The pediatric ankle and foot: a review of common injuries in the pediatric athlete and their treatments
Introduction
Injuries of the ankle and foot are among the most common injuries in the pediatric athlete. These injuries may account for up to 30% of visits to sports medicine clinics in this population (1). It has been reported that over 4.5 million sport-related injuries occur in the United States each year with approximately 45 million children between 6 and 18 years old participating in at least one organized sport (2,3). As the number of pediatric athletes increases and the subset who specialize in a sport continue to rise, a concomitant rise in pediatric ankle and foot injuries is expected (4). The ankle and foot complex is a nuanced structure requiring the interplay of bony, ligamentous and muscular structures. This complex composes the fundamental base for activity. In the case of pediatric athletes, this structure is developing and changing, and this difference manifests in a wide range of conditions including ligamentous injuries, instabilities, physeal and bony injuries that may differ from the adult population. The goal of this chapter is to review common pediatric ankle and foot injuries, and to detail the current practices with regard to their prevention, diagnosis, and treatment.
Ankle physeal/bone fractures
Amongst fractures involving the physis, ankle physeal fractures are the most common in the lower extremity, but are the third most common after finger and distal radial physeal fractures (5,6). Overall, ankle physeal fractures represent about 5% of all fractures in children, and about 15–20% of physeal injuries (5-7). These injuries are common in athletes who play sports that require sudden changes in direction (i.e., football, basketball) or extreme sports (i.e., skateboards, scooters) (8).
Ankle fractures are commonly caused by direct trauma, twisting, and compression. The talus is wider anteriorly which results in increased stability of the tibiotalar joint in dorsiflexion than plantarflexion (9). Therefore, rotational injuries to the ankle are much more likely to occur in a plantarflexed position. In children, the ligamentous structures are typically stronger than the open physis. Mechanisms that may cause ligamentous injuries in adults often cause physeal fractures or avulsion fractures in children (9,10). Patients presenting with injuries due to high-energy mechanisms, such as falls from height, may also have hidden physeal crush injuries (Salter-Harris V) that may not be visible on initial radiographs. Symptoms include ankle pain, focal tenderness to palpation, swelling, ecchymosis, and inability to bear weight.
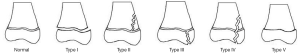
The Salter-Harris classification is the most commonly used system to classify physeal injuries (Figure 1) (11,12). Traditionally there are five types of Salter-Harris fractures, with Salter-Harris II being the most common and representing 32–40% of distal tibial physeal fractures (13).
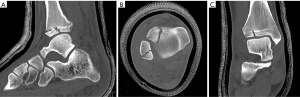
Two pediatric-specific distal tibial fractures can be described using the Salter-Harris Classification: triplane (Figure 2) and juvenile Tillaux fractures (Figure 3). The triplane fracture is characterized by fracture lines visible in all three planes (axial, coronal, and sagittal). The fracture most often appears as a Salter-Harris III injury on AP radiographs and a Salter-Harris II injury on lateral radiographs. Therefore, it is often classified as a Salter-Harris IV injury. There can be a variable number of fragments (2-4) as well as variable articular involvement (intra vs. extra-articular) (14). A juvenile Tillaux fracture is a Salter-Harris III injury characterized by an avulsion fracture of the anterolateral distal tibial tubercle extending into the epiphysis secondary to pull by the anterior inferior tibiofibular ligament during forced external rotation (15). The pathophysiology of triplane and juvenile Tillaux fractures is unique due to the asymmetric closure of the pediatric distal tibial physis. The fracture configuration will vary depending on the timing and magnitude of the mechanism of injury (14). The distal tibial physis closes between the ages of 12 and 16 in females and 14 and 19 in males. This closure follows a typical pattern of central to medial closure with lateral closure terminally (15). The unfused lateral physis is more vulnerable to injury compared to the already ossified portions of the physis. This helps explain why, in a juvenile Tillaux fracture, it is the anterolateral distal tibial tubercle that is avulsed. Advanced imaging such as CT scans may be helpful for diagnosis, assessment of fracture reduction, and pre-operative planning. In regards to triplane fractures, a study by Jones et al. (16) found that after viewing the CT scan, all surgeons questioned changed their surgical planning for screw placement.
Treatment for ankle physeal fractures depends upon classification. Nondisplaced fractures of the tibial physis can be treated conservatively with non-weightbearing in a short (8) or long leg (17) cast for 4–6 weeks. Displaced fractures must first be closed reduced to minimize injury to the physis (18). Barriers to closed reduction, such as soft tissue interposition, may mandate open reduction as multiple attempts at closed reduction are discouraged due to the risk of additional physeal injury (19). Depending on the amount of displacement in extra-articular injuries (Salter-Harris I and II), open reduction may be warranted to prevent premature physeal closure. In a study by Rohmiller et al. (20), the average residual displacement in patients with Salter-Harris Type I and II fractures who experienced premature physeal closure was 3.2 mm compared to 2 mm for those who did not. Similarly, Barmada et al. (19) found that 60% of fractures with greater than 3 mm of displacement after closed reduction showed premature physeal closure, compared to 17% in patients with less than 3 mm of displacement.
