
The current management of patients with patellofemoral pain from the physical therapist’s perspective
Patellofemoral pain (PFP) is exceedingly common. Annual prevalence for PFP approaches 23% in the general population and is approximately 29% among adolescents, with female athletes being at particularly high risk (1). Participation in recreationally running or military training, both of which may lead to high patellofemoral joint contact forces (2), is associated with an especially high incidence of PFP (1). Persistent symptoms are common and 57% of individuals with PFP report unfavorable outcomes five to eight years after their initial diagnosis (3). As such, it is important for individuals with PFP to receive optimal rehabilitation with the goal of achieving positive short- and long-term outcomes and preventing the transition from a transient, acute episode into a recurrent, chronic problem.
The purpose of this review is to provide an overview of the physical therapist’s management, including the evaluation and treatment, of the patient with PFP. We begin with a brief overview of symptom onset, then discuss the importance of considering the complexities of the painful experience when rehabilitating individuals with PFP, particularly among those with episodic or recalcitrant symptoms. We then present our rehabilitation approach for a systematic physical therapy examination including a thorough subjective history and objective clinical, functional, and patient-reported outcome measures. Finally, we present a comprehensive treatment approach that draws heavily from recently published literature and clinical trials.
Symptom onset
PFP, or anterior knee pain, is an amalgam of conditions that are typically non-traumatic in origin and result in peripatellar and/or retropatellar knee pain. A number of structures in and around the patellofemoral and tibiofemoral joints, such as the synovium or infrapatellar fat pad, may individually or collectively contribute to PFP (4). The patellofemoral articular cartilage itself, however, is not painful when probed directly sans anesthesia (5), likely due to its lack of free nerve endings (6). While a variety of factors may also contribute to symptom onset, disruption of tissue homeostasis via acute injury or repetitive overloading (i.e., high-frequency moderate loading or an isolated very high loading event) may exceed tissue homeostasis, or the envelope of function, for a given structure(s) and lead to pathology and pain (7,8). Conservative management may initially promote relative rest and avoidance of activities that exacerbate the patient’s pain while attempting to limit loss of muscle strength, ROM, or function. PFP, however, often persists for months or even years (3,9), requiring a more complex rehabilitation approach.
The complex pain experience
Throughout the successful management of PFP and especially when symptoms are chronic in nature, rehabilitation specialists must appreciate the complexity of the pain experience (10). In his 2016 Maley Lecture, physical therapist and pain science researcher Steven George, PT, PhD, calls for a shift in physical therapist education, research, and clinical practice from the traditional direct link among pain, nociception, and injury to a more inclusive biopsychosocial model that incorporates pain with movement (10). Healthcare professionals must consider not only the patient’s underlying knee pathology (e.g., structural abnormalities, muscle dysfunction) but also the patient’s psychological distress and pain neurophysiology when evaluating the clinical pain experience (11). In chronic musculoskeletal conditions, as can often become the case with PFP, symptoms may outlive their usefulness; although no clear definition exists, chronic pain is generally described as pain that lasts “beyond the body’s usual healing time” and is typically three months or greater (12). Clinicians must recognize the difference between acute (protective) pain and chronic pain, which may limit function and inhibit progress. Encouraging regular movement and exercise within the pain-free envelope of function (7) and, when appropriate, such as in the chronic case, even beyond the pain-free range, may be necessary to optimize function in patients with PFP. In such cases, graded exposure (13) may help maximize function even in the absence of full symptom resolution.
Conscientious monitoring and progression of interventions and other activities throughout rehabilitation is thus essential to achieving optimal outcomes. The remainder of this review article will delineate strategies for conducting a thorough evaluation and creating an appropriate, progressive, and individualized treatment approach for PFP.
Evaluation
History
A thorough history is critical for appropriately diagnosing (14) and optimally managing PFP (15). While one may accurately identify the relatively young, active woman with atraumatic onset of anterior knee pain as the most likely candidate, men and women of all activity levels across a wide age range may develop PFP (16). The rehabilitation specialist should ask the patient to identify the date of symptom onset, mechanism of injury and/or antecedent events, location and quality of pain, exacerbating and alleviating symptoms, relevant past medical history including prior lower extremity and low back symptoms, diagnostic imaging, occupational demands, recreational activities, footwear including use of orthotics, and patient goals (Table 1). Pertinent past medical history may include not only previous knee symptoms but also ankle, hip, and lumbar pain, as radiculopathy from the spine to the knee is possible. Referred knee pain may be present due to hip pathology, such as osteoarthritis or predominantly pediatric conditions like slipped capital femoral epiphysis (17,18), thus subjective questioning and physical examination should consider the hip, particularly when the practitioner is unable to provoke the patient’s symptoms during a thorough, targeted knee evaluation. Gradual and even insidious onset of anterior knee pain are common in PFP whereas acute onset of knee pain secondary to a traumatic event merits further evaluation of the integrity of the knee ligaments, tendons, menisci, and bone. Clinicians should refer their patients to an appropriate specialist if they suspect serious pathology (e.g., fracture or osteomyelitis) or non-musculoskeletal origin (e.g., cancer or infection) due to the presence of red flags (i.e., fever, unremitting night pain, or increased temperature and swelling around the knee; or, among adolescents or children, a leg length discrepancy, limp, and limited hip ROM possibly indicative of Perthes disease or a slipped capital femoral epiphysis) (16). Physician referral is also warranted in the case of unremitting or worsening symptoms despite appropriate physical therapy and activity modification.
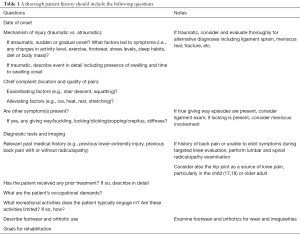
Full table
Clinical examination
Physical examination should incorporate a variety of measures including ROM, muscle length, effusion, resisted isometrics, strength, balance and postural control, movement quality assessments, special tests, palpation, functional evaluation, and patient reported outcome measures. Objective assessments should guide treatment, progression, and clinical decision-making. An individualized rehabilitation program that addresses the patient’s specific impairments and functional limitations is regarded as best practice (9).
