Combined open and arthroscopic approaches in hip preservation surgery
Introduction
Despite recent advances in diagnostic and imaging techniques, misdiagnoses still occur in the hip and groin regions. Numerous studies have been published on nonsurgical and surgical treatments to manage associated pain and/or mechanical symptoms and to preserve the hip joint. Increased sports participation, often year-round and beginning at a young age, as well as improved recognition by medical practitioners with high resolution imaging techniques have all contributed to a rise in the diagnosis of nonarthritic hip related pathology. However, hip pathologies are not always isolated and may be associated with a mix of intra- and extra-articular abnormalities. It is sometimes difficult to distinguish intra-articular from extra-articular pathologies including snapping, muscle spasm, and neurological problems (1). Recently, Draovitch et al. developed the layer concept approach to help determine where pain and pathology originates (2). Layer I, the osseous layer; layer II, the inert tissue layer; layer III, the contractile layer and layer IV, the neuro-mechanical layer have been described (2).
There are studies looking at the correlation among layer III with layer I and II pathologies. Casartelli et al. have shown that patients with femoroacetabular impingement (FAI) have considerable muscle weakness compared to healthy control patients. The authors have demonstrated that contractile muscular dysfunction can occur as a result of structural pathology and pain (3). Compensatory muscular responses to layer 1 and II pathology may also cause muscle-related pathology including snapping or tendinitis. In order to address these concurrent pathologies, open and arthroscopic approaches in hip preservation surgery have been used in combination. The purpose of this article is to review the surgical techniques and usefulness of combined open and arthroscopic procedures in hip preservation surgery and describe novel technical approaches to nerve entrapment syndromes of the groin and deep gluteal region.
Layer 1, osseous layer
There are two major pathologies in layer 1, the osseous layer. FAI has been recognized as one of the most common source of groin pain, which is associated with intra-articular pathologies including labral tears and cartilage damage from dynamic abutment of the proximal femur with the acetabular rim (4,5). Arthroscopic hip surgery has evolved over recent years as an effective surgical approach to manage patients presenting with FAI (6,7). Other causes of groin pain are developmental pathologies including developmental dysplasia of the hip (DDH) and abnormalities of femoral version, acetabular version and/or femoral inclination. Recent studies have shown that patients with DDH have generally less favorable outcomes following hip arthroscopy. The evidence has reported high rates of progressive lateral subluxation of the hip and high reoperation rates with conversion to total hip arthroplasty after hip arthroscopy for patients with DDH (8,9). One study advised against performing hip arthroscopy for DDH when pre-operative radiographs indicate a break in Shenton’s line, femoral neck shaft angle >140 degrees, lateral center edge (LCE) angle <19 degrees, body mass index (BMI) >23 kg/m2 or when severe cartilage damage is present at the time of surgery (10). Similarly, Hatakeyama et al. have shown that preoperative predictors of worsened clinical outcomes following hip arthroscopic labral preservation, capsular plication and cam osteoplasty in the setting of borderline DDH (BDDH) are age ≥42 years, broken Shenton’s Line, osteoarthritis (Tonnis ≥ grade 1), Tonnis angle ≥15 degrees and vertical center anterior (VCA) angle ≤17 degrees on preoperative radiographs (11). Poor outcomes were defined as conversion to total hip arthroplasty, rotational acetabular osteotomy, endoscopic shelf acetabuloplasty, or progression to of osteoarthritis (Tonnis >2) at a minimum of 2 years post-surgery. Furthermore, the authors reported that intra-operative predictors of poor outcomes were severe cartilage delamination of the acetabulum and mild cartilage damage at the femoral head. However, recent studies have shown that hip arthroscopy may be beneficial for treating patients with BDDH (12-15). Future research is needed to refine the surgical indications to both open and arthroscopic techniques in the setting of DDH.
