Surgical technique of direct anterior approach for total hip arthroplasty on a standard operating room table
Introduction
The direct anterior approach (DAA) was first popularized at least as early as the beginning of the 20th century (1), more recently popularized in the last several decades (2), with classic description by Matta et al., with the use of a specialized fracture table (3). Regardless of the technique employed, the DAA has received great attention in the literature of late. Reported benefits of the DAA for total hip arthroplasty (THA) include improved outcomes early in the recovery period (4), earlier discharge and potential improved resource utilization (5), favorable postoperative stability profile (6-8), potentially decreased muscle damage (9), and higher likelihood of radiographically acceptable component placement (10).
Comparisons between the DAA with or without a specialized fracture table have been limited. There does not seem to be a difference between the rate of intraoperative femur fractures between the two techniques (11), which can be a concern with the approach, especially for surgeons lacking experience. Purported advantages of performing the DAA on a standard operating room table include facile, direct, clinical evaluation of leg lengths, easy intraoperative testing of component stability, and the obviation of the need for an expensive, specialized fracture table.
However, the DAA for THA is a technically challenging operation, with a well-described learning curve (12,13), and a number of distinct, described complications and difficulties, including femur fractures (14), greater trochanter fractures and femoral canal perforations (15), and lateral femoral cutaneous nerve injury (16). It is therefore imperative to perform the THA via the DAA with caution and appropriate technique to avoid complications and ensure reliably excellent outcomes. Here the authors describe our preferred technique in performing THA using the DAA on a regular operating room table.
Set up and positioning
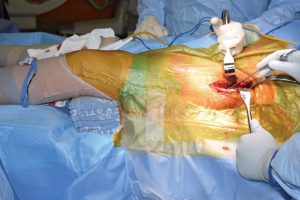
The patient is positioned supine on a regular operating room table. Variations exist with positioning, including authors that advocate placing the lumbosacral junction directly at the flexion point of the table, or a bump placed under the lumbosacral area, centered under the anterior superior iliac spine (ASIS) (17), in order to facilitate femoral broaching and which may help with obtaining fluoroscopy images. With the use of the regular table, the feet can be lowered during the course of the operation to have a similar effect.
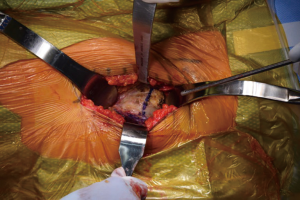
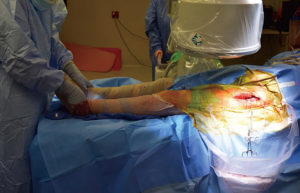
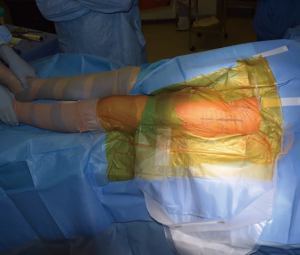
Both lower extremities are sterilely prepped and draped into the field to allow for manual comparison of limb lengths intraoperatively as well as for stability testing. The planned incision is marked, using the ASIS as a guide to the location. The incision is typically approximately 8–10 cm in length, depending on the patient’s surrounding soft tissue envelope (Figure 1). The authors also recommend palpation of the tensor fascia lata (TFL), with the incision planned over the muscle belly.
Surgical approach
Once the skin is incised, the soft tissues are dissected deep to the level of the fascia over the TFL. A bipolar sealant may be used to help control intraoperative bleeding during the approach. Once the fascia is cleared, it is sharply incised. The medial aspect of the fascia overlying the TFL is then bluntly dissected free from the muscle belly medially. Once the TFL is free from the fascia, the Hueter interval between the TFL and the rectus femoris is developed. The fascial split is carried proximally up to the origin of the TFL and distally to the vastus to maximize the working window. Within this interval the ascending branches of the lateral femoral circumflex artery are encountered and coagulated with electrocautery (Figure 2). In rare circumstances suture ligatures may be required. A bump is placed under the ipsilateral knee to relieve tension on the rectus femoris to allow easier dissection from the underlying hip capsule (Figure 3). The rectus is elevated with electrocautery and a retractor is placed under the rectus and over top of the anterior column of the acetabulum.