ROM and muscle length testing
ROM of the knee as well as the ankle and hip should be assessed. The physical therapist should evaluate at a minimum both active and passive ROM measurements of tibiofemoral flexion and extension, talocrural dorsiflexion, and femoroacetabular extension, internal and external rotation, and flexion; other motions (e.g., hip abduction and adduction) or joints (e.g., subtalar eversion and inversion and lumbar flexion and extension) may also be considered.
Muscle length testing is also an important consideration as soft tissue tightness (i.e., limited flexibility) is prevalent in individuals with PFP and may contribute to symptoms (19). Evaluation of the rectus femoris, hip flexors (1- and 2-joint muscles), tensor fascia lata and iliotibial band, hamstrings, gastrocnemius, and soleus should be performed.
Effusion
Knee joint effusion can easily be evaluated using the stroke test (Table 2). The stroke test is a reliable grading scale that assesses the presence of intracapsular swelling (20). While effusion is not often present, mild effusion can occur among individuals with PFP; significant effusion is likely indicative of more serious pathology (e.g., ligament rupture, meniscus tear, fracture) and merits further evaluation. Effusion monitoring may help determine appropriate clinical progression (21,22). Increased effusion can indicate when rehabilitation has exceeded the patient’s current envelope of function (7,23) and thus rehabilitation exercises or activity should be reduced or not progressed further. Tracking or asking the patient about outside activities is critical in determining whether or not the prescribed exercises or home exercise program contributed to an exacerbation of effusion and/or other symptoms or whether other factors are more likely culpable. For example, asking a student about activities such as walking around school or campus or attending a party may be pertinent. The use of activity trackers to monitor movement outside of therapy is becoming increasingly possible and should be considered as a more accurate way to quantify activity and joint loading (24).

Full table
Resisted isometrics
Resisted isometrics at various angles of knee flexion may be used during the early portions of the clinical examination to determine what type of structure(s) is most likely involved. A finding of “strong and painful” with resisted isometric knee extension is most likely to support the diagnosis of PFP, although weakness is also possible, particularly in the acute phase (pain-mediated) or in long-standing, chronic cases. The clinician should evaluate resisted isometrics at multiple angles of knee flexion to see if there is a range that is more or less painful for the individual patient. The clinician may use these findings to inform subsequent strength evaluations as well as treatment, selecting ranges of motion that are least provocative to the patient to improve muscle strength and activation while avoiding exacerbation of symptoms.
Strength
Strength assessments should evaluate not only the muscles crossing the knee joint but also the surrounding hip and ankle musculature. Knee extensor and hip extensor, abductor, and external rotator muscle strength and activation are of utmost importance given their roles in dynamically controlling hip and knee motion and the association of PFP with weakness of these muscles (25-29), although cause and effect are unknown (28). Interestingly, Kindel and Challis found that patients with PFP have weaker hip extensors and poorer neuromuscular control with the knee flexed but not extended compared to healthy controls (30), suggesting knee position may be important when evaluating hip musculature. A thorough evaluation should also strength of the core muscles, knee flexors, ankle plantarflexors and dorsiflexors, and hip flexors, internal rotators, and adductors.
Given the strength of the lower extremity muscles, clinicians should evaluate lower extremity muscle, particularly quadriceps, strength using an electromechanical dynamometer when possible. When an electromechanical dynamometer is not available, one-rep max testing on knee extension machine for quadriceps strength or hand-held dynamometer secured with a strap are acceptable alternatives, although they overestimate strength of the involved quadriceps (31). Electrical burst superimposition may be used to evaluate quadriceps muscle activation (i.e., inhibition) (32), but requires relatively expensive equipment that is unavailable to many clinicians (Figure 1). In contrast to the usual order, we recommend that clinicians test the (most) involved limb first to determine the angle of knee flexion that is pain-free or least provocative; the clinician can subsequently evaluate the contralateral limb in the same position. Clinicians may also use patellar taping (see below) to facilitate strength evaluation, enabling some patients to complete testing with less or no pain. While we most often use a limb symmetry index [i.e., involved limb strength/uninvolved limb strength × 100 (%)] for comparison, PFP is often a bilateral condition thus clinicians should interpret limb symmetry indexes with caution. Additional evaluation using manual muscle testing of the hip and knee muscles may provide additional insight, especially in the case of bilateral weakness.

Balance and postural control
Balance and postural control may be impaired in patients with PFP compared to healthy controls (33-35) during a variety of tasks including dynamic standing balance (33), postural stability during a stepping up and down task (34), and stair climbing (35). Static balance during single leg stance is also impaired on the involved compared to uninvolved limb among women with PFP (36). Fatigue of the hip abductors and to a lesser degree the knee extensors is associated with greater balance instability during dynamic standing balance (33). Patients with PFP may also exhibit especially poor postural control with their eyes closed (37). In light of these findings, it is important to assess both static balance with eyes opened and closed as well as dynamic balance on both the (most) involved and contralateral limb. To assess static balance, we evaluate single leg stance, which can be progressed in difficulty by having the patient stand on an unstable surface such as a foam pad; document the time to error and/or number of errors in a given time (e.g., 30 seconds). Dynamic balance may be assessed using the reliable Star Excursion Balance Test (38,39).
