30%. Another paper out of Denmark looked exclusively at knee injuries in a cohort of 8 to 15 years old (7). They identified 952 injuries and found that 85% of them were overuse compared to 15% traumatic. The majority of these injuries were secondary to traction apophysitis and they established significant risk factors as being female gender and previous knee injury. O’kane et al. (8) studied 351 female youth soccer players and found that 47% of the injuries were in the knee and that the incidence rate for first-time lower extremity overuse injuries was 1.7 per 1,000 athlete-exposure hours (AEH) and for repeat injuries was 3.4 per 1,000 AEH. They also found that athletes who were on more than 1 team had a 2.5-fold increased risk for overuse knee injury. These studies indicate that a large proportion of children participating in sports will have to deal with a knee-related overuse injury at some point in their career. Establishing good epidemiologic data is essential to create effective injury prevention programs, as evidenced by initiatives that have lead to decreased rate of spine injuries in football players and decreased incidence of eye injuries in hockey players (9).
Patellofemoral pain syndrome
Idiopathic anterior knee pain, otherwise known as patellofemoral syndrome (PFS), is considered the most common cause of knee pain in the athletic adolescent population. It refers to non-specific activity related knee pain and is a “diagnosis of exclusion” once other pathologies have been ruled out. The exact etiology is unknown but likely secondary to highly repetitive and excessive loading of the joint as evidenced by higher rates of PFS in the athletic population (10). It should be noted that one third of all adolescents with this diagnosis participate in no athletics at all, thus challenging the notion that the etiology is completely load dependent (11). A recent prospective study suggests that landing mechanics, as measured by knee abduction moment (KAM), may also contribute PFS (12). The authors found that a KAM greater than 15 Nm was associated with a 6.8% risk to develop PFS compared with 2.9% in those athletes with a lower KAM. Landing mechanics are influenced by neuromuscular coordination between the anterior and posterior muscular chain, thus providing a possible target for therapy protocols.
Considered to be a benign and self-limited condition, PFS is almost exclusively managed non-surgically. Initial treatment consists of modifying activity levels, local icing and short courses of anti-inflammatory medication to help with pain control. Complete activity cessation and knee immobilization are not recommended treatment protocols. With regards to knee taping techniques, Logan et al. (13) recently reviewed five level 1 studies and found that the evidence supports taping only as an adjunct to traditional exercise therapy and not as an isolated treatment.
Exercise therapy is the mainstay of treatment and focuses on increasing the flexibility, strength, endurance and neuromuscular retraining of the anterior and posterior muscular chains of the lower extremity. Much has been published about the efficacy of therapy for PFS in the adult literature, however only 1 RCT over the past 20 years has looked at an all-pediatric population. Rathleff et al. (14) randomized 121 adolescents with PFP into two treatment arms, one with patient education alone and the other with patient education plus a guided exercise therapy program. They found that at all time points patient education with exercise therapy was more effective than patient education alone. They also found that there was a dose-response relationship suggesting that better adherence to a prescribed therapy program would increase efficacy. Another significant finding was that at the final time point of two years, only 44% in the exercise group and 22% in the education group reported resolution of symptoms, meaning a significant portion of patients continued to have pain.
The same author then compared the results of this study to the two largest RCT’s of adult PFS to compare efficacy of treatment (11). Despite having similar treatment protocols, the success rate for adolescents was much lower compared to adults. They proposed that this may be secondary to a “cause-effect relationship” between strength and the onset of PFS, suggesting that in younger patients reduced hip and knee strength is a result of the PFS. They suggested that neuromuscular control and coordination control in addition to strength training might improve outcomes in adolescents.
OSD
OSD is a traction apophysitis of the tibial tubercle. It occurs secondary to repetitive contractions of the knee extensor mechanism leading to microavulsions at the chondro-fibro-osseous tibial tuberosity. As activity persists the mechanical strain leads to a chronic avulsion of the apophysis causing a fibrous localized nonunion with persistent ossicles and painful bony enlargements of the tibial tuberosity.