Intra-articular fractures, such as displaced Salter-Harris Type III and IV fractures, should be treated with open reduction and internal fixation (ORIF). A study by Kling et al. (21) noted that patients with Salter-Harris Type III and IV fractures treated with closed reduction were more likely to have premature physeal closure when compared to those who underwent ORIF. Similarly, studies have shown ORIF to produce good outcomes as long as articular congruity was well restored (22). Although 2 mm is the commonly cited acceptable incongruity, the exact amount of acceptable displacement is under debate. After fracture reduction, then the patient should be placed in a short leg cast for 4–6 weeks (18).
Because of the nature of the injury, Salter-Harris Type V tibial fractures are frequently missed and only become diagnosed when the child presents with a leg length discrepancy. If identified early, excision of the damaged physis and replacement with a fat graft may prevent deformity and future leg length discrepancy (17,23). These patients should undergo increased surveillance with repeat radiographs to monitor for any premature physeal closure, even with normal initial radiographs.
Most distal fibular fractures are Salter-Harris Type I and II (13). Reduction is rarely required and most can be treated conservatively with a short leg cast or walking boot for 3–4 weeks (17,18). If a distal fibular fracture has a concomitant distal tibia fracture [Salter-Harris Type I and II fibular fractures are commonly associated with Salter-Harris Type III and IV distal tibial fractures (17)], reduction of the tibia often causes the fibula to reduce as well (8,18,24). If the fibula is still unstable, Kirschner wires may be required for fixation (8).
Non-displaced or minimally displaced (<2 mm) extra-articular triplane fractures can be treated conservatively with a long leg cast with the ankle in internal rotation. Fractures with greater than 2 mm of displacement should be reduced. A study by Ertl et al. (25) showed worsening symptoms at follow up with >2 mm of intra-articular step-off. These displaced fractures can be treated with closed reduction and percutaneous fixation with Kirschner wires, percutaneous screw fixation, or ORIF (23).
Nondisplaced or minimally displaced (<2 mm) Tillaux fractures may be treated with a long leg cast for 4 weeks (8,18,23). If, after attempted reduction, the displacement is greater than 2 mm, additional fixation with either percutaneous wires, percutaneous screws, or ORIF is indicated (8,26). It is important to note that if the child still has considerable growth potential, the physis should respected and all attempts should be made to avoid violation of the physis with screws or wires.
Growth arrest due to premature closure of the physis can occur, causing limb-length discrepancies and possible angular deformities. This is less of a concern with triplane and juvenile Tillaux fractures, as these patients are nearing physeal closure at the time of injury. Close follow-up with repeat radiographs is recommended when indicated. Surgical interventions may eventually be required depending on the severity of the growth arrest. Treatment options depend on the amount of predicted growth remaining and the percentage of physeal involvement. Possible options include bar resection, epiphysiodesis with or without contralateral epiphysiodesis, or open wedge corrective osteotomy (17).
Ankle fractures can also lead to osteochondral defects (27). History and physical exam at follow-up will reveal continued pain after ankle fracture. Imaging of choice to evaluate the articular cartilage surface is a magnetic resonance imaging (MRI) and treatments may include lesion stabilization, microfracture, autologous chondrocyte implantation, or osteochondral autograft/allograft transplantation (28).
Metatarsal fractures
Metatarsal fractures are the most common pediatric foot fractures, accounting for 60% of foot fractures (29). The most common mechanism for metatarsal fractures in the pediatric population include fall (from either a level surface or from a height), crush injury, twisting, athletic activities, or traffic accidents (30,31). The first and fifth metatarsals are most frequently fractured in children, and commonly occur in isolation, representing 58–67% (30-32) of all pediatric metatarsal fractures overall. The second, third, and fourth metatarsals are commonly associated with other concomitant metatarsal fractures (31,32) and in cases of more than one metatarsal fracture, the fractures occurred in contiguous bones (31). Children under the age of 5 sustain 50–60% of all first metatarsal fractures, and first metatarsal fractures make up 50–70% of metatarsal fractures in these children (30,32). Children over the age of 5 are more likely to have a 5th metatarsal fracture, which account for 90% of all 5th metatarsal fractures in children (30). Patients may present with pain, swelling, and inability to bear weight.