Movement assessments
Clinicians should consider a variety of movement quality assessments concordant with the patient’s complaints and activity limitations given that aberrant mechanics and neuromuscular activation patterns are often present in individuals with PFP (23,26,40-44). The position of dynamic knee valgus, characterized by hip adduction and internal rotation, may be associated with PFP (23,40,44,45), thus clinicians should pay particular attention for these aberrant mechanics. Clinicians should consider evaluating multi-joint lower extremity movements including but not limited to double and single leg squatting, drop jump landing, hopping, walking, stair ascent and descent, and running. Identification of movement impairments may guide not only targeted strengthening but also and perhaps more importantly neuromuscular activation exercises and movement retraining (23,40,46,47).
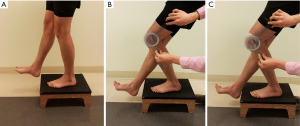
Step test
We recommend using a modification of the previously described step test (Figure 2). The step test involves standing on a 15 centimeter block with hands on hips and using the involved limb to “slowly” and “smoothly” eccentrically lower the body until the contralateral heel touches the floor (48). A positive result is reproduction of the patient’s PFP; a positive finding is prevalent in 74% (57 of 77) of individuals with PFP (49) and has a modest positive likelihood ratio of 2.34 (48). In the authors’ clinical experience, we modify the test by recording the angle at which pain first occurs and asking the patient to rate the pain on an 11-point numeric pain rating scale. If the test is positive, we often evaluate the patient again on the modified step test after applying patellar taping (described below) to determine whether or not patellar taping provides immediate relief of symptoms and may therefore be beneficial in facilitating increased function in the short-term.
Palpation
Individuals with PFP often have pain in or around the patella that may be reproduced with palpation. Clinicians should also palpate other nearby structures, such as the patellar and quadriceps tendons, to rule out other sources of anterior knee pain. For example, reproduction of pain with palpation of the patellar tendon may indicate patellar tendinopathy; pain at the distal pole of the patella in adolescents may indicate Sinding-Larsen-Johansson Syndrome (50); and swelling and point tenderness around the tibial tuberosity in adolescents may indicate Osgood-Schlatter Disease (16,50).
Functional testing
Functional testing may evaluate tasks that are important to the patient and are currently limited. Examples of functional testing include the stair climb test, sit to stand test, and 6-minute walk test. Performance as well as symptoms should be documented.
Objective measures for evaluation, treatment progression, and clinical decision-making
Evaluation, treatment progression, and clinical decision-making like discharge and return-to-sport clearance should be based as much as possible on objective measures while simultaneously considering the patient’s needs and goals. As mentioned above, an increase in or the presence of new effusion indicates that the activity has exceeded the current envelope of function and should not be progressed further. Clinicians may also use the soreness rules (Table 3), initially developed by Fees et al. (51) and later adapted to the lower extremity by Adams et al. (21), to monitor appropriate progression of activities. (While avoiding pain and symptom exacerbation is critical during the early management of acute PFP, clinicians may set a threshold of acceptable symptoms (e.g., 5/10 on numeric pain rating scale) for individuals with chronic PFP, focusing on increasing function rather than complete avoidance of symptoms). Successful completion of a running progression (Table 4) (21) should be pre-requisite to initiating higher level activities.

Full table

Full table
Valid and reliable patient reported outcome measures should be completed at initial evaluation and periodically throughout rehabilitation to monitor progress and inform rehabilitation. The Visual Analog Scale for usual pain or worst pain and the Kujala Anterior Knee Pain Scale (52) are reliable, valid, and responsive in individuals with PFP (53); the Kujala Anterior Knee Pains Scale is also valid and reliable in adolescent female athletes with anterior knee pain (54).
Throughout the rehabilitation process, the clinicians must appreciate the impact of psychological factors (e.g., kinesiophobia) (55) and other factors (e.g., stress, sleep) on pain, particularly when a patient reports a transient increase in symptoms. Anxiety, depression, catastrophizing, and kinesiophobia may be present in individuals with PFP and correlate with higher pain ratings and reduced physical function (56); appropriate referral or consultation may be beneficial. Stress levels (57) and sleep duration (58) also influence pain; for example, too much (>9 hours) or too little (<6 hours) sleep the previous night is associated with greater pain the following day (58). Asking and educating patients about these factors is important when determining whether to progress, maintain, or reduce interventions.
Treatment
Patients with PFP present with a wide variety of underlying pathophysiology and associated impairments (25,47). It is thus imperative to individually assess each patient to identify and subsequently address his or her impairments, functional limitations, and activity restrictions. Management of PFP should consist of an individualized (47), multi-modal approach with exercise therapy as the hallmark of the plan (9,16,26,59-61).
Exercise therapy: strengthening, stretching, and aerobic exercise
According to the 2016 consensus statement from the International Patellofemoral Pain Research Committee, exercise therapy is the “treatment of choice” for individuals with PFP (9). High-quality evidence supports exercise therapy to improve pain and function in the short-, medium-, and long-term; exercise was the only intervention that received such a high recommendation (9). Exercise therapy should include both hip and knee strengthening (9,27,62,63) using both open (non-weight-bearing) and closed (weight-bearing) kinetic chain exercises (9,62). Open kinetic chain exercises include straight leg raises (progress by adding ankle weights), short arc quadriceps strengthening, knee extensions, side-lying hip abduction straight leg raise, and clamshells. Closed kinetic chain exercises include wall sits, double- and single-leg squats, lateral step-downs, and leg press. Strengthening of the core (47,64) and ankle musculature should be included if the patient exhibits deficits or imbalances in these areas.
Appropriate selection of open and closed chain strengthening exercises should consider the patellofemoral joint contact forces in each mode. Steinkamp et al. found that comparison of patellofemoral joint contact forces during closed (i.e., body weight squat) and open (i.e., 9 kg weighted boot) kinetic chain exercises resulted in relatively less patellofemoral contact force in the closed kinetic chain condition in less than 48° knee flexion and relatively less patellofemoral contact force in the open kinetic chain condition in more than 48° knee flexion (65). Similar findings have been more recently produced by Powers et al., who added that patellofemoral joint contact force was less during quadriceps strengthening using a constant resistance knee extension machine compared to squatting at angles greater than approximately 45° (66). Therefore, particularly during the early stages of rehabilitation, patients may benefit from performing open kinetic chain exercises in deeper ranges of knee flexion (e.g., 50°–90°) and closed kinetic chain exercises in shallower ranges (e.g., 0°–45°) (66).