Endoscopic shelf acetabuloplasty
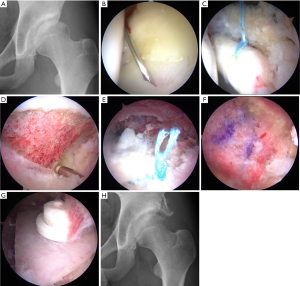
In order to address some of the arthroscopic pitfalls of managing DDH, Uchida et al. developed a new technique of endoscopic shelf acetabuloplasty. The authors described a procedure for direct endoscopic visualization of the anterolateral osseous undercoverage of the socket during self acetabuloplasty (16). They have shown in a series of 32 active patients with DDH that endoscopic shelf acetabuloplasty provided promising clinical outcome and a high rate of return to sports (average follow-up: 32.3±3.0 months; range, 24–48 months) (17) (Figure 1). They noted that endoscopic shelf acetabuloplasty is a less invasive option, than open shelf procedure or peri-acetabular osteotomy (PAO) that compliments concurrent arthroscopic procedures including labral repair, subspinal decompression and cam osteoplasty (17).
Endoscopy-assisted PAO (eaPAO)
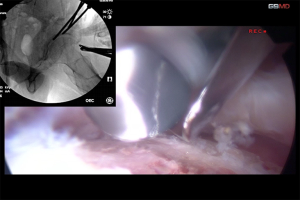
Although endoscopic shelf acetabuloplasty is less invasive, it appears to be limited for severe dysplasia with femoral deformities including femoral version and inclination. Open PAO is beneficial for patients with moderate and severe dysplasia but has the disadvantages of being a more invasive surgery with higher volume of blood loss and rates of complications including sciatic nerve injury. Recent cadaveric and clinical studies have highlighted techniques to successful achievement of eaPAO using mini incisions and customized guides and osteotomes without creating iatrogenic damage to the acetabulum and vital structures (18-20). The technical advantages of eaPAO are direct endoscopic visualization for improved accuracy and safety of key ischial and posterior column osteotomy cuts with protection of the adjacent sciatic nerve, improved accuracy of acetabular fragment repositioning and subsequent construct stability, and visualization of the dynamic interaction of the repositioned acetabular rim with the proximal femur enables the detection of PAO-induced FAI (Figure 2). As with the endoscopic shelf surgery, arthroscopic hip procedures can address co-existing intra-articular pathology in a less invasive manner with the potential for decreased hospital stay and accelerated rehabilitation. However, at the present time there are only technical descriptions and case reports available in the literature. Long-term cohort studies that are sufficiently powered are required to clarify whether eaPAO can be safe and provide favorable clinical outcomes for patients with DDH.
Layer 3: contractile layer
Snapping hip
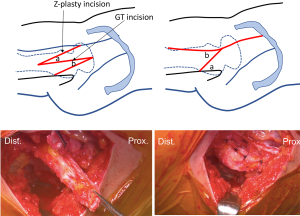
Layer 3 consists of all contractile muscle surrounding hips, trunk stabilizers and pelvic floor stabilizers. The main aim of this layer is to create controlled synchronized movements and provide dynamic stability to the hip joint. Snapping hip may occur in the extra-articular peri-trochanteric location. This process is known as coxa sultans externus, and in contrast to internal snapping hip, this condition is typically visible and palpable, but not audible. Numerous open surgical procedures have been described to either resect variable amounts of the posterior portion of the iliotibial band (ITB) over the greater trochanter (GT) or to Z-lengthen the ITB in attempts to decrease the tension of the ITB (21). Recent literature has described endoscopic procedures to release the ITB or gluteus sling over the ITB (22,23).
In our practice, we have experienced a proportion of DDH patients that also have painful recalcitrant external snapping hip. Compensatory muscular responses to DDH and/or hip instability may cause musculotendinous-related pathologies including snapping hip. In these situations, we prefer to perform endoscopic shelf acetabuloplasty and open Z-plasty of the ITB via a small incision simultaneously (Figure 3).