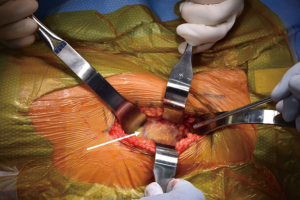
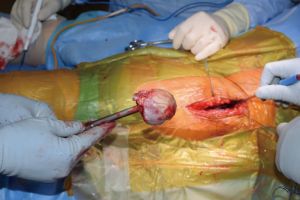
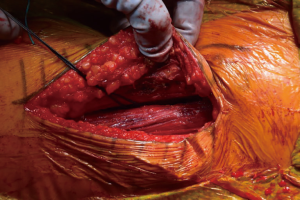
At this point, the pericapsular fat is removed and the capsulotomy to enter the hip joint proper is planned (Figure 4). The capsule is incised and the flaps are tagged for later repair following completion of component placement. Some authors prefer a capsulectomy at this point, which has been demonstrated to have equal outcomes to capsulotomy and repair one year after surgery (18). Retractors are placed along the superior and inferior aspects of the femoral neck within the capsular flaps previously developed. At this point, the neck cut is made. In the event of difficulty with femoral head removal, a so-called “napkin ring” cut can be performed to create more space within the joint. The femoral head is impaled with a corkscrew device and extracted from the acetabulum (Figure 5).
Acetabular preparation and component placement
Acetabular retractors are placed in the anterosuperior, anteroinferior, and posterior locations. For a left hip, these are at the 11, 7, and 4 o’clock positions, respectively. The labrum is excised with electrocautery or osteotome if ossified. This serves to allow improved positioning of the acetabular reamers and component. Tight bands of the capsule may be released at this time to allow visualization and access of the acetabulum. The initial reamer is placed and medialized to the level of the teardrop, as denoted by the floor of the acetabular fossa. Once sufficiently medialized, successively larger reamers are sequentially employed to enlarge the acetabular bed until bleeding cancellous bone is encountered circumferentially. The authors prefer using native anatomy as landmarks, with the anterior and posterior walls guiding appropriate version and the superior lip to guide acetabular component inclination.
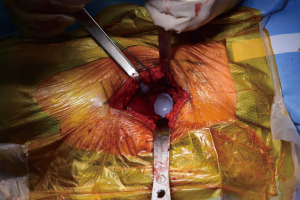
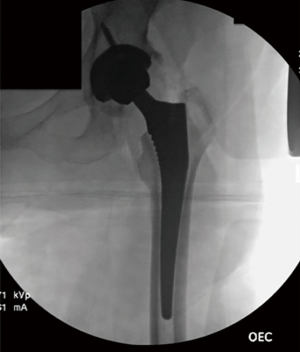
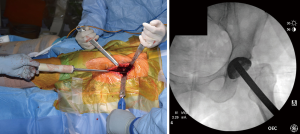
After bony preparation of the acetabulum, the field is cleansed with irrigation and the bony bed is cleared of all soft tissue that might become interposed during component placement. The cup is preliminarily impacted, and checked via fluoroscopy to ensure appropriate positioning (Figure 6). Final impaction is completed in the desired orientation determined by fluoroscopy. The authors prefer at this point to place accessory screws through the cup to increase the stability of the cup. The acetabular liner is placed (Figure 7) and attention is turned to the femoral side.
Femoral preparation and component placement
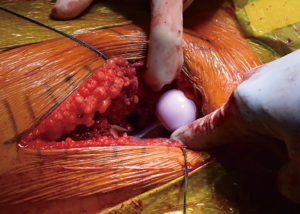
Facilitation of proximal femoral positioning to improve access and visualization is critical to avoid known complications while preparing the femur, including canal perforation, calcar fractures, and greater trochanter fractures (19). The authors prefer to begin the femoral work by releasing the superior capsule (20), and, in the majority of cases in which it is hypertrophied from the arthritis process, debulking it to improve visualization. Release of the medial femoral neck is done just enough to allow placement of a two-prong retractor along its border, with the tines abutting the posteromedial neck of the cut femur. The release of the posterior structures is limited, but a vertical episiotomy type incision through the posterior capsule is made in line with and down to the greater trochanter, extending superior to its apex. Limiting the releases done posteriorly is thought to impart improved stability by maintaining posterior structural integrity. The release is extended to the piriformis fossa, but not beyond so as to avoid violating the conjoined tendon. At this point, a posterior retractor is placed along the anterolateral aspect of the trochanter (Figure 8). With these releases completed and retractors in place, the exposed, elevated cut neck of the femur is in excellent position for visualization and instrumentation. The native version of the femur is also readily identifiable and will be used to guide broaching. If visualization is still limited, a titrated sequential release of the conjoined tendon, with or without the piriformis tendon may be needed. Care must be taken to avoid release of the obturator externus tendon, which is a dynamic stabilizer of the hip.