Throughout the rehabilitation process, clinicians should design appropriate exercises that maximize muscle strength while minimizing symptom exacerbation, using the soreness rules (Table 3) to guide progression. A recent study by van Rossom and colleagues provides peak and mean patellofemoral joint contact forces during gait plus nine functional exercises and may serve as a guide for appropriately and gradually progressing loading during rehabilitation (67). While initially during the acute stage of rehabilitation a clinician may strive to perform only exercises that are pain-free, the goal of completely eliminating movement-related pain in the chronic condition may be not only unrealistic but also a disservice to the patient’s recovery (10). In such cases, setting an acceptable threshold of symptoms based on the patient’s presentation may be appropriate.
Stretching is another important component of rehabilitation, as individuals with PFP often have limited ROM, particularly around the hip (19) and knee and perhaps also the ankle (25). Treatments should address the specific ROM and muscle length restrictions identified during the evaluation and may include the quadriceps, hip flexors, hamstrings, tensor fascia lata/iliotibial band, gastrocnemius, and/or soleus.
Joint mobilizations
Joint mobilizations may be effective in improving pain and function among individuals with PFP when joint mobilizations are directed at the knee (i.e., patellofemoral and tibiofemoral joint) and combined with a comprehensive treatment approach including exercise (59). A case study by Lantz et al. highlights the potential benefit of tibiofemoral mobilizations in an individual with chronic PFP (68).
Patellofemoral taping
Conflicting evidence exists regarding the efficacy of patellofemoral taping (60,69-72). We recommend using taping in conjunction with a multi-modal, comprehensive treatment plan if taping alleviates pain during exercises in rehabilitation and/or functional activities. Clinicians should evaluate the immediate effectiveness of patellofemoral taping within an individual by assessing a functional task pre- and post-taping that is specific to that patient’s symptoms; if pain is alleviated then taping may help the patient complete functional activities and exercises which may in turn facilitate recovery. While we recommend first evaluating medial patellar glide therapeutic taping (73), placebo taping plus exercise may be similarly beneficial to therapeutic tension taping plus exercise (60). The use of patellar taping in isolation is not recommended (9,16,60,61,69,70,73).
Neuromuscular electrical stimulation (NMES)
A 2017 Cochrane Review by Martimbianco et al. found limited, low-quality regarding the effect of NMES for the treatment of PFP (74). The review concluded that very low-quality evidence suggests NMES reduces pain at the end of treatment (3 to 12 weeks) but the improvement may not be clinically relevant given the small magnitude of change (1.63 out of 10 on the visual analog scale). The authors found even less support for NMES on strength or function, concluding that “insufficient and inconclusive evidence” exists for the effect of NMES on treating individuals with PFP (74). While one pilot study has found no statistically significant differences between 38 athletes (19 per group) who completed physiotherapy or physiotherapy plus electrical stimulation, limitations including study design, follow-up, and stimulation parameters limit its applicability (75). Given the dose response relationship between electrical stimulation intensity and quadriceps femoris muscle torque (76), we recommend using higher NMES intensity levels to facilitate muscular strength and activation development. A 2010 systematic review on NMES on quadriceps strength in individuals after anterior cruciate ligament reconstruction found that NMES combined with exercise is more effective than exercise alone at improving quadriceps muscle strength (77). We therefore recommend using NMES in conjunction with a comprehensive rehabilitation program in individuals who have PFP and deficits in quadriceps strength and/or activation. We recommend the following parameters: 10.2 cm × 12.7 cm pads on the vastus medialis and proximal vastus lateralis muscles; 15 electrically elicited, isometric contractions of the quadriceps at about 65° knee flexion (or the most comfortable position for the patient), 75 bursts per second; 10” on, 50” off, 2” ramp; and the maximum tolerated intensity that elicits at least 50% maximum volitional isometric contraction (21,76).
Neuromuscular training
Neuromuscular activation deficits are common in individuals with PFP, especially in the hip abductors and external rotators, knee extensors, and core musculature (23,26,40,44,45). Evaluating movements during functional tasks (described above) is essential to identifying and treating neuromuscular activation deficits. Strengthening alone seldom changes mechanics (78), thus task-specific movement retraining is likely necessary (23,40,79,80). Use of resistance tubing bands may promote activity of specific muscle groups; for example, using resistance tubing bands around the knees during a squat may facility hip abduction and external rotation. NMES may facilitate neuromuscular training, as improvements in kinematics and muscle activity have been observed in a small group (N=15) of women with PFP (46).
Running mechanics and gait retraining in patients with patellofemoral pain have received significant attention likely due in part to the high incidence of PFP among runners (1). Running mechanics are often altered in individuals with PFP and young women may be especially prone to altered mechanics such as excessive hip adduction and internal rotation leading to dynamic knee valgus (23,41-43,81). Gait retraining may be considered in individuals with PFP who have aberrant running mechanics and should address the specific deficits in the individual (43). Sagittal plane trunk mechanics (82) and footwear (as described by the Minimalist Index) (83) are related to patellofemoral joint stress during running, thus should also be considered during gait analysis and running retraining; forward trunk lean (82) and more minimalist shoes (83) are associated with reduced patellofemoral joint stress. A systematic review by Agresta and Brown found the use of real-time auditory and visual feedback in conjunction with therapeutic exercise to be effective in improving lower extremity kinematics in runners with patellofemoral, although no single method of feedback was deemed superior (84).