Layer 4: neuromuscular layer
Groin-related neuropathy
Peripheral nerve entrapment in the groin is commonly caused by compression of three main nerves that include the obturator, femoral and lateral femoral cutaneous nerves (LFCN). In this article, we show typical obturator nerve entrapment that can co-exist with intra-articular pathologies. Although femoral neuropathy and LFCN neuropathy are oftentimes seen in our practice, surgical decompression should be avoided because surgical outcomes remain unsatisfactory (24). Meralgia paresthetica (MP) is a painful disorder as a result of an entrapment of LFCN near the lateral aspect of the inguinal ligament. Usually non-traumatic in etiology, MP is commonly associated with obesity, pregnancy and tight-fitting clothing. A recent case report demonstrated the effectiveness of an ultrasound-guided percutaneous technique of hydrodissection [hydrorelease (HR)] for treating MP (25).
Obturator nerve entrapment
Obturator neuropathy has been recognized as a source of unexplained groin pain or loss of sensation of medial thigh (26). Obturator neuropathy is a rare mononeuropathy, which is associated with pelvic fractures, pelvic hematomas, retroperitoneal masses and intrapelvic tumors (26). In addition, several studies have reported obturator neuropathy as a potential complication associated with gynecological, urological and orthopedic surgeries (27,28). Anatomically, the obturator nerve arises from the ventral divisions of the second, third, and fourth lumbar nerves in the lumbar plexus. It descends through the fibers of the psoas major, emerges medially near the brim of the pelvis and runs along the lateral wall of the lesser pelvis, above and in front of the obturator vessels, to the upper part of the obturator foramen (26). The nerve then passes through a fibro-osseous tunnel under the pubic ramus. Within the tunnel, the nerve divides into anterior and posterior branches, as well as a branch to the obturator externus (26,29).
Clinical signs and symptoms of obturator nerve entrapment often include post-exercise medial thigh pain and adductor muscle weakness. Paresthesia and/or sensory loss in the medial thigh area is usually observed. The diagnosis with electromyography (EMG) is based on fibrillation potentials or high amplitude waves limited to muscles innervated by the obturator nerve. Ultrasound-guided obturator nerve block with local anesthesia is useful for diagnosing and relieving the pain and symptoms (30,31). If conservative treatment including nerve block, non-steroid anti-inflammatory agents and physiotherapy fail to improve the symptoms, surgical intervention may be considered. We have experienced some patients with FAI who underwent hip arthroscopic procedures with obturator nerve entrapment after initial surgery. Lower extremity traction during hip arthroscopy may cause obturator nerve entrapment in those patients and emphasizes the general principle of minimizing traction time and force during hip arthroscopic procedures.
Our preferred surgical approach to obturator nerve decompression is described below with a case example. The patient was placed in the supine position on a standard operating table. An oblique incision was made 2-cm distal and parallel to the inguinal ligament on the upper adductor region. The pectineus and adductor longus was identified and the superficial fascia was incised. The two muscles were then separated by blunt dissection. The anterior branch of the obturator nerve was identified by intraoperative neurological monitoring over the adductor brevis and the overlying fascia was released to decompress the nerve (Figure 4). The nerve was found to be strangulated by the overlying adhesive fascia. The overlying fascia was released to decompress the entrapped nerve. Bradshaw et al. demonstrated, in a series of 32 patients with obturator neuropathy, the excellent clinical outcomes and high return to sport rate after this surgical neurolysis treatment (32).
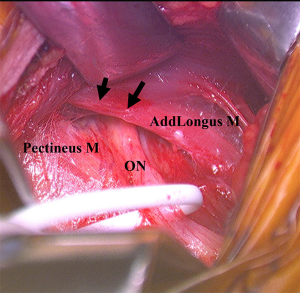
Neuropathy of the deep subgluteal space
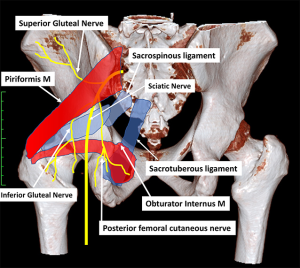
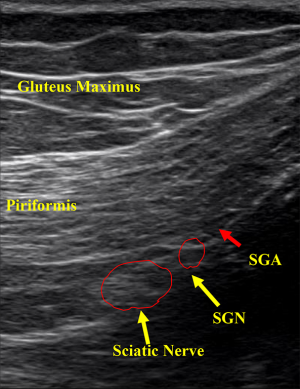
Deep gluteal syndrome is an entity characterized by pain and paresthesia in the buttock area, posterior thigh and hip region caused by non-discogenic sciatic nerve neuropathy in the subgluteal space. Multiple nerves including sciatic, superior gluteal, inferior gluteal, posterior femoral cutaneous and pudendal nerve can become entrapped in the piriformis area (Figure 5). There is a paucity of knowledge regarding how to deal with these pathological entities.