It used to be that males were predominantly affected by OSD but with increasing activity levels in the young female population, incidence rates are starting to equalize (15). Skeletal maturity determines the age at which it affects adolescents, girls are affected younger around ages 8–13 and boys ages 12–15.
Treatment of OSD is guided by the severity of symptoms. OSD is self-limiting and generally ceases with skeletal maturity. Most patients are able to continue with athletics and only a small portion have symptoms severe enough that cause a change in sport or position. Conservative treatment involves modification of activities that reproduce pain, physical therapy, stretching, anti-inflammatory drugs and tibial tubercle padding.
Conservative management of OSD is successful in the vast majority of patients, however, as many as 10% of patients may have symptoms persisting into adulthood despite conservative measures (15). Surgical treatment is reserved only for these refractory cases and is usually directed at debriding a painful ossicle. As is the case in most of sports medicine, there has been a recent trend towards using arthroscopic techniques for surgical OSD treatment. Circi and Beyzadeoglu (16) just published the first case series on arthroscopic treatment of OSD. They looked at 11 athletes who underwent arthroscopy for isolated pain over the tibial tubercle that had failed conservative treatment. All athletes were able to return the same level of athletic activity and degree of participation as they had before the operation, and they returned to full activity after an average of 6.7 weeks. The authors suggested that arthroscopic treatment of unresolved OSD is a reliable option that has added benefit of faster recovery and less patella tendon morbidity when compared to open techniques (17).
Another recent article describes a novel technique designed to address residual hypertrophied tibial tubercle after OSD. Pagenstert et al. (18) hypothesize that a prominent tibial tubercle may be the cause of pain in patients with residual OSD. They proposed that a closing wedge osteotomy of the tubercle would provide significant reduction of the prominence and ultimate resolution of symptoms. In their series of seven patients who underwent the procedure, they found that the tibial tubercle prominence was successfully reduced in all patients with a resultant decrease in pain scores and improved function. This study provides promising results and a potentially new target for surgical management of OSD.
Juvenile OCD
Another common cause of knee pain in the active pediatric population is OCD. OCD is a pathological condition that consists of destruction of subchondral bone with secondary damage to the overlying cartilage. It can occur in adults but when it occurs in a patient with open physes it is specifically referred to as JOCD (Figures 1,2). Older studies estimate the prevalence of JOCD between 15 to 29 cases per 100,000 pediatric patients, but this pathology is seen more frequently now in association with the increase in sport participation and training regimens (19). The etiology of JOCD is multifactorial with traumatic, ischemic and genetic components, however, a recent juvenile animal model has suggested that repetitive mechanical stresses is likely the main contributing factor (20).
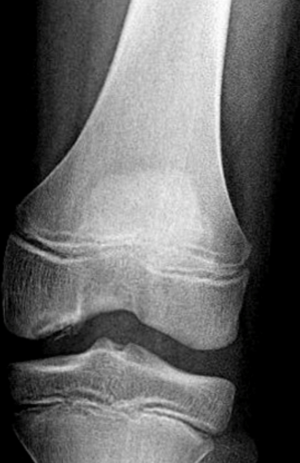
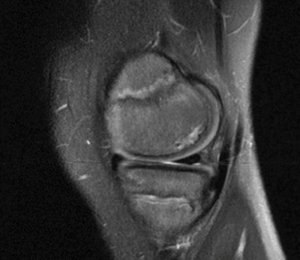
Both non-surgical and surgical management play a role in treating JOCD lesions. In a child with minimal symptoms and a lesion determined to be stable, non-operative treatment consisting of activity restriction has been shown to have a 50% resolution rate at 10–18 months, with younger age being the best prognostic factor (21).
Failure of non-surgical treatment or the presence of an unstable lesion is widely accepted as an indication for surgical treatment, however there is no consensus on which techniques are superior for cartilage restoration. They can be broken down into general categories of reparative versus restorative, and the following will go through the recent literature as it pertains to various surgical treatments.