Activity modification and gradual loading
During the acute phase, activity modification characterized by relative rest is likely appropriate to allow healing to occur. Reintegration of loading, however, must be implemented and should be done in a systematic way to gradually increase and restore the envelope of function. Chen et al. evaluated patellofemoral joint reaction forces using an MRI-informed subject-specific three-dimensional model, finding that, among the four tasks evaluated, patellofemoral joint reaction forces were highest during running [58.2 N/kg-body weight (bwt)], followed by stair ascent (33.9 N/kg-bwt), stair descent (27.9 N/kg-bwt), and walking (10.1 N/kg-bwt) (2). In light of these findings, it may be inappropriate for an individual with acute PFP to run if stair descent is painful, although individual evaluation and clinical judgment should be considered. Recently, van Rossom et al. added to Chen’s findings by evaluating peak and mean patellofemoral joint contact forces during ten functional tasks; peak patellofemoral joint contact forces were lowest during gait and progressively higher in sit down, stand up, squat, forward lunge, stair ascent, stair descent, single leg hop weight acceptance phase, sideward lunge, and single leg hop push-off phase (67).
Other interventions
Numerous other interventions have been proposed as adjuvants or stand-alone treatments for individuals with PFP and may be considered as part of a comprehensive plan of care if impairments warrant or symptoms have been intractable to the more evidence-based approaches outlined above. Foot orthotics may be beneficial in reducing pain and improving function (16). Dry needling does not appear to provide any additional benefit when added to a multi-modal treatment approach including manual therapy and strengthening exercise compared to manual therapy and strengthening exercise alone (85).
Appropriate progression and discharge
Rehabilitation should be progressive and rooted in objective clinical findings. Monitoring effusion and soreness should occur throughout rehabilitation and guide progression. Use of gradual, return-to-activity training protocols, such as the running progression (Table 4) (21), may facilitate appropriate progression and aid clinical decision-making.
Discharge from physical therapy should occur when the patient has achieved his or her goals and is equipped to transition to self-management or management by an athletic trainer, strength and conditioning coach, or personal trainer if available. Patient education is thus critical at this time-point and throughout the rehabilitation process; the patient should know what exercises to perform and how to progress activity while adhering to basic principles such as the soreness rules. Although research on return-to-sport criteria in patients with PFP is lacking, we recommend athletes achieve limb symmetry index scores of 90% of greater for quadriceps strength and all four hop tests (single, crossover, triple, and 6 meter timed) (86) prior to resuming full participation; limb symmetry indexes, however, have limitations (87) particularly in individuals with bilateral involvement thus should be interpreted with caution.
Conclusions
Early, appropriate rehabilitation may be critical to preventing poor outcomes (88) and optimizing function for individuals with PFP. We strongly recommend exercise therapy, including hip and knee strengthening and stretching, to improve short-, medium-, and long-term outcomes in individuals with PFP (9,16,26,27). A multi-modal, individually tailored rehabilitation program should be designed to target the patient’s specific impairments and functional limitations identified during the evaluation (47). Treatments may include open- and closed-chain exercises, strengthening, stretching, aerobic exercise, patellofemoral and tibiofemoral mobilizations, patellar taping, high-intensity NMES, neuromuscular training, and gait retraining. Although short-term changes or reductions in movement often are necessary in a protective capacity, the persistence of altered movement is a key characteristic of chronic pain. PFP etiology is largely movement related and a comprehensive conservative treatment using movement can be successful.
Acknowledgments
Funding: JJ Capin receives funding from the Foundation for Physical Therapy (Promotion of Doctoral Studies Level I Scholarship) and the University of Delaware (Doctoral Fellowship Award). L Snyder-Mackler receives funding from the National Institutes of Health: NICHD (R44-HD068054, R37-HD037985, and T32-HD007490), NIAMS (R01-AR048212), and NIGMS (U54-GM104941).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vicente Sanchis-Alfonso and Scott F. Dye) for the series “The Patellofemoral Joint” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.04.11). The series “The Patellofemoral Joint” was commissioned by the editorial office without any funding or sponsorship. LSM serves as an unpaid editorial board member of Annals of Joint from Aug 2017 to Jul 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith BE, Selfe J, Thacker D, et al. Incidence and prevalence of patellofemoral pain: a systematic review and meta-analysis. PLoS One 2018;13:e0190892 [Crossref] [PubMed]
- Chen YJ, Scher I, Powers CM. Quantification of Patellofemoral Joint Reaction Force During Functional Activities Using a Subject-Specific Three-Dimensional Model. J Appl Biomech 2010;26:415-23. [Crossref] [PubMed]