First-line treatment should be a conservative treatment consisting of rest, anti-inflammatory agents and physiotherapy. In a few cases, endoscopic resection of piriformis is ultimately required (33). A systematic review of mostly case series and reports reported consistently positive results with improvement in pain and a low incidence of complications, particularly for endoscopic procedures (34).
Superior gluteal nerve (SGN) entrapment
SGN arises from posterior divisions of fourth lumbar through first sacral ventral rami. It passes the pelvis through the greater sciatic foramen, superior to the piriformis and runs laterally between the gluteus medius and minimus muscles with the deep branch of the SGN to innervate the gluteus medius, gluteus minimus, tensor fascia lata and the hip joint (35). This nerve has no cutaneous sensory distribution (Figure 5).
SGN entrapment is a rare but recognized condition presenting as buttock pain secondary to local buttock trauma, pelvic fracture, hip surgery, and as a sequelae of repeated buttock injections (36,37). There are three noticeable symptoms of the SGN entrapment including spontaneous gluteal pain, weakness in abduction of the lower limb and tenderness over the superior gluteal region corresponding to the greater sciatic notch (36). Significant weakness of hip abduction muscular may cause a Trendelenburg sign and gait.
The first line treatment for the SGN entrapment is conservative including non-steroidal anti-inflammatory agents and physiotherapy. Recently, HR has been defined as a technique to release adhesive tissue by introducing normal saline under pressure into tissue planes causing dissection (25). This technique helps to decompress the area of entrapment by creating previous nonexistent planes. It has been used to preserve perforating arteries surrounding entrapment nerve. We have treated some patients with the SGN entrapment undergoing ultrasound-guided HR.
The technique involves placing patients in the prone position on a table. Since the exit of the SGN is located approximately at the medial one third of the line connecting the posterior inferior iliac spine (PIIS) and GT, the transducer is placed at the intersection of these anatomic landmarks. The transducer is gently moved in a caudal direction until the echo signature of the SGN is located. Once the SGN is visualized in a transverse plane, the nerve is traced proximally and distally to confirm its appropriate course toward lateral gluteal region. A sterile 22-gauge needle is reached out to the inter-fascia plane under the direct ultrasound visualization in a longitudinal transverse view. We then inject 6 mL of sterile normal saline to bluntly dissect the nerve away from the surrounding soft tissue (Figure 6).