In low-grade OCDs with minimal chondral separation (Guhl I & II), drilling is a technique that can enhance fragment healing by creating vascular channels in a devitalized region. Arthroscopic assisted extra-articular drilling is one technique that is described and has an advantage of avoiding any damage to the chondral surface. Adachi et al. (22) performed a retrospective study on their use of arthroscopically assisted drilling in the treatment of stable JOCD. They looked at 20 adolescents and found that 95% showed radiographic healing with only one “poor” clinical outcome at final follow up. There is no data to suggest that extra-articular drilling has any different outcome whether you drill through the physis or avoid the physis.
High-grade, unstable OCD lesions (Guhl 3 & 4) are not amenable to non-operative treatment or drilling techniques. The preferred management is internal fixation especially if the fragment has viable cartilage or bone remaining. Fixation options include metal versus bioabsorbable pins and screws. A recent publication from Adachi et al. (23) looked at 30 patients who underwent fixation of unstable JOCD lesions with bioabsorbable pins. They’re healing rate was found to 97% and it occurred at an average of 2.4 months on plain radiographs and 4.2 months on MRI. Other studies have also showed excellent healing rates of OCD lesions after primary fixation, and this technique is considered the standard of care in lesions that are amenable (24,25).
Sanders et al. (26) just published a cohort study detailing the degenerative consequences of knee OCD lesions that are treated with excision compared to preservative techniques such as drilling or fixation. They found that patients treated with fragment excision had a significantly higher rate of osteoarthritis and knee arthroplasty at long-term follow-up, compared to patients with fragment preservation. Predictors associated with worse outcome were BMI greater than 25 kg/m2 and older age. These findings provide further support for the recommendation to preserve native articular fragments when possible in JOCD patients.
Microfracture is a bone marrow stimulation technique that is a valid option when restorative intervention is indicated. There are no definite established indications for microfracture, but it is often performed at the time of arthroscopic removal of a fragment that is determined to be non-fixable. Several factors figure into decision making when deciding whether to microfracture after a fragment excision including, size and depth of the defect, location, and how well the defect is contained by healthy cartilage. A recently published PRCT demonstrated that that at 1 year, 83% of pts who underwent microfracture had good to excellent clinical outcomes, however at 4 years follow-up this rate dropped to 63% with almost half of the patients requiring a re-operation (27). These results highlight an important point regarding interpretation of literature where restorative treatment is performed as a primary operation. The removal of the fragment itself is a confounding variable when trying to assess the biologic treatment of the defect bed, as most patients will respond favorably to just fragment resection alone.
Osteochondral autograft transfer (OAT) is a restorative method used as primary treatment in cases with small defects (<2.5 cm) and severe compromise of the subchondral bone, or as secondary treatment after failed microfracture. Only one PRCT has looked at outcomes of OAT in patients with JOCD. Gudas et al. (27) studied 25 pediatric patients and found that at 1 and 4 years follow-up, good to excellent clinical outcomes were found in 86% and 83% respectively. Post-operative MRI corroborated the good clinical results as 91% of the repairs were determined to be excellent.
In patients with bigger defects, in which the osteochondral donor site would be unacceptably large, fresh-frozen osteochondral allograft transplantation is an option. This technique can be used for patients who have large defects not amenable to other techniques, as well as in those who have failed prior procedures. Osteochondral allograft transplantation has recently been shown to be effective in a cohort of patients younger than 18 years. Murphy et al. (28) showed 90% graft survivorship at 10 years with 88% of the patients having good to excellent clinical outcomes. They did not analyze radiographic results but did conclude that fresh allograft is a viable treatment option in pediatric patients.
Autologous chondrocyte implantation (ACI) is another restorative technique that is typically used for larger defects. Again, high quality studies looking at ACI in patients with JOCD are scarce. One case series looked at 34 patients with unresolved JOCD who had failed at least one non-ACI treatment (29). They found that after treatment with ACI clinical outcome scores were significantly increased at all time points up to 48 months compared to baseline. Their success rate of 85% was consistent with other published outcomes seen in patients with adult OCD and traumatic chondral lesions (29).
Major limitations of ACI are the harvest site morbidity and that it requires two surgical procedures performed months apart. To address these issues biomimetic scaffolds have been created that once implanted induce both subchondral bone and cartilage regeneration. These scaffolds allow biologic restoration with a single procedure and no need for autograft or allograft. Promising short term-results have been seen in adult OCD patients treated with this technique, with studies looking at JOCD in the works (30).
