Surgical approaches for total hip arthroplasty
Introduction
Total hip arthroplasty (THA) has been lauded as one of the most successful surgeries since its inception in the 1950s (1,2). The Charnley low-friction arthroplasty revolutionized treatment for disabling hip arthritis (3). Over the decades since, THA has evolved greatly and has proven to be a reliable operation in relieving pain and dysfunction associated with severe hip arthrosis (4-10). Cost-effectiveness for THA for significant disability has been evaluated and found to be cost-saving per episode of hip arthrosis (11). The demand for THA is projected to increase in future decades (12-14).
Surgical approach for THA is an area of interest in the current orthopaedic literature (15-30). The surgeon must have a thorough understanding of the anatomy in order to optimize exposure and implore precise technique to minimize complications and optimize patient outcomes. The most commonly used approaches worldwide for THA include the posterior approach (PA), direct lateral approach (DLA), and the direct anterior approach (DAA) (31). The purpose of this review is to outline the anatomy and technique for each of these approaches while highlighting the differences and similarities in complication profiles and outcomes amongst these three popular approaches.
Posterior approach
The PA is the most commonly used surgical approach for THA worldwide (31). There have been several iterations of the PA since it was first described by von Langenbeck in 1874 (32). The modern-day PA was popularized by Moore in 1957 and referred to as the “Southern” approach (33). As the first modern-day approach, its intent was to limit bone and soft-tissue damage and avoid non-union often seen with the transtrochanteric approach. This approach spares the abductor musculature while providing the opportunity for wide exposure of the acetabulum and femur (34).
The PA is performed with the patient positioned in the lateral decubitus position on a traditional operating room (OR) table. Studies have shown that the pelvis must be stabilized properly when in the lateral decubitus position to avoid “pelvic drift” during the surgery (35,36). Asayama et al. (35) demonstrated that the pelvis frequently shifts 14.5° anteriorly during the operation from placement of an anterior pelvic retractor, pulling the femur anterior during exposure of the acetabulum. If this movement is not appreciated, the acetabular component may be placed in a relative retroversion (36). Firm stabilization of the pelvis is achieved with proper positioning via a peg board or padded hip positioners (32) (Figure 1A). An axillary roll is placed under the contralateral axilla in order to prevent a brachial plexopathy. The limb is then prepped and draped according to surgeon preference. While there is not a wide consensus on the optimal skin preparation, it is thought that a preparatory stick or solution with alcohol may be superior (37). Moreover, when iodine-impregnated, adhesive plastic draping is used, studies have reported a decreased incidence of drape lift off during the procedure when DuraPrep (3M Health Care, Minneapolis, MN, USA) was used as opposed to ChloraPrep (CareFusion, Inc., Leawood, KS, USA) or povidine-iodine scrub and paint (38,39). Lift-off of the adhesive drape facilitates bacterial entry into the wound. Alexander et al. demonstrated that a decreased incidence of drape lift-off during the procedure decreases the risk of surgical site infection six-fold (40).
The length of skin incision with any surgical approach is variable based on patient obesity, severity of joint destruction and stiffness as well as comfort level of the surgeon. Minimal differences in skin incision length have not been associated with clinical outcomes and therefore it is wise for the surgeon to extend the incision if exposure difficulties are encountered. The senior author has utilized incision lengths of 3–6 inches while utilizing the PA. Based on observations of excessive scaring in revision of THAs performed with 3-inch skin incisions, believed secondary to excessive retraction forces, he favors a skin length of 4–5 inches in most cases.