Most metatarsal fractures can be treated conservatively. Treatment should first consist of an attempt at closed reduction followed by closed management in a cast. In a retrospective review, Mahan et al. (33) found that all their patients with extra-articular fractures with less than 75% of displacement achieved healing without surgical intervention. Clear indications for operative management include delayed union, open fractures, and compartment syndrome. However, there is a paucity of evidence in regard to fracture displacement, angulation and their indications for surgical management. Robertson et al. (32) found that patients undergoing surgical treatment compared to the non-surgical group showed evidence of multiple metatarsal fractures (70% vs. 28%) and had significantly greater fragment translation (84% vs. 28%). Fragment angulation was not found to significantly correlate with a decision for surgical management. Outcomes were the same for both the operative and non-operative groups in terms of time to return to full activities, with complete union achieved in 84.6% of patients. If operative management is indicated, treatments include closed reduction and percutaneous pinning or ORIF. Fractures near the physis should be monitored closely as this may lead to shortening of the affected bone. Non-unions are also not uncommonly seen, with up to 15% of patients in one study showing delayed union (32). However, only 2 of the 50 patients eventually required operative fixation.
Special attention has been given to fractures of the base of the fifth metatarsal, which are commonly seen in children, and are classified according to the zone (1, 2, or 3) where the fracture occurs (Figure 4). However most of the literature pertains to adult patients and pediatric studies are limited. Herrera-Soto et al. (34) performed a retrospective review of 103 children with fifth metatarsal fractures, 45% of which had fifth metatarsal base fractures. Pseudo-Jones fractures (Figure 4), an avulsion fracture of the fifth metatarsal base (Zone 1), were successfully treated with a short-leg walking cast for 3–6 weeks depending on symptoms and displacement (34). Jones fractures (Figure 4), a fracture of the proximal diaphyseal region (Zone 2), are notoriously difficult to treat because of its limited vascular supply. Treatment is controversial, but a trial of conservative treatment with a non-walking cast for 6 weeks followed by 2 weeks of progressive weight bearing is acceptable, although refracture is common (34). Operative treatment includes reduction and internal fixation with or without bone grafting.

Osteochondritis dissecans (OCD)
OCD of the talus in children is a relatively rare occurrence. One study reported only 85 out of 1,068,215 (0.008%) pediatric patients were diagnosed with OCD of the talus (35). As such, data is relatively scarce.
The most frequent site of OCD on the talus is on the medial border (36), occurring roughly four times more frequently there than compared to the lateral border (36,37). Lesions on the lateral talar dome tend to be associated with a history of trauma (36,38). However, the exact etiology of OCD is not fully understood and there may be more than one mechanism leading to the development of OCD, including but not limited to traumatic, micro-traumatic, and ischemic (39). It is thought that chronic repetitive micro-traumas may eventually lead to the development of OCD by devascularizing a region of the talus (37,40). Eventual aseptic necrosis of the subchondral bone may lead to a free intraarticular body due to failed re-integration of the necrotic subchondral bone (40). Classically, OCD lesions were described by Berndt and Harty in 4 stages (Figure 5) (41).
The diagnosis of OCD is difficult as it can remain asymptomatic for long periods of time. Often it is diagnosed accidentally while the patient is being worked up for another condition. Symptoms depend upon the severity of the disease, but the most common complaint is pain, with 92–97% of all pediatric patients indicating its presence (36,42). Other symptoms consist of swelling (36), instability (37,43), and decreased ROM (37). If the fragment becomes detached the patient could experience a marked increase in symptoms including, but not limited to, “intense pain (“articular crisis”), joint swelling, instability during walking, and possible locking” (40).
Initial treatment depends upon the Berndt-Harty stage, but is mainly non-operative. Conservative treatment is recommended for lesions up to Berndt-Harty stage III, while Berndt-Harty stage IV lesions represent unstable lesions and require operative fixation (36,37,42,44). A 6-month trial of non-operative treatment is recommended before considering surgical fixation. Non-operative approaches include partial weight bearing and activity restriction, ankle bracing without weightbearing, and below-the-knee cast immobilization. Outcomes have generally been shown to be good. Higuera et al. (37) had only 1/12 patients require surgical intervention after initial conservative treatment. Perumal et al. (42) had 10/31 require eventual surgical treatment.
Indications for surgical management include failure of conservative treatment, Berndt-Harty stage IV lesions, progression of Berndt-Harty stage, or displacement of osteochondral fragment (36,37,44). Common surgical procedures include bone marrow stimulation techniques (drilling, microfracture), bone marrow-derived cell transplantation, transplantation techniques [bone grafting, osteochondral transplantation, autologous chondrocyte transplantation (ACI)], excision of fragment, or fixation of fragment (28,44,45).