Discoid meniscus
Increased athletic participation of the young population has led to an increased incidence of meniscal injuries (31). Meniscal injuries in children are usually traumatic with an identifiable inciting event (32). The lower rate of meniscal injury in children can be attributed to increased vascularity and healing potential of the meniscus (31). However, meniscal injuries in adolescents may have an overuse component as that seen in adults, since the meniscal architecture essentially reaches an adult pattern by age 10 (32).
The lateral meniscus is more commonly involved than medial in patients under the age of 30 due to association with sport injuries and with discoid lateral menisci pathologies (32) (Figure 3). Pathology associated with discoid lateral menisci often present as a snapping, locking, or bulging at the lateral knee that present spontaneously and sometimes progressively (33).Younger children tend to present with a complete discoid meniscus with a painless knee that cannot fully extend, while adolescents present with a popping knee with pain suggestive of an associated tea (34). It is unclear the proportion of patients who have discoid menisci that report knee symptoms. However, a majority of young and adolescent patients presenting with lateral meniscus pathology requiring arthroscopy have discoid lateral menisci (33). As it is now accepted that they are a dysplastic anatomic variant that occasionally cause symptoms, clinical management of discoid menisci is usually guided by symptomology (35).
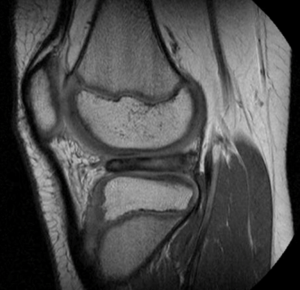
Traditional therapy for discoid lateral meniscus is total or subtotal meniscectomy (36). However, children who have undergone total or subtotal meniscectomy often develop chondromalacia due to increased contact stresses in a meniscus-deficient knee. A number of older literature of at least sixteen-year follow up of children and adolescents who have undergone total meniscectomy demonstrate that a majority of patients develop pain, stiffness, swelling, and instability within one to two decades of surgery and though most patients report satisfactory subjective outcomes, over 40% of patients fail objective measures and a large number report limitations with work and sports (37-39).
Current literature supports the use of “partial meniscectomy”, “saucerization”, “meniscal reshaping” or “meniscoplasty” with or without repair of tears as the mainstay of treatment (31). However until recently there was no long term clinical data supporting the use of meniscoplasty or meniscal repair in children, though biomechanical studies of meniscoplasty have demonstrated return to near-normal contact stresses in the knee joint (40). As it can take many years for detectable degenerative changes to occur, long-term clinical data is needed to justify use of meniscoplasty methods in treating symptomatic discoid menisci.
In one case series involving 38 children (48 knees) of mean age of 9.9 and average follow up of 10.1 years, 23%, 39%, and 88% of knees were found to have undergone radiological degenerative changes at final follow up in partial meniscectomy, partial meniscectomy with repair, and subtotal meniscectomy groups, respectively (41). Another study involving 43 knees with average follow up of 4.3 years also found statistically significant differences between subtotal/total meniscectomy versus partial meniscectomy groups in radiological outcomes (42). Both studies also demonstrated increased arthritic changes in all study groups at final follow up compared to time of surgery. Neither study discussed symptomatic degeneration at final follow up; clinical knee scores consistently improved. To date there is no clear data demonstrating long term symptomatic degeneration status-post meniscoplasty in the same manner as total meniscectomy (37). At a glance, meniscoplasty appears to provide a clinically significant improvement in outcomes as compared to traditional therapy. This finding is limited by the small volume of available data in current clinical literature and the lack of randomized controlled studies.