- Lankhorst NE, van Middelkoop M, Crossley KM, et al. Factors that predict a poor outcome 5-8 years after the diagnosis of patellofemoral pain: a multicentre observational analysis. Br J Sports Med 2016;50:881-6. [Crossref] [PubMed]
- Biedert RM, Sanchis-Alfonso V. Sources of anterior knee pain. Clin Sports Med 2002;21:335-47. [Crossref] [PubMed]
- Dye SF, Vaupel GL, Dye CC. Conscious Neurosensory Mapping of the Internal Structures of the Human Knee Without Intraarticular Anesthesia. Am J Sports Med 1998;26:773-7. [Crossref] [PubMed]
- Witoński D, Wagrowska-Danielewicz M. Distribution of substance-P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surg Sports Traumatol Arthrosc 1999;7:177-83. [Crossref] [PubMed]
- Dye SF. The knee as a biologic transmission with an envelope of function: a theory. Clin Orthop Relat Res 1996;10-8. [Crossref] [PubMed]
- Post WR, Dye SF. Patellofemoral Pain: An Enigma Explained by Homeostasis and Common Sense. Am J Orthop (Belle Mead NJ) 2017;46:92-100. [PubMed]
- Crossley KM, Middelkoop M, Van , Callaghan MJ, et al. 2016 Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 2: Recommended physical interventions (exercise, taping, bracing, foot orthoses and combined interventions). Br J Sports Med 2016;50:844-52. [Crossref] [PubMed]
- George SZ. Pain Management: Road Map to Revolution. Phys Ther 2017;97:217-26. [PubMed]
- Kittelson AJ, George SZ, Maluf KS, et al. Future Directions in Painful Knee Osteoarthritis: Harnessing Complexity in a Heterogeneous Population. Phys Ther 2014;94:422-32. [Crossref] [PubMed]
- Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev 2017;4:CD011279 [PubMed]
- Nijs J, Lluch Girbés E, Lundberg M, et al. Exercise therapy for chronic musculoskeletal pain: Innovation by altering pain memories. Man Ther 2015;20:216-20. [Crossref] [PubMed]
- Décary S, Frémont P, Pelletier B, et al. Validity of Combining History Elements and Physical Examination Tests to Diagnose Patellofemoral Pain. Arch Phys Med Rehabil 2018;99:607-614.e1. [Crossref] [PubMed]
- Werner S. Anterior knee pain: an update of physical therapy. Knee Surg Sports Traumatol Arthrosc 2014;22:2286-94. [Crossref] [PubMed]
- Crossley KM, Callaghan MJ, Van Linschoten R. Patellofemoral pain. Br J Sports Med 2016;50:247-50. [Crossref] [PubMed]
- Herngren B, Stenmarker M, Vavruch L, et al. Slipped capital femoral epiphysis: A population-based study. BMC Musculoskelet Disord 2017;18:304. [Crossref] [PubMed]
- Hatfield SJ, Baxter RE. Slipped Capital Femoral Epiphysis in a Patient With Knee Pain. J Orthop Sports Phys Ther 2012;42:482. [Crossref] [PubMed]
- Hamstra-Wright KL, Earl-Boehm J, Bolgla L, et al. Individuals with patellofemoral pain have less hip flexibility than controls regardless of treatment outcome. Clin J Sport Med 2017;27:97-103. [Crossref] [PubMed]
- Sturgill LP, Snyder-Mackler L, Manal TJ, et al. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther 2009;39:845-9. [Crossref] [PubMed]
- Adams D, Logerstedt D, Hunter-Giordano A, et al. Current concepts for anterior cruciate ligament reconstruction: a criterion-based rehabilitation progression. J Orthop Sports Phys Ther 2012;42:601-14. [Crossref] [PubMed]
- Capin JJ, Behrns W, Thatcher K, et al. On-Ice Return-to-Hockey Progression After Anterior Cruciate Ligament Reconstruction. J Orthop Sports Phys Ther 2017;47:324-33. [Crossref] [PubMed]
- Willy RW, Meira EP. Current Concepts in Biomechanical Interventions for Patellofemoral Pain. Int J Sports Phys Ther 2016;11:877-90. [PubMed]
- Shull PB, Jirattigalachote W, Hunt MA, et al. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014;40:11-9. [Crossref] [PubMed]
- Witvrouw E, Werner S, Mikkelsen C, et al. Clinical classification of patellofemoral pain syndrome: Guidelines for non-operative treatment. Knee Surg Sports Traumatol Arthrosc 2005;13:122-30. [Crossref] [PubMed]
- Saltychev M, Dutton R, Laimi K, et al. Effectiveness of conservative treatment for patellofemoral pain syndrome: A systematic review and meta-analysis. J Rehabil Med 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Nascimento LR, Teixeira-Salmela LF, Souza RB, et al. Hip and Knee Strengthening is More Effective Than Knee Strengthening Alone for Reducing Pain and Improving Activity in Individuals With Patellofemoral Pain: A Systematic Review With Meta-Analysis. J Orthop Sports Phys Ther 2018;48:19-31. [Crossref] [PubMed]
- Rathleff MS, Rathleff CR, Crossley KM, et al. Is hip strength a risk factor for patellofemoral pain? A systematic review and meta-analysis. Br J Sports Med 2014;48:1088. [Crossref] [PubMed]
- Giles LS, Webster KE, McClelland JA, et al. Does Quadriceps Atrophy Exist in Individuals With Patellofemoral Pain? A Systematic Literature Review With Meta-analysis. J Orthop Sports Phys Ther 2013;43:766-76. [Crossref] [PubMed]
- Kindel C, Challis J. Joint Moment-Angle Properties of the Hip Extensors in Subjects With and Without Patellofemoral Pain. J Appl Biomech 2018;34:159-66. [PubMed]
- Sinacore JA, Evans AM, Lynch BN, et al. Diagnostic Accuracy of Handheld Dynamometry and 1-Repetition-Maximum Tests for Identifying Meaningful Quadriceps Strength Asymmetries. J Orthop Sports Phys Ther 2017;47:97-107. [Crossref] [PubMed]