Zwingmann et al. (28) conducted a meta-analysis review of 54 studies involving 1,105 patients. Their main outcome parameters were clinical outcome and success rate of the individual surgical technique applied. They found that the overall treatment success rate was 79% and noted that success rate seemed dependent on the Berndt-Harty stage, with stage IV lesions showing the lowest success rate (stage I: 82%; stage II; 86%; stage III: 83%; stage IV: 76%). They also examined the overall success rates of various surgical procedures, with transplantation procedures showing the highest success rates (ACI/osteochondral grafts: 84%; fragment fixation: 82%; drilling/microfracture: 76%; debridement: 71%).
Despite resolution of clinical symptoms, radiographical lesions are often still evident. Higuera et al. (37) reported good or excellent clinical outcomes in 94.8% of their patients, but only 68.5% of those showed good to excellent outcomes according to radiographic outcome measures. However, the overall rate of development of degenerative changes to the ankle is relatively low (43,46), with a study by Bruns et al. (43) noting that none of their adolescent patients exhibited signs of degenerative changes after surgical management. Complicating treatment, medial lesions must typically be accessed through an open approach, typically requiring an osteotomy of the medial malleolus. This can increase the risk for post-operative osteoarthritis (47), in addition to causing physeal arrest and angular deviation (36). It is therefore not recommended for use in pediatric patients.
Ligamentous injuries
In the skeletally immature, bony injuries (especially those involving the physis) rather than ligamentous injuries are significantly more common. However, a strong understanding of ligamentous injuries of the ankle and foot in the young athlete is essential in order to provide the appropriate treatment. Here we describe two major categories of ligamentous injuries—ankle sprains and midfoot sprains (Lisfranc injuries).
Ankle sprains (lateral ankle)
Ankle sprains account for nearly half of all ankle injuries in the United States (49.3%) (48). These are typically sports related activities with basketball (41.1%), football (9.3%), and soccer (7.9%) representing the most common mechanisms. Peak age of injury occurs in patients 15 to 19 years of age (48).
Ankle sprains are often divided into lateral, medial, and high ankle sprains, with lateral ankle sprains representing the vast majority of ankle sprains in this patient population. The most common mechanism is a combination of inversion and plantarflexion that results in injuries to the anterior talofibular ligament (ATFL) and calcaneofibular ligament (CL) (1). Conversely, eversion/external rotation injuries can result in deltoid and syndesmotic injuries (medial and high ankle sprains respectively) (1).
Proper treatment of these injuries is contingent on adequate diagnosis with thorough review of the patient’s history and physical exam. The Ottawa ankle rules (OAR) were developed to present a highly sensitive test for fracture detection while limiting unnecessary exposure to radiation (Figure 6) (49). Multiple studies have validated the efficacy of the OAR in both pediatric and adult populations (50,51). As the vast majority of ankle sprains and literature evaluating ankle sprains are of the lateral ankle, we focus here on sprains of the lateral ankle.
Broadly, lateral ankle sprains are often classified into one of three grades depending on their level of ligamentous injury and related morbidity (52). The determination for each grade of injury has been described previously (53). Typically, lateral ankle sprains are treated non-operatively with the majority of injuries treated by conservative measures. In these methods, use of the “RICE” method which involves rest, ice, compression, and elevation is often employed with the goal of reducing swelling and minimizing patient discomfort (54). Non-operative methods have proven to be highly successful suggesting that in most cases, surgery may not be indicated. A 2007 Cochrane Systematic Review sought to compare surgical and conservative treatment methods for the treatment of lateral ligamentous ankle injuries in adults. They found that no treatment was able to demonstrate a significantly superior outcomes. However, they noted a statistically significant decrease in rates of re-injury in patients who received surgical intervention when compared to those who did not.
While the majority of patients do well with non-operative management, surgical intervention may be indicated for patients with high grade injuries that have failed conservative management, have continued pain and instability, or are high-level athletes who require higher functional stability of their ankle joints. Operative techniques are aimed at improving stability in the ankle. The Brostrum procedure, and the modified Brostrum procedure, were developed with the purpose of anatomically reconstructing the lateral ankle ligaments in order to minimize pain while stabilizing and improving function in the ankle (55). Multiple studies have demonstrated the relative success of this procedure and its modifications over the years. A study by Bell et al. (56) followed 31 patients and 32 ankles treated with the Brostrum procedure and demonstrated very positive results with 91% of patients describing their ankle function as good or excellent with a 26-year-follow-up. Gould et al. (57) later modified this approach by attaching a supplementary lateral portion of the extensor retinaculum to the fibula. Since then, multiple studies with further modifications and addition of arthroscopic techniques have exhibited success (55,58). Similar efficacy was demonstrated in pediatric populations; Kocher et al. (59) examined 31 patients under the age of 18 who underwent the modified Brostrum technique for chronic lateral ankle instability. Of the 31 patients, 22 (71%) achieved good-to-excellent results as demonstrated by their American Orthopedic Foot and Ankle Society (AOFAS) score (greater than 80).