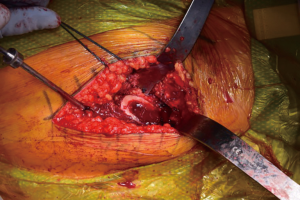
The authors prefer at this point to sound the canal with an instrument placed by hand, which we believe may help to avoid canal perforation during subsequent steps. Broaching commences with sequentially increasing broaches being sure to adequately lateralize until adequate fit is established from medial to lateral cortex. Version of the broach placement is determined in most cases by matching the patient’s native anatomy. Trial neck ball are placed. Gross estimation of leg lengths is done by direct observation of the patient’s limbs distally at the level of the medial malleoli and plantar foot surface. Stability is assessed with manual manipulation of the limb into extension, external rotation figure-4, and flexion/internal rotation (Figure 9). The authors believe that this surgeon-directed manipulation and assessment without the requisite directives to an unscrubbed assistant as in the case of use of a specialized table eliminates unnecessary steps and may expedite the operation. Fluoroscopy may be used at this point to check trial component position. Once satisfactory limb length and stability is established, the trial components are dislocated and the final matching components are placed (Figure 10) by repeating the exposure and retractor placement previously mentioned. Final leg lengths are again examined (Figure 11) and final components are checked with fluoroscopy (Figure 12), which obviates the need for postoperative X-rays in the recovery room.
With appropriate retractor placement, damage to the surrounding musculature, and the TFL in particular, should be minimal (Figure 13). The authors prefer to close the capsular flaps previously tagged with stitches. If desired, local anesthetic can be injected into the field. Regardless, the authors experience is that patients undergoing the DAA for THA typically have good pain control (21) and trend towards earlier discharge, in keeping with some published data on the topic (5,22). The fascia overlying the TFL is then repaired, and the subcutaneous tissue is closed, followed by a subcuticular skin stitch. The authors prefer to use a wound sealant surgical glue on the wound and do not currently employ a drain (23).
Conclusions
The technique described here we believe limits unnecessary steps, allows direct, manual as well as radiographic evaluation of leg lengths, and limits the amount of fluoroscopic radiation exposed to the patient and surgeon (24). In the senior author’s experience, only a maximum of five or six fluoroscopic images are necessary to obtain in a typical case. Additionally, in an increasingly cost-conscious healthcare landscape, this technique does not require purchase of an expensive specialized table to complete. We believe this technique provides surgeons with a means of performing the DAA for THA in a reproducible and accessible manner, affording the benefits the approach may render for patients.
Acknowledgments
The authors would like to acknowledge Roseann Johnson for her assistance in manuscript preparation and submission.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)”. The article has undergone external peer review.
Conflicts of Interest: The series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” was commissioned by the editorial office without any funding or sponsorship. CCY served as the unpaid Guest Editor of the series and serves as an unpaid associate editor of Annals of Joint from Dec 2016 to Dec 2018. CCY is a paid presenter or speaker for Zimmer Biomet, Medtronic, and DePuy, a Johnson & Johnson company, a paid consultant for DePuy, and receives research support from DePuy. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith-Peterson MN. A New Supra-Articular Subperiosteal Approach to the Hip. Am. J Orthop Surg 1917;15:592-5.