Conclusions
Endoscopic assisted PAO and shelf acetabuloplasty are less invasive treatment strategies for DDH. Hydrorelease used to decompress nerve entrapment surrounding the hip joint can improve pain and function in patients with groin and deep gluteal space neuropathy. There is a dearth of knowledge regarding entrapment neuropathy in groin and deep gluteal space. Further investigation is necessary to clarify the long-term clinical outcomes of these innovative techniques.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.03.14). SU is a consultant for Smith & Nephew and Zimmer-Biomet and receive research fund from Smith & Nephew, Pfizer and Johnson & Johnson. DKM is a consultant for Zimmer-Biomet. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tibor LM, Sekiya JK. Differential diagnosis of pain around the hip joint. Arthroscopy 2008;24:1407-21. [Crossref] [PubMed]
- Draovitch P, Edelstein J, Kelly BT. The layer concept: utilization in determining the pain generators, pathology and how structure determines treatment. Curr Rev Musculoskelet Med 2012;5:1-8. [Crossref] [PubMed]
- Casartelli NC, Maffiuletti NA, Item-Glatthorn JF, et al. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthritis Cartilage 2011;19:816-21. [Crossref] [PubMed]
- Ganz R, Parvizi J, Beck M, et al. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;112-20. [PubMed]
- Ito K, Leunig M, Ganz R. Histopathologic features of the acetabular labrum in femoroacetabular impingement. Clin Orthop Relat Res 2004;262-71. [Crossref] [PubMed]
- Casartelli NC, Leunig M, Maffiuletti NA, et al. Return to sport after hip surgery for femoroacetabular impingement: a systematic review. Br J Sports Med 2015;49:819-24. [Crossref] [PubMed]
- Murata Y, Uchida S, Utsunomiya H, et al. A comparison of clinical outcome between athletes and non-athletes undergoing hip arthroscopy for femoroacetabular impingement. Clin J Sport Med 2017;27:349-56. [Crossref] [PubMed]
- Matsuda DK, Khatod M. Rapidly progressive osteoarthritis after arthroscopic labral repair in patients with hip dysplasia. Arthroscopy 2012;28:1738-43. [Crossref] [PubMed]
- Parvizi J, Bican O, Bender B, et al. Arthroscopy for labral tears in patients with developmental dysplasia of the hip: a cautionary note. J Arthroplasty 2009;24:110-3. [Crossref] [PubMed]
- Uchida S, Utsunomiya H, Mori T, et al. Clinical and Radiographic Predictors for Worsened Clinical Outcomes After Hip Arthroscopic Labral Preservation and Capsular Closure in Developmental Dysplasia of the Hip. Am J Sports Med 2016;44:28-38. [Crossref] [PubMed]
- Hatakeyama A, Utsunomiya H, Nishikino S, et al. Predictors of Poor Clinical Outcome After Arthroscopic Labral Preservation, Capsular Plication, and Cam Osteoplasty in the Setting of Borderline Hip Dysplasia. Am J Sports Med 2018;46:135-43. [Crossref] [PubMed]
- Domb BG, Chaharbakhshi EO, Perets I, et al. Hip Arthroscopic Surgery With Labral Preservation and Capsular Plication in Patients With Borderline Hip Dysplasia: Minimum 5-Year Patient-Reported Outcomes. Am J Sports Med 2018;46:305-13. [Crossref] [PubMed]
- Domb BG, Stake CE, Lindner D, et al. Arthroscopic capsular plication and labral preservation in borderline hip dysplasia: two-year clinical outcomes of a surgical approach to a challenging problem. Am J Sports Med 2013;41:2591-8. [Crossref] [PubMed]
- Fukui K, Briggs KK, Trindade CA, et al. Outcomes After Labral Repair in Patients With Femoroacetabular Impingement and Borderline Dysplasia. Arthroscopy 2015;31:2371-9. [Crossref] [PubMed]
- Nawabi DH, Degen RM, Fields KG, et al. Outcomes After Arthroscopic Treatment of Femoroacetabular Impingement for Patients With Borderline Hip Dysplasia. Am J Sports Med 2016;44:1017-23. [Crossref] [PubMed]
- Uchida S, Wada T, Sakoda S, et al. Endoscopic Shelf Acetabuloplasty Combined With Labral Repair, Cam Osteochondroplasty, and Capsular Plication for Treating Developmental Hip Dysplasia. Arthroscopy Techniques 2014;3:e185-91. [Crossref] [PubMed]