Tarsometatarsal (TMT) joint injuries: the Lisfranc injury
TMT joint injuries, including ligamentous and fractures-dislocation injuries, otherwise known as Lisfranc injuries, are rare injuries reported to occur in 1 of 50,000 to 55,000 adults in the United States per year and account for 0.2% of all fractures (60). While the reported incidence is rare, and even more rare within the pediatric population (the exact number has not been reported), there is some concern that the true incidence is severely underestimated due to missed diagnosis (61). Missed diagnoses, especially in the skeletally immature, can result in significant deformity and long-term complications.
Lisfranc fracture-dislocations and ligamentous dislocations occur when the articulation of the medial cuneiform and base of the second metatarsal is disrupted. This disruption may involve a fracture of the 2nd metatarsal base or a disruption of the Lisfranc ligament—the interosseous ligament connecting the medial cuneiform and the plantar base of the second metatarsal. The TMT joint has intrinsic stability due to this ligament and to the bony configuration of the 2nd TMT joint lying proximal to the first TMT joint. As the bony ossification has not been completed in the pediatric population, it is often difficult to radiographically determine whether or not an injury has occurred to the TMT joint. Accordingly, various studies have attempted to elucidate methods of detecting these injuries radiographically with high sensitivity and specificity.
Typically, these injuries are thought to occur primarily due to high velocity accidents such as motor vehicle accidents (MVA) and falls from height, however, they can sometimes occur in low velocity scenarios due to seemingly innocuous mechanisms such as missing a step off of a sidewalk.
Management decisions are typically based upon weight bearing and stress radiographs and clinical assessment of the patient’s stability. Instability is an indication for operative management including ORIF or arthrodesis. With the low incidence of these injuries, there is little literature that has evaluated the various treatment options in the pediatric Lisfranc injuries. Accordingly, treatment is based upon studies performed on the adult population or rely on small case series of pediatric or adolescent patients (62).
In pediatric patients, instability is a common indication for operative fixation. A pediatric study retrospectively reviewing 56 children treated over a 12-year period found that 82% and 51% of ligamentous and bony Lisfranc injuries were sports-related (63). Of note, none of the patients who developed ligamentous injuries were treated operatively where as 49% (19/39) of the bony injuries were managed operatively. In pediatric patients, internal fixation with Kirschner wires are often preferred for younger children and cannulated screws for older children with sufficient bone stock. Another case series of 7 Lisfranc injuries occurring in adolescents who were treated operatively supported the good anatomic reduction following the procedure (64). However, a majority of the patients complained of discomfort and pain at the last follow-up (3–26 months). Wiley (65) reported on 18 children who developed Lisfranc injuries of which seven required closed reduction. However, Wiley did report residual pain in only 4 of 18 patients despite short follow-up (3–8 months).
Other injuries of the ankle and foot
Sever’s disease/calcaneal apophysitis
Calcaneal apophysitis, also known as Sever’s disease, is a traction apophysitis that is a common cause of heel pain in immature athletes who participate in running and jumping sports. Multiple studies have evaluated whether the identification of Sever’s disease is reliable purely through radiographic methods alone. In one such study, 80 radiographs (50 with Sever’s disease and 30 healthy controls) demonstrated that with the absence of clinical information they could not reliably diagnose this condition (66).
Treatment of Sever’s disease is often treated by conservative therapies, including rest, stretching and occasionally orthotic interventions such as heel lifts. A systematic review sought to compare the efficacy of these various treatment modalities in 9 peer reviewed articles (67). Overall, despite the lack of rigor in many of these studies, they suggested that indicated orthoses with a brim (heel cup) and medial arch support, along with taping was effective in reducing pain in sporting activities as compared to no treatment (67). Another study which reviewed 85 patients treated with physical therapy and orthotics demonstrated excellent results with all patients returning to their sport of choice 2 months following diagnosis (68). Of note, a cross-sectional study by James et al. (69) found that children with higher body mass index, increased weight, and greater height were more likely to have a greater pain severity, and a greater period of pain.
Iselin disease
Iselin disease (traction apophysitis of the tuberosity of the fifth metatarsal) is thought to be caused by repetitive traction of the peroneus brevis tendon at the site of its attachment. Treatment of this disease is similar to Sever’s disease in that conservative management including rest, footwear modification, and physical therapy have demonstrated good results (70-72). As above, proper management is contingent on effective diagnosis of the condition.