- Light TR, Keggi KJ. Anterior approach to hip arthroplasty. Clin Orthop Relat Res 1980;255-60. [PubMed]
- Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res 2005;115-24. [Crossref] [PubMed]
- Berend KR, Lombardi AV Jr, Seng BE, et al. Enhanced early outcomes with the anterior supine intermuscular approach in primary total hip arthroplasty. J Bone Joint Surg Am 2009;91:107-20. [Crossref] [PubMed]
- Joseph NM, Roberts J, Mulligan MT. Financial impact of total hip arthroplasty: a comparison of anterior versus posterior surgical approaches. Arthroplast Today 2016;3:39-43. [Crossref] [PubMed]
- Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty 2009;24:999-1005. [Crossref] [PubMed]
- Kennon RE, Keggi JM, Wetmore RS, et al. Total hip arthroplasty through a minimally invasive anterior surgical approach. J Bone Joint Surg Am 2003;85-A:39-48. [Crossref] [PubMed]
- Siguier T, Siguier M, Brumpt B. Mini-incision anterior approach does not increase dislocation rate: a study of 1037 total hip replacements. Clin Orthop Relat Res 2004;164-73. [Crossref] [PubMed]
- Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am 2011;93:1392-8. [Crossref] [PubMed]
- Lin TJ, Bendich I, Ha AS, et al. A Comparison of Radiographic Outcomes After Total Hip Arthroplasty Between the Posterior Approach and Direct Anterior Approach With Intraoperative Fluoroscopy. J Arthroplasty 2017;32:616-23. [Crossref] [PubMed]
- Cohen EM, Vaughn JJ, Ritterman SA, et al. Intraoperative Femur Fracture Risk During Primary Direct Anterior Approach Cementless Total Hip Arthroplasty With and Without a Fracture Table. J Arthroplasty 2017;32:2847-51. [Crossref] [PubMed]
- Masonis J, Thompson C, Odum S. Safe and accurate: learning the direct anterior total hip arthroplasty. Orthopedics 2008;31: [PubMed]
- D’Arrigo C, Speranza A, Monaco E, et al. Learning curve in tissue sparing total hip replacement: comparison between different approaches. J Orthop Traumatol 2009;10:47-54. [Crossref] [PubMed]
- De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: complications and early outcome in a series of 300 cases. Acta Orthop Belg 2013;79:166-73. [PubMed]
- Jewett BA, Collis DK. High Complication Rate With Anterior Total Hip Arthroplasties on a Fracture Table. Clin Orthop Relat Res 2011;469:503-7. [Crossref] [PubMed]
- Homma Y, Baba T, Sano K, et al. Lateral femoral cutaneous nerve injury with the direct anterior approach for total hip arthroplasty. Int Orthop 2016;40:1587-93. [Crossref] [PubMed]
- Post ZD, Orozco F, Diaz-Ledezma C, et al. Direct anterior approach for total hip arthroplasty: indications, technique, and results. J Am Acad Orthop Surg 2014;22:595-603. [Crossref] [PubMed]
- Curtin BM, Edwards PK, Odum S, et al. Anterior Capsulectomy vs. Repair in Direct Anterior Total Hip Arthroplasty. AAHKS Annual Meeting, Poster Presentation, 2016.
- Barnett SL, Peters DJ, Hamilton WG, et al. Is the Anterior Approach Safe? Early Complication Rate Associated With 5090 Consecutive Primary Total Hip Arthroplasty Procedures Performed Using the Anterior Approach. J Arthroplasty 2016;31:2291-4. [Crossref] [PubMed]
- Matsuura M, Ohashi H, Okamoto Y, et al. Elevation of the femur in THA through a direct anterior approach: cadaver and clinical studies. Clin Orthop Relat Res 2010;468:3201-6. [Crossref] [PubMed]
- Goebel S, Steinert AF, Schillinger J, et al. Reduced postoperative pain in total hip arthroplasty after minimal-invasive anterior approach. Int Orthop 2012;36:491-8. [Crossref] [PubMed]
- Schweppe ML, Seyler TM, Plate JF, et al. Does surgical approach in total hip arthroplasty affect rehabilitation, discharge disposition, and readmission rate? Surg Technol Int 2013;23:219-27. [PubMed]
- Suarez JC, McNamara CA, Barksdale LC, et al. Closed Suction Drainage Has No Benefits in Anterior Hip Arthroplasty: A Prospective, Randomized Trial. J Arthroplasty 2016;31:1954-8. [Crossref] [PubMed]
- McArthur BA, Schueler BA, Howe BM, et al. Radiation Exposure during Fluoroscopic Guided Direct Anterior Approach for Total Hip Arthroplasty. J Arthroplasty 2015;30:1565-8. [Crossref] [PubMed]
Cite this article as: Holst DC, Angerame MR, Yang CC. Surgical technique of direct anterior approach for total hip arthroplasty on a standard operating room table. Ann Joint 2018;3:34.