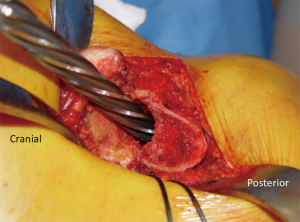
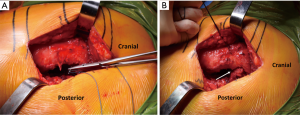
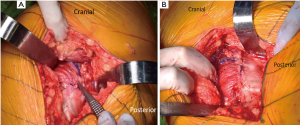
With the hip flexed 60°–70°, a straight, 4–5 inch skin incision is extended from approximately one inch distal to the vastus lateralis tubercle and continued proximally over the greater trochanter, in line with the longitudinal axis of the femoral diaphysis. Moving the incision proximally enhances exposure for femoral canal preparation which shifting it distally facilitates acetabular preparation. The iliotibial band and gluteal fascia overlying the gluteus maximus muscle (GMM) are incised. The GMM is then split longitudinally along the axis of its muscle fibers. A Charnley retractor is placed to retract the GMM for exposure. When placing the posterior arm of the Charnley retractor, the surgeon must be mindful of the sciatic nerve as it runs immediately posterior to the short external rotators (SERs) but might not be visible. The SERs and piriformis tendons are then identified and tenotomized with electrocautery at their insertion on the proximal femur. The capsule is initially incised along the proximal edge of the piriformis and extended to the piriformis insertion into the piriformis recess (Figure 1B,C). This is critical to avoid shortening of the SER tendons due to incising them posterior to their true attachment onto the proximal femur. The incision is then extended distally to complete the SER and posterior capsular release. This results in an “L” shaped SER-posterior capsular flap (Figure 1D). In most cases, the SER release involves the piriformis, superior and inferior gemellus, and obturator internus muscles. If exposure difficulties are encountered, the release can extend distally into the quadratus femoris muscle. In rare cases, partial or complete release of the conjoined tendon of the GMM tendon can be performed to gain adequate mobilization of the proximal femur and acetabular exposure.
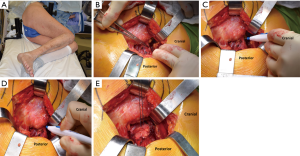
A heavy, non-absorbable suture is used to secure the proximal corner of the posterior capsule and the piriformis and obturator tendons (Figure 1E). Additional SER anchoring sutures can be added if the surgeon desires. Next, the hip is dislocated by an assistant with flexion, adduction, internal rotation, and gentle traction of the leg (Figure 2A). Using a hip hook around the femoral neck is wise during dislocation to reduce torque and possible proximal femoral fracture. If dislocation is difficult, osteophytes around the neck or acetabulum may be removed. In the setting of a severely contracted hip, partial or full release of the GMM insertion, rectus femoris tendon, and/or incision of the inferior capsule may be necessary. An in situ femoral neck osteotomy may also be used to avoid risk of iatrogenic fracture of the femoral neck. This is more frequently seen in the setting of coxa profunda. The neck osteotomy should be carried out using an oscillating or reciprocating saw blade oriented perpendicular to the femoral neck and according to the coronal plane template (Figure 2B,C).
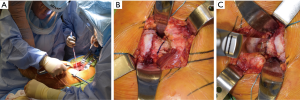
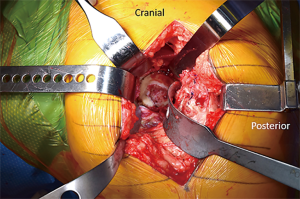
Acetabular exposure is accomplished by anteriorly retracting the femur with a retractor placed over the anterior column at the 2 or 10 o’clock position, based on the hip side being operated on (Figure 3A). To facilitate anterior mobilization of the femur, the tensioned superior capsule is sharply incised (Figure 3B). The posterior joint capsule is retracted using a posterior acetabular retractor or a self-retaining retractor, such as a Charnley peg, placed into the base of the ischium. A sharp, bent retractor or an additional Charnley peg may be used at the 12 o’clock position should a robust gluteus medius muscle be obscuring proper visualization. Lastly, a Hohmann-type of retractor is placed in the region of the transverse acetabular ligament (TAL) to complete acetabular exposure (Figure 3C). Following exposure of the acetabulum, the labrum and pulvinar are sharply dissected. Next, acetabular reaming (Figure 3D) and component placement with proper anteversion and inclination are carried out utilizing landmarks, such as the posterior wall and TAL, as well as orientation of the reamer shaft relative to the floor.