Arthroscopic approaches to the discoid lateral meniscus have become standard, and a variety of resection and suture repair techniques exist without clear comparative superiority. Generally, a 6–8 mm portion of the residual meniscus should remain to provide favorable function, and separated/unstable tears that appear chronic and do not readily reduce should be resected. Smaller residual meniscal widths of less than 5.0 mm has been implicated in worse knee degeneration in a cohort of a mean age of 12 that underwent arthroscopic meniscal saucerization with or without suture fixation at 39.6 months’ follow up (43). This is likely secondary to the immediate, significant, and persistent change in the mechanical alignment of the knee seen after discoid tissue removal (44). Therefore, large, Wrisberg-type discoid meniscal tears may warrant cautious tissue reconstruction rather than resection wherever possible to retain adequate shock absorption.
In younger children with open physes, a more traditional, less retentive index surgical procedure can be taken with less risk for severe pathologic consequences, perhaps attributable to the increased ability for the body to compensate during the years of increased growth (45). Care should be taken in resecting healthy tissue, as there is a tendency to overestimate the post-resection meniscus size (46). Postoperative recovery varies greatly on the degree of meniscal injury; in the case of a large posterior tear, the therapy regimen should consist of limiting knee flexion by the use of a hinged knee brace to allow gradual return to 90 degrees of flexion at 6 weeks, and gradual return to sport after least 3 months to allow full tissue integration (47).
Conclusions
Knee injuries in children are very common and unfortunately the prevalence is rising with increased participation and time dedicated to sports. Although most overuse injuries are self-limiting, they have the potential to lead to persistent pain, a decrease in quality of life, and occasionally long-term health effects that could require more aggressive intervention. Because of this, it is important for clinicians to comprehensively evaluate and manage these injuries. The recent literature is starting to reflect the increase in these injuries however there is still a need for higher quality studies especially as it relates to epidemiology and long term outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Alexis Chiang Colvin and Diana Patterson) for the series “Orthopaedic Sports Injuries in Youth” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.02.06). The series “Orthopaedic Sports Injuries in Youth” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Comstock RD, Currie DW, Pierpoint LA. National High School Sports-Related Injury Surveillance Study. Colorado School of Public Health. Available online: http://www.ucdenver.edu/academics/colleges/PublicHealth/research/ResearchProjects/piper/projects/RIO/Pages/default.aspx
- Darrow CJ, Collins CL, Yard EE, et al. Epidemiology of severe injuries among United States high school athletes: 2005-2007. Am J Sports Med 2009;37:1798-805. [Crossref] [PubMed]
- Backx FJ, Erich WB, Kemper AB, et al. Sports injuries in school-aged children. An epidemiologic study. Am J Sports Med 1989;17:234-40. [Crossref] [PubMed]
- Caine D, Caine C, Maffulli N. Incidence and distribution of pediatric sport-related injuries. Clin J Sport Med 2006;16:500-13. [Crossref] [PubMed]
- Roos KG, Marshall SW. Definition and usage of the term "overuse injury" in the US high school and collegiate sport epidemiology literature: a systematic review. Sports Med 2014;44:405-21. [Crossref] [PubMed]
- Jespersen E, Rexen CT, Franz C, et al. Musculoskeletal extremity injuries in a cohort of schoolchildren aged 6-12: a 2.5-year prospective study. Scand J Med Sci Sports 2015;25:251-8. [Crossref] [PubMed]
- Junge T, Runge L, Juul-Kristensen B, et al. Risk Factors for Knee Injuries in Children 8 to 15 Years: The CHAMPS Study DK. Med Sci Sports Exerc 2016;48:655-62. [Crossref] [PubMed]
- O’Kane J, Neradilek M, Polissar N, et al. Risk Factors for Lower Extremity Injuries in Female Youth Soccer Players. Orthop J Sports Med 2017;5:2325967117733963 [PubMed]
- Caine D, Caine C, Linder K. The epidemiologic approach to sports injuries. In: Epidemiology of Sports Injuries. Human Kinetics, 1996:1-13.