Conclusions
Injuries to the ankle and foot are common in the immature athlete. These injuries are uniquely different from the adult population because of the presence of the physis. Practitioners must be aware of the effect of physeal damage on growth potential and treat accordingly. Fortunately, pediatric patients have tremendous remodeling potential and many injuries can be managed with conservative treatment. Despite this, the practitioner needs to be aware of the indications for operative intervention. We have highlighted conditions that we believe are commonly seen or are unique to the pediatric population with the goal of providing practitioners with a solid foundation on which to build upon with regard to ankle and foot injuries in the athletic pediatric population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Orthopaedic Sports Injuries in Youth”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.02.02). The series “Orthopaedic Sports Injuries in Youth” was commissioned by the editorial office without any funding or sponsorship. DP served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Erickson JB, Samora WP, Klingele KE. Ankle Injuries in the Pediatric Athlete. Sports Med Arthrosc 2016;24:170-7. [Crossref] [PubMed]
- Fernandez WG, Yard EE, Comstock RD. Epidemiology of lower extremity injuries among U.S. high school athletes. Acad Emerg Med 2007;14:641-5. [Crossref] [PubMed]
- Biber R, Gregory A. Overuse injuries in youth sports: is there such a thing as too much sports? Pediatr Ann 2010;39:286-92. [Crossref] [PubMed]
- Gottschalk AW, Andrish JT. Epidemiology of sports injury in pediatric athletes. Sports Med Arthrosc 2011;19:2-6. [Crossref] [PubMed]
- Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma 1972;12:275-81. [Crossref] [PubMed]
- Peterson HA, Madhok R, Benson JT, et al. Physeal fractures: Part 1. Epidemiology in Olmsted County, Minnesota, 1979-1988. J Pediatr Orthop 1994;14:423-30. [Crossref] [PubMed]
- Worlock P, Stower M. Fracture patterns in Nottingham children. J Pediatr Orthop 1986;6:656-60. [Crossref] [PubMed]
- Blackburn EW, Aronsson DD, Rubright JH, et al. Ankle fractures in children. J Bone Joint Surg Am 2012;94:1234-44. [Crossref] [PubMed]
- Kay RM, Mattbys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg 2001;9:268-78. [Crossref] [PubMed]
- Su AW, Larson AN. Pediatric ankle fractures: concepts and treatment principles. Foot Ankle Clin 2015;20:705-19. [Crossref] [PubMed]
- Salter RB, Harris R. Injuries involving the epiphyseal plate. J Bone Joint Surg Am 1963;45:587-622. [Crossref]
- Vahvanen V, Aalto K. Classification of ankle fractures in children. Arch Orthop Trauma Surg 1980;97:1-5. [Crossref] [PubMed]
- Spiegel PG, Cooperman DR, Laros GS. Epiphyseal fractures of the distal ends of the tibia and fibula. A retrospective study of two hundred and thirty-seven cases in children. J Bone Joint Surg Am 1978;60:1046-50. [Crossref] [PubMed]
- Schnetzler KA, Hoernschemeyer D. The pediatric triplane ankle fracture. J Am Acad Orthop Surg 2007;15:738-47. [Crossref] [PubMed]
- Stefanich RJ, Lozman J. The juvenile fracture of Tillaux. Clin Orthop Relat Res 1986;219-27. [PubMed]
- Jones S, Phillips N, Ali F, et al. Triplane fractures of the distal tibia requiring open reduction and internal fixation: Pre-operative planning using computed tomography. Injury 2003;34:293-8. [Crossref] [PubMed]
- Podeszwa DA, Mubarak SJ. Physeal fractures of the distal tibia and fibula (Salter-Harris Type I, II, III, and IV fractures). J Pediatr Orthop 2012;32:S62-8. [Crossref] [PubMed]
- Kay RM, Matthys GA. Pediatric ankle fractures: evaluation and treatment. J Am Acad Orthop Surg 2001;9:268-78. [Crossref] [PubMed]
- Barmada A, Gaynor T, Mubarak SJ. Premature physeal closure following distal tibia physeal fractures: a new radiographic predictor. J Pediatr Orthop 2003;23:733-9. [Crossref] [PubMed]
- Rohmiller MT, Gaynor TP, Pawelek J, et al. Salter-Harris I and II fractures of the distal tibia: does mechanism of injury relate to premature physeal closure? J Pediatr Orthop 2006;26:322-8. [Crossref] [PubMed]
- Kling TF Jr, Bright RW, Hensinger RN. Distal tibial physeal fractures in children that may require open reduction. J Bone Joint Surg Am 1984;66:647-57. [Crossref] [PubMed]
- Schurz M, Binder H, Platzer P, et al. Physeal injuries of the distal tibia: long-term results in 376 patients. Int Orthop 2010;34:547-52. [Crossref] [PubMed]