- Snyder-Mackler L, De Luca PF, Williams PR, et al. Reflex inhibition of the quadriceps femoris muscle after injury of reconstuction of the anterior cruciate ligament. J Bone Joint Surg Am 1994;76:555-60. [Crossref] [PubMed]
- Negahban H, Etemadi M, Naghibi S, et al. The effects of muscle fatigue on dynamic standing balance in people with and without patellofemoral pain syndrome. Gait Posture 2013;37:336-9. [Crossref] [PubMed]
- de Moura Campos Carvalho-E-Silva AP. Dynamic postural stability and muscle strength in patellofemoral pain: Is there a correlation? Knee 2016;23:616-21. [Crossref] [PubMed]
- de Oliveira Silva D, Magalhães FH, Pazzinatto MF, et al. Contribution of altered hip, knee and foot kinematics to dynamic postural impairments in females with patellofemoral pain during stair ascent. Knee 2016;23:376-81. [Crossref] [PubMed]
- Citaker S, Kaya D, Yuksel I, et al. Static balance in patients with patellofemoral pain syndrome. Sports Health 2011;3:524-7. [Crossref] [PubMed]
- Zeinalzadeh A, Talebian S, Naghdi S, et al. Effects of vision and cognitive load on static postural control in subjects with and without patellofemoral pain syndrome. Physiother Theory Pract 2018;34:276-85. [Crossref] [PubMed]
- Gribble PA, Hertel J, Plisky P. Using the star excursion balance test to assess dynamic postural-control deficits and outcomes in lower extremity injury: A literature and systematic review. J Athl Train 2012;47:339-57. [Crossref] [PubMed]
- Kinzey SJ, Armstrong CW. The Reliability of the Star-Excursion Test in Assessing Dynamic Balance. J Orthop Sports Phys Ther 1998;27:356-60. [Crossref] [PubMed]
- Neal BS, Barton CJ, Gallie R, et al. Runners with patellofemoral pain have altered biomechanics which targeted interventions can modify: A systematic review and meta-analysis. Gait Posture 2016;45:69-82. [PubMed]
- Noehren B, Pohl MB, Sanchez Z, et al. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech (Bristol, Avon) 2012;27:366-71. [Crossref] [PubMed]
- Noehren B, Sanchez Z, Cunningham T, et al. The effect of pain on hip and knee kinematics during running in females with chronic patellofemoral pain. Gait Posture 2012;36:596-9. [Crossref] [PubMed]
- Willy RW, Manal KT, Witvrouw EE, et al. Are mechanics different between male and female runners with patellofemoral pain? Med Sci Sports Exerc 2012;44:2165-71. [Crossref] [PubMed]
- Willson JD, Kernozek TW, Arndt RL, et al. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech (Bristol, Avon) 2011;26:735-40. [Crossref] [PubMed]
- Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med 2009;43:584-8. [Crossref] [PubMed]
- Glaviano NR, Huntsman S, Dembeck A, et al. Improvements in kinematics, muscle activity and pain during functional tasks in females with patellofemoral pain following a single patterned electrical stimulation treatment. Clin Biomech (Bristol, Avon) 2016;32:20-7. [Crossref] [PubMed]
- Glaviano NR, Saliba S. Impairment based rehabilitation for patellofemoral pain patients. Phys Sportsmed 2016;44:311-23. [Crossref] [PubMed]
- Nijs J, Van Geel C, Van Der Auwera C, et al. Diagnostic value of five clinical tests in patellofemoral pain syndrome. Man Ther 2006;11:69-77. [Crossref] [PubMed]
- Selfe J, Harper L, Pedersen I, et al. Four Outcome Measures for Patellofemoral Joint Problems. Physiotherapy 2001;87:507-15. [Crossref]
- Peace KAL, Lee JC, Healy J. Imaging the infrapatellar tendon in the elite athlete. Clin Radiol 2006;61:570-8. [Crossref] [PubMed]
- Fees M, Decker T, Snyder-Mackler L, et al. Upper extremity weight-training modifications for the injured athlete: A clinical perspective. Am J Sports Med 1998;26:732-42. [Crossref] [PubMed]
- Kujala UM, Jaakkola LH, Koskinen SK, et al. Scoring of patellofemoral disorders. Arthroscopy 1993;9:159-63. [Crossref] [PubMed]
- Crossley KM, Bennell KL, Cowan SM, et al. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch Phys Med Rehabil 2004;85:815-22. [Crossref] [PubMed]
- Ittenbach RF, Huang G, Barber Foss KD, et al. Reliability and Validity of the Anterior Knee Pain Scale: Applications for Use as an Epidemiologic Screener. PLoS One 2016;11:e0159204 [Crossref] [PubMed]
- Sanchis-Alfonso V. Holistic approach to understanding anterior knee pain. Clinical implications. Knee Surg Sports Traumatol Arthrosc 2014;22:2275-85. [Crossref] [PubMed]
- Maclachlan LR, Collins NJ, Matthews MLG, et al. The psychological features of patellofemoral pain: A systematic review. Br J Sports Med 2017;51:732-42. [Crossref] [PubMed]
- Østerås B, Sigmundsson H, Haga M. Perceived stress and musculoskeletal pain are prevalent and significantly associated in adolescents: An epidemiological cross-sectional study Chronic Disease epidemiology. BMC Public Health 2015;15:1-10. [Crossref] [PubMed]
- Edwards RR, Almeida DM, Klick B, et al. Duration of sleep contributes to next-day pain report in the general population. Pain 2008;137:202-7. [Crossref] [PubMed]
- Jayaseelan DJ, Scalzitti DA, Palmer G, et al. The effects of joint mobilization on individuals with patellofemoral pain: a systematic review. Clin Rehabil 2018;269215517753971 [Epub ahead of print]. [PubMed]
- Logan CA, Bhashyam AR, Tisosky AJ, et al. Systematic Review of the Effect of Taping Techniques on Patellofemoral Pain Syndrome. Sports Health 2017;9:456-61. [Crossref] [PubMed]
- Collins NJ, Bisset LM, Crossley KM, et al. Efficacy of Nonsurgical Interventions for Anterior Knee Pain. Sports Med 2012;42:31-49. [Crossref] [PubMed]