- Myer GD, Ford KR, Barber Foss KD, et al. The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech (Bristol, Avon) 2010;25:700-7. [Crossref] [PubMed]
- Rathleff MS, Vicenzino B, Middelkoop M, et al. Patellofemoral Pain in Adolescence and Adulthood: Same Same, but Different? Sports Med 2015;45:1489-95. [Crossref] [PubMed]
- Myer GD, Ford KR, Di Stasi SL, et al. High knee abduction moments are common risk factors for patellofemoral pain (PFP) and anterior cruciate ligament (ACL) injury in girls: is PFP itself a predictor for subsequent ACL injury? Br J Sports Med 2015;49:118-22. [Crossref] [PubMed]
- Logan CA, Bhashyam AR, Tisosky AJ, et al. Systematic Review of the Effect of Taping Techniques on Patellofemoral Pain Syndrome. Sports Health 2017;9:456-61. [Crossref] [PubMed]
- Rathleff MS, Roos EM, Olesen JL, et al. Exercise during school hours when added to patient education improves outcome for 2 years in adolescent patellofemoral pain: a cluster randomised trial. Br J Sports Med 2015;49:406-12. [Crossref] [PubMed]
- Circi E, Atalay Y, Beyzadeoglu T. Treatment of Osgood-Schlatter disease: review of the literature. Musculoskelet Surg 2017;101:195-200. [Crossref] [PubMed]
- Circi E, Beyzadeoglu T. Results of arthroscopic treatment in unresolved Osgood-Schlatter disease in athletes. Int Orthop 2017;41:351-6. [Crossref] [PubMed]
- Pihlajamäki HK, Mattila VM, Parviainen M, et al. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men. J Bone Joint Surg Am 2009;91:2350-8. [Crossref] [PubMed]
- Pagenstert G, Wurm M, Gehmert S, et al. Reduction Osteotomy of the Prominent Tibial Tubercle After Osgood-Schlatter Disease. Arthroscopy 2017;33:1551-7. [Crossref] [PubMed]
- Ganley T, Flynn J. Osteochondritis Dessecans of the Knee. In: Micheli LJ, Kocher MS, editors. The Pediatric and Adolescent Knee. Philadelphia: Saunders/Elsevier, 2006: 273–93.
- Stone AV, Little KJ, Glos DL, et al. Repetitive Stresses Generate Osteochondral Lesions in Skeletally Immature Rabbits. Am J Sports Med 2016;44:2957-66. [Crossref] [PubMed]
- Hefti F, Beguiristain J, Krauspe R, et al. Osteochondritis dissecans: a multicenter study of the European Pediatric Orthopedic Society. J Pediatr Orthop B 1999;8:231-45. [PubMed]
- Adachi N, Deie M, Nakamae A, et al. Functional and radiographic outcome of stable juvenile osteochondritis dissecans of the knee treated with retroarticular drilling without bone grafting. Arthroscopy 2009;25:145-52. [Crossref] [PubMed]
- Adachi N, Deie M, Nakamae A, et al. Functional and radiographic outcomes of unstable juvenile osteochondritis dissecans of the knee treated with lesion fixation using bioabsorbable pins. J Pediatr Orthop 2015;35:82-8. [Crossref] [PubMed]
- Tabaddor RR, Banffy MB, Andersen JS, et al. Fixation of juvenile osteochondritis dissecans lesions of the knee using poly 96L/4D-lactide copolymer bioabsorbable implants. J Pediatr Orthop 2010;30:14-20. [Crossref] [PubMed]
- Kocher MS, Czarnecki JJ, Andersen JS, et al. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med 2007;35:712-8. [Crossref] [PubMed]
- Sanders TL, Pareek A, Obey MR, et al. High Rate of Osteoarthritis After Osteochondritis Dissecans Fragment Excision Compared With Surgical Restoration at a Mean 16-Year Follow-up. Am J Sports Med 2017;45:1799-805. [Crossref] [PubMed]
- Gudas R, Simonaityte R, Cekanauskas E, et al. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop 2009;29:741-8. [Crossref] [PubMed]
- Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med 2014;42:635-40. [Crossref] [PubMed]