- Wuerz TH, Gurd DP. Pediatric physeal ankle fracture. J Am Acad Orthop Surg 2013;21:234-44. [Crossref] [PubMed]
- Su AW, Larson AN. Pediatric Ankle Fractures: Concepts and Treatment Principles. Foot Ankle Clin 2015;20:705-19. [Crossref] [PubMed]
- Ertl JP, Barrack RL, Alexander AH, et al. Triplane fracture of the distal tibial epiphysis. Long-term follow-up. J Bone Joint Surg Am 1988;70:967-76. [Crossref] [PubMed]
- Schlesinger I, Wedge JH. Percutaneous reduction and fixation of displaced juvenile Tillaux fractures: a new surgical technique. J Pediatr Orthop 1993;13:389-91. [Crossref] [PubMed]
- O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med 2010;38:392-404. [Crossref] [PubMed]
- Zwingmann J, Sudkamp NP, Schmal H, et al. Surgical treatment of osteochondritis dissecans of the talus: a systematic review. Arch Orthop Trauma Surg 2012;132:1241-50. [Crossref] [PubMed]
- Rammelt S, Heineck J, Zwipp H. Metatarsal fractures. Injury 2004;35:SB77-86. [Crossref] [PubMed]
- Owen RJ, Hickey FG, Finlay DB. A study of metatarsal fractures in children. Injury 1995;26:537-8. [Crossref] [PubMed]
- Singer G, Cichocki M, Schalamon J, et al. A study of metatarsal fractures in children. J Bone Joint Surg Am 2008;90:772-6. [Crossref] [PubMed]
- Robertson NB, Roocroft JH, Edmonds EW. Childhood metatarsal shaft fractures: treatment outcomes and relative indications for surgical intervention. J Child Orthop 2012;6:125-9. [Crossref] [PubMed]
- Mahan ST, Lierhaus AM, Spencer SA, et al. Treatment dilemma in multiple metatarsal fractures: when to operate? J Pediatr Orthop B 2016;25:354-60. [Crossref] [PubMed]
- Herrera-Soto JA, Scherb M, Duffy MF, et al. Fractures of the fifth metatarsal in children and adolescents. J Pediatr Orthop 2007;27:427-31. [Crossref] [PubMed]
- Kessler JI, Weiss JM, Nikizad H, et al. Osteochondritis dissecans of the ankle in children and adolescents: demographics and epidemiology. Am J Sports Med 2014;42:2165-71. [Crossref] [PubMed]
- Letts M, Davidson D, Ahmer A. Osteochondritis dissecans of the talus in children. J Pediatr Orthop 2003;23:617-25. [Crossref] [PubMed]
- Higuera J, Laguna R, Peral M, et al. Osteochondritis dissecans of the talus during childhood and adolescence. J Pediatr Orthop 1998;18:328-32. [Crossref] [PubMed]
- Canale ST, Belding RH. Osteochondral lesions of the talus. J Bone Joint Surg Am 1980;62:97-102. [Crossref] [PubMed]
- Schenck RC Jr, Goodnight JM. Osteochondritis dissecans. J Bone Joint Surg Am 1996;78:439-56. [Crossref] [PubMed]
- Zanon G, DI, Vico G, Marullo M. Osteochondritis dissecans of the talus. Joints 2014;2:115-23. [Crossref] [PubMed]
- Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am 1959;41:988-120. [Crossref] [PubMed]
- Perumal V, Wall E, Babekir N. Juvenile osteochondritis dissecans of the talus. J Pediatr Orthop 2007;27:821-5. [Crossref] [PubMed]
- Bruns J, Rosenbach B. Osteochondrosis dissecans of the talus. Comparison of results of surgical treatment in adolescents and adults. Arch Orthop Trauma Surg 1992;112:23-7. [Crossref] [PubMed]
- Buda R, Pagliazzi G, Castagnini F, et al. Treatment of Osteochondritis Dissecans of the Talus in Skeletally Immature Population: A Critical Analysis of the Available Evidence. Foot Ankle Spec 2016;9:265-70. [Crossref] [PubMed]
- Vannini F, Cavallo M, Baldassarri M, et al. Treatment of juvenile osteochondritis dissecans of the talus: current concepts review. Joints 2014;2:188-91. [PubMed]
- Reilingh ML, Kerkhoffs GM, Telkamp CJ, et al. Treatment of osteochondral defects of the talus in children. Knee Surg Sports Traumatol Arthrosc 2014;22:2243-9. [Crossref] [PubMed]
- Mukherjee SK, Young AB. Dome fracture of the talus. A report of ten cases. J Bone Joint Surg Br 1973;55:319-26. [Crossref] [PubMed]
- Waterman BR, Owens BD, Davey S, et al. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am 2010;92:2279-84. [Crossref] [PubMed]
- Stiell IG, Greenberg GH, McKnight RD, et al. A study to develop clinical decision rules for the use of radiography in acute ankle injuries. Ann Emerg Med 1992;21:384-90. [Crossref] [PubMed]
- Plint AC, Bulloch B, Osmond MH, et al. Validation of the Ottawa Ankle Rules in children with ankle injuries. Acad Emerg Med 1999;6:1005-9. [Crossref] [PubMed]
- Gravel J, Hedrei P, Grimard G, et al. Prospective validation and head-to-head comparison of 3 ankle rules in a pediatric population. Ann Emerg Med 2009;54:534-40 e1.