- Uchida S, Hatakeyama A, Kanezaki S, et al. Endoscopic Shelf Acetabuloplasty Can Improve Clinical Outcomes and Achieve Return to Sports-related Activity in Active Patients with Hip Dysplasia. Knee Surg Sports Traumatol Arthrosc 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Inan M, Gokce A, Ustunkan F. Endoscopy-assisted periacetabular osteotomy: a preliminary cadaveric study. Clin Orthop Relat Res 2008;466:862-70. [Crossref] [PubMed]
- Matsuda DK, Matsuda NA. Endoscopic hip osteotomies: less invasive approaches to peri-acetabular, proximal femoral and pubic symphyseal procedures. J Hip Preserv Surg 2015;2:108-15. [Crossref] [PubMed]
- Matsuda DK, Martin HD, Parvizi J. Endoscopy-Assisted Periacetabular Osteotomy. Arthrosc Tech 2016;5:e275-80. [Crossref] [PubMed]
- Provencher MT, Hofmeister EP, Muldoon MP. The surgical treatment of external coxa saltans (the snapping hip) by Z-plasty of the iliotibial band. Am J Sports Med 2004;32:470-6. [Crossref] [PubMed]
- Ilizaliturri VM Jr, Chaidez C, Villegas P, et al. Prospective randomized study of 2 different techniques for endoscopic iliopsoas tendon release in the treatment of internal snapping hip syndrome. Arthroscopy 2009;25:159-63. [Crossref] [PubMed]
- Polesello GC, Queiroz MC, Domb BG, et al. Surgical technique: Endoscopic gluteus maximus tendon release for external snapping hip syndrome. Clin Orthop Relat Res 2013;471:2471-6. [Crossref] [PubMed]
- Fargo MV, Konitzer LN. Meralgia paresthetica due to body armor wear in U.S. soldiers serving in Iraq: a case report and review of the literature. Mil Med 2007;172:663-5. [Crossref] [PubMed]
- Mulvaney SW. Ultrasound-guided percutaneous neuroplasty of the lateral femoral cutaneous nerve for the treatment of meralgia paresthetica: a case report and description of a new ultrasound-guided technique. Curr Sports Med Rep 2011;10:99-104. [Crossref] [PubMed]
- McCrory P, Bell S. Nerve entrapment syndromes as a cause of pain in the hip, groin and buttock. Sports Med 1999;27:261-74. [Crossref] [PubMed]
- DeHart MM, Riley LH Jr. Nerve injuries in total hip arthroplasty. J Am Acad Orthop Surg 1999;7:101-11. [Crossref] [PubMed]
- Hakoiwa S, Hoshi T, Tanaka M, et al. Case of bilateral obturator neuropathy after caesarean section. Masui 2011;60:721-3. [PubMed]
- Tipton JS. Obturator neuropathy. Curr Rev Musculoskelet Med 2008;1:234-7. [Crossref] [PubMed]
- Anagnostopoulou S, Kostopanagiotou G, Paraskeuopoulos T, et al. Obturator nerve block: from anatomy to ultrasound guidance. Anesth Analg 2008;106:350-author reply 350-1. [Crossref] [PubMed]
- Saranteas T, Anagnostopoulou S, Chantzi C. Obturator nerve anatomy and ultrasound imaging. Reg Anesth Pain Med 2007;32:539-40. [Crossref] [PubMed]
- Bradshaw C, McCrory P, Bell S, et al. Obturator nerve entrapment. A cause of groin pain in athletes. Am J Sports Med 1997;25:402-8. [Crossref] [PubMed]
- Hernando MF, Cerezal L, Perez-Carro L, et al. Deep gluteal syndrome: anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol 2015;44:919-34. [Crossref] [PubMed]
- Kay J, de Sa D, Morrison L, et al. Surgical Management of Deep Gluteal Syndrome Causing Sciatic Nerve Entrapment: A Systematic Review. Arthroscopy 2017;33:2263-78.e1. [Crossref] [PubMed]
- Apaydin N, Kendir S, Loukas M, et al. Surgical anatomy of the superior gluteal nerve and landmarks for its localization during minimally invasive approaches to the hip. Clin Anat 2013;26:614-20. [Crossref] [PubMed]
- Rask MR. Superior gluteal nerve entrapment syndrome. Muscle Nerve 1980;3:304-7. [Crossref] [PubMed]
- Diop M, Parratte B, Tatu L, et al. Anatomical bases of superior gluteal nerve entrapment syndrome in the suprapiriformis foramen. Surg Radiol Anat 2002;24:155-9. [Crossref] [PubMed]
Cite this article as: Uchida S, Matsuda DK, Miya E, Saho A, Sakai A. Combined open and arthroscopic approaches in hip preservation surgery. Ann Joint 2018;3:33.