- Cole BJ, DeBerardino T, Brewster R, et al. Outcomes of autologous chondrocyte implantation in study of the treatment of articular repair (STAR) patients with osteochondritis dissecans. Am J Sports Med 2012;40:2015-22. [Crossref] [PubMed]
- Delcogliano M, Menghi A, Placella G, et al. Treatment of osteochondritis dissecans of the knee with a biomimetic scaffold. A prospective multicenter study. Joints 2014;2:102-8. [Crossref] [PubMed]
- Bellisari G, Samora W, Klingele K. Meniscus tears in children. Sports Med Arthrosc Rev 2011;19:50-5. [Crossref] [PubMed]
- Francavilla ML, Restrepo R, Zamora KW, et al. Meniscal pathology in children: differences and similarities with the adult meniscus. Pediatr Radiol 2014;44:910-25; quiz 907-9. [Crossref] [PubMed]
- Ellis HB Jr, Wise K, LaMont L, et al. Prevalence of Discoid Meniscus During Arthroscopy for Isolated Lateral Meniscal Pathology in the Pediatric Population. J Pediatr Orthop 2017;37:285-92. [Crossref] [PubMed]
- Stilli S, Marchesini Reggiani L, Marcheggiani Muccioli GM, et al. Arthroscopic treatment for symptomatic discoid lateral meniscus during childhood. Knee Surg Sports Traumatol Arthrosc 2011;19:1337-42. [Crossref] [PubMed]
- Wasser L, Knörr J, Accadbled F, et al. Arthroscopic treatment of discoid meniscus in children: clinical and MRI results. Orthop Traumatol Surg Res 2011;97:297-303. [Crossref] [PubMed]
- Hagino T, Ochiai S, Senga S, et al. Arthroscopic treatment of symptomatic discoid meniscus in children. Arch Orthop Trauma Surg 2017;137:89-94. [Crossref] [PubMed]
- Räber DA, Friederich NF, Hefti F. Discoid lateral meniscus in children. Long-term follow-up after total meniscectomy. J Bone Joint Surg Am 1998;80:1579-86. [Crossref] [PubMed]
- Wroble RR, Henderson RC, Campion ER, et al. Meniscectomy in children and adolescents. A long-term follow-up study. Clin Orthop Relat Res 1992;180-9. [PubMed]
- Abdon P, Turner MS, Pettersson H, et al. A long-term follow-up study of total meniscectomy in children. Clin Orthop Relat Res 1990;166-70. [PubMed]
- Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--Part I: Tibial surface of the knee. J Biomech Eng 1983;105:216-25. [Crossref] [PubMed]
- Ahn JH, Kim KI, Wang JH, et al. Long-term results of arthroscopic reshaping for symptomatic discoid lateral meniscus in children. Arthroscopy 2015;31:867-73. [Crossref] [PubMed]
- Lee DH, Kim TH, Kim JM, et al. Results of subtotal/total or partial meniscectomy for discoid lateral meniscus in children. Arthroscopy 2009;25:496-503. [Crossref] [PubMed]
- Yamasaki S, Hashimoto Y, Takigami J, et al. Risk Factors Associated With Knee Joint Degeneration After Arthroscopic Reshaping for Juvenile Discoid Lateral Meniscus. Am J Sports Med 2017;45:570-7. [Crossref] [PubMed]
- Wang J, Xiong J, Xu Z, et al. Short-term effects of discoid lateral meniscectomy on the axial alignment of the lower limb in adolescents. J Bone Joint Surg Am 2015;97:201-7. [Crossref] [PubMed]
- Shieh AK, Edmonds EW, Pennock AT. Revision Meniscal Surgery in Children and Adolescents: Risk Factors and Mechanisms for Failure and Subsequent Management. Am J Sports Med 2016;44:838-43. [Crossref] [PubMed]
- Mayer-Wagner S, von Liebe A, Horng A, et al. Discoid lateral meniscus in children: magnetic resonance imaging after arthroscopic resection. Knee Surg Sports Traumatol Arthrosc 2011;19:1920-4. [Crossref] [PubMed]
- Jose J, Buller LT, Rivera S, et al. Wrisberg-variant discoid lateral meniscus: current concepts, treatment options, and imaging features with emphasis on dynamic ultrasonography. Am J Orthop (Belle Mead NJ) 2015;44:135-9. [PubMed]
Cite this article as: DeBellis N, Cong GT, Mikhail C, Gladstone J. Overuse injuries of the knee. Ann Joint 2018;3:17.