- Chorley JN, Hergenroeder AC. Management of ankle sprains. Pediatr Ann 1997;26:56-64. [Crossref] [PubMed]
- Maffulli N, Ferran NA. Management of acute and chronic ankle instability. J Am Acad Orthop Surg 2008;16:608-15. [Crossref] [PubMed]
- Letts M, Davidson D, Mukhtar I. Surgical management of chronic lateral ankle instability in adolescents. J Pediatr Orthop 2003;23:392-7. [Crossref] [PubMed]
- Nery C, Raduan F, Del Buono A, et al. Arthroscopic-assisted Brostrom-Gould for chronic ankle instability: a long-term follow-up. Am J Sports Med 2011;39:2381-8. [Crossref] [PubMed]
- Bell SJ, Mologne TS, Sitler DF, et al. Twenty-six-year results after Brostrom procedure for chronic lateral ankle instability. Am J Sports Med 2006;34:975-8. [Crossref] [PubMed]
- Gould N, Seligson D, Gassman J. Early and late repair of lateral ligament of the ankle. Foot Ankle 1980;1:84-9. [Crossref] [PubMed]
- Lui TH. Modified arthroscopic Brostrom procedure. Foot Ankle Surg 2015;21:216-9. [Crossref] [PubMed]
- Kocher MS, Fabricant PD, Nasreddine AY, et al. Efficacy of the Modified Brostrom Procedure for Adolescent Patients With Chronic Lateral Ankle Instability. J Pediatr Orthop 2017;37:537-42. [Crossref] [PubMed]
- Gallagher SM, Rodriguez NA, Andersen CR, et al. Anatomic predisposition to ligamentous Lisfranc injury: a matched case-control study. J Bone Joint Surg Am 2013;95:2043-7. [Crossref] [PubMed]
- Mayich DJ, Mayich MS, Daniels TR. Effective detection and management of low-velocity Lisfranc injuries in the emergency setting: principles for a subtle and commonly missed entity. Can Fam Physician 2012;58:1199-204, e620-5.
- Zgonis T. Pediatric foot deformities. Clin Podiatr Med Surg 2013;30:xi. [PubMed]
- Duzhii ID, Borodenko MM. Rare manifestations of spontaneous pneumothorax and pleural effusion. Klin Khir 1994;58-9. [PubMed]
- Veijola K, Laine HJ, Pajulo O. Lisfranc injury in adolescents. Eur J Pediatr Surg 2013;23:297-303. [Crossref] [PubMed]
- Wiley JJ. Tarso-metatarsal joint injuries in children. J Pediatr Orthop 1981;1:255-60. [Crossref] [PubMed]
- Kose O, Celiktas M, Yigit S, et al. Can we make a diagnosis with radiographic examination alone in calcaneal apophysitis (Sever's disease)? J Pediatr Orthop B 2010;19:396-8. [Crossref] [PubMed]
- James AM, Williams CM, Haines TP. Effectiveness of interventions in reducing pain and maintaining physical activity in children and adolescents with calcaneal apophysitis (Sever's disease): a systematic review J Foot Ankle Res 2013;6:16. [Crossref] [PubMed]
- Micheli LJ, Ireland ML. Prevention and management of calcaneal apophysitis in children: an overuse syndrome. J Pediatr Orthop 1987;7:34-8. [Crossref] [PubMed]
- James AM, Williams CM, Luscombe M, et al. Factors Associated with Pain Severity in Children with Calcaneal Apophysitis (Sever Disease). J Pediatr 2015;167:455-9. [Crossref] [PubMed]
- Sylvester JE, Hennrikus WL. Treatment outcomes of adolescents with Iselin's apophysitis. J Pediatr Orthop B 2015;24:362-5. [Crossref] [PubMed]
- Canale ST, Williams KD. Iselin's disease. J Pediatr Orthop 1992;12:90-3. [Crossref] [PubMed]
- Forrester RA, Eyre-Brook AI, Mannan K. Iselin's Disease: A Systematic Review. J Foot Ankle Surg 2017;56:1065-9. [Crossref] [PubMed]
Cite this article as: Du P, Chen K, Patterson D, Ranade S. The pediatric ankle and foot: a review of common injuries in the pediatric athlete and their treatments. Ann Joint 2018;3:35.