- Lack S, Barton C, Sohan O, et al. Proximal muscle rehabilitation is effective for patellofemoral pain: A systematic review with metaanalysis. Br J Sports Med 2015;49:1365-76. [Crossref] [PubMed]
- Kooiker L, Van De Port IG, Weir A, et al. Effects of Physical Therapist-Guided Quadriceps-Strengthening Exercises for the Treatment of Patellofemoral Pain Syndrome: A Systematic Review. J Orthop Sports Phys Ther 2014;44:391-B1. [Crossref] [PubMed]
- Ferber R, Bolgla L, Earl-Boehm JE, et al. Strengthening of the hip and core versus knee muscles for the treatment of patellofemoral pain: A multicenter randomized controlled trial. J Athl Train 2015;50:366-77. [Crossref] [PubMed]
- Steinkamp LA, Dillinghan MF, Markel MD, et al. Biomechanical considerations in patellofemoral joint rehabilitation. Am J Sports Med 1993;21:438-44. [Crossref] [PubMed]
- Powers CM, Ho K-Y, Chen Y-J, et al. Patellofemoral Joint Stress During Weight-Bearing and Non—Weight-Bearing Quadriceps Exercises. J Orthop Sports Phys Ther 2014;44:320-7. [Crossref] [PubMed]
- van Rossom S, Smith CR, Thelen DG, et al. Knee Joint Loading in Healthy Adults During Functional Exercises: Implications for Rehabilitation Guidelines. J Orthop Sports Phys Ther 2018;48:162-73. [Crossref] [PubMed]
- Lantz JM, Emerson-Kavchak AJ, Mischke JJ, et al. Tibiofemoral Joint Mobilization in the Successful Management of Patellofemoral Pain Syndrome: a Case Report. Int J Sports Phys Ther 2016;11:450-61. [PubMed]
- Crossley K, Cowan SM, Bennell KL, et al. Patellar taping: Is clinical success supported by scientific evidence? Man Ther 2000;5:142-50. [Crossref] [PubMed]
- Aminaka N, Gribble PA. Patellar Taping, patellofemoral pain syndrome, lower extremity kinematics, and dynamic postural control. J Athl Train 2008;43:21-8. [Crossref] [PubMed]
- Ho K-Y, Epstein R, Garcia R, et al. Effects of Patellofemoral Taping on Patellofemoral Joint Alignment and Contact Area During Weight Bearing. J Orthop Sports Phys Ther 2017;47:115-23. [Crossref] [PubMed]
- Edmonds DW, McConnell J, Ebert JR, et al. Biomechanical, neuromuscular and knee pain effects following therapeutic knee taping among patients with knee osteoarthritis during walking gait. Clin Biomech (Bristol, Avon) 2016;39:38-43. [Crossref] [PubMed]
- Crossley KM, Marino GP, Macilquham MD, et al. Can patellar tape reduce the patellar malalignment and pain associated with patellofemoral osteoarthritis? Arthritis Rheum 2009;61:1719-25. [Crossref] [PubMed]
- Martimbianco ALC, Torloni MR, Andriolo BN, et al. Neuromuscular electrical stimulation (NMES) for patellofemoral pain syndrome. Cochrane Database Syst Rev 2017;12:CD011289 [PubMed]
- Bily W, Trimmel L, Mödlin M, et al. Training Program and Additional Electric Muscle Stimulation for Patellofemoral Pain Syndrome: A Pilot Study. Arch Phys Med Rehabil 2008;89:1230-6. [Crossref] [PubMed]
- Snyder-Mackler L, Delitto A, Stralka SW, et al. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther 1994;74:901-7. [Crossref] [PubMed]
- Kim KM, Croy T, Hertel J, et al. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. J Orthop Sports Phys Ther 2010;40:383-91. [Crossref] [PubMed]
- Snyder KR, Earl JE, O’Connor KM, et al. Resistance training is accompanied by increases in hip strength and changes in lower extremity biomechanics during running. Clin Biomech (Bristol, Avon) 2009;24:26-34. [Crossref] [PubMed]
- Willy RW, Halsey L, Hayek A, et al. Patellofemoral joint and achilles tendon loads during overground and treadmill running. J Orthop Sports Phys Ther 2016;46:664-72. [Crossref] [PubMed]
- Bowersock CD, Willy RW, DeVita P, et al. Reduced step length reduces knee joint contact forces during running following anterior cruciate ligament reconstruction but does not alter inter-limb asymmetry. Clin Biomech (Bristol, Avon) 2017;43:79-85. [Crossref] [PubMed]
- Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med 2011;45:691-6. [Crossref] [PubMed]
- Teng H-L, Powers CM. Sagittal Plane Trunk Posture Influences Patellofemoral Joint Stress During Running. J Orthop Sports Phys Ther 2014;44:785-92. [Crossref] [PubMed]
- Esculier JF, Dubois B, Bouyer LJ, et al. Footwear characteristics are related to running mechanics in runners with patellofemoral pain. Gait Posture 2017;54:144-7. [Crossref] [PubMed]
- Agresta C, Brown A. Gait Retraining for Injured and Healthy Runners Using Augmented Feedback: A Systematic Literature Review. J Orthop Sports Phys Ther 2015;45:576-84. [Crossref] [PubMed]
- Espí-López GV, Serra-Añó P, Vicent-Ferrando J, et al. Effectiveness of Inclusion of Dry Needling in a Multimodal Therapy Program for Patellofemoral Pain: A Randomized Parallel-Group Trial. J Orthop Sports Phys Ther 2017;47:392-401. [Crossref] [PubMed]
- Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med 2016;50:804-8. [Crossref] [PubMed]
- Wellsandt E, Failla MJ, Snyder-Mackler L. Limb Symmetry Indexes Can Overestimate Knee Function After Anterior Cruciate Ligament Injury. J Orthop Sports Phys Ther 2017;47:334-8. [Crossref] [PubMed]
- Matthews M, Rathleff MS, Claus A, et al. Can we predict the outcome for people with patellofemoral pain? A systematic review on prognostic factors and treatment effect modifiers. Br J Sports Med 2017;51:1650-60. [Crossref] [PubMed]
Cite this article as: Capin JJ, Snyder-Mackler L. The current management of patients with patellofemoral pain from the physical therapist’s perspective. Ann Joint 2018;3:40.