The proximal femur is exposed with an assistant controlling the leg in a flexed, internally rotated, and adducted fashion. A two-pronged retractor is commonly placed along the medial calcar while a Hohmann retractor is positioned at the posterior aspect of the tip of the greater trochanter to retract the gluteal muscles and allow easy access to the femoral medullary canal (Figure 4). Any remaining soft tissue along the saddle of the femoral neck as it confluences with the greater trochanter is remove to facilitate lateralization of the femoral component (FC) during preparation. FC preparation and implantation is then carried out in this position. Following component placement and reconstitution of hip stability, the posterior hip capsule and SERs are repaired in anatomic position through a series of transosseous bone tunnels and/or a transtendinous stitch through the gluteus medius insertion at the piriformis recess (Figure 5). Repair of the posterior capsule and SERs have been shown to decrease post-operative dislocation rates following PA THA (41,42). The fascia lata, GMM, and iliotibial band are then closed with a running, barbed suture or interrupted sutures followed by routine subcutaneous and skin closure.
Direct lateral approach
The DLA is the second most common exposure for THA (31). The modern iteration of the DLA was first described by Hardinge in 1982 (43). This approach allows for sufficient exposure of the acetabulum and femur, while allowing latitude for an extensible exposure of the femur if needed (34). Perhaps the most significant purported benefit of the DLA compared to other popular approaches is a low dislocation rate presumably due to the preservation of the posterior stabilizers of the hip joint (17,44,45).
Similar to the PA, the patient is positioned and padded in the lateral decubitus position. A specialized hip drape, or a sterile bag, is incorporated in the draping process to allow the operative leg to be and hang over the side of the operating table to aid the exposure and maintain sterility during femoral preparation.
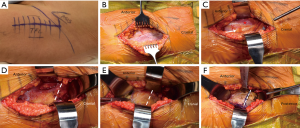
With the hip flexed to 45°, a straight, 4–5 inch skin incision is centered over the greater trochanter. The iliotibial band is incised, centered over the femur, with care taken to not inadvertently cut the gluteus medius muscle. The fascia lata is incised to the proximal extent of the skin incision. Next, a Charnley retractor is placed at the level of the greater trochanter to retract the incised iliotibial band. The greater trochanteric bursa is incised and reflected posteriorly to better visualize the “fan-like” orientation of the gluteus medius muscle fibers (Figure 6A). At the junction of the anterior one-third and posterior two-thirds of the muscle belly, the fibers of the gluteus medius muscle become more vertically oriented. At this junction, the muscle belly is incised sharply along its fibers (Figure 6B). This split is carried down to the greater trochanter, approximately one centimeter distal to its tip. As the split is carried into the tendinous portion of the insertion on the trochanter, it is sharply angled distally along the vastus ridge with care not to disrupt the vastus lateralis. It is critical to leave a cuff of tendon intact to repair the tenotomy at the end of the case. The interval between the gluteus medius and gluteus minimus muscles is then sharply developed (Figure 6C). Next, the assistant controlling the operative extremity will slightly flex, externally rotate and abduct the leg to place tension on the gluteus minimus muscle (Figure 6D). The gluteus minimus insertion is then tenotomized with care to preserve a cuff of tendon on the greater trochanter for later repair. The gluteus minimus tendon is tagged at the proximal and distal corners of the tenotomy. A Meyerding retractor is used to retract the tenotomized gluteus medius and minimus muscles. The joint capsule should be clearly apparent at this point without additional exposure. At this point, surgeons may perform a partial capsulectomy to aid in dislocation of the hip. The capsule is split along the extent of femoral neck carried distal to the vastus ridge. The inferior portion of the capsule is then excised with electrocautery. Finally, the hip is dislocated with flexion, external rotation, and adduction (Figure 6E). Once dislocated, the foot is placed in the sterile pouch on the side of the table opposite the surgeon. A femoral neck osteotomy is next completed with an oscillating or reciprocating saw after placement of cobra retractors on either side of the neck (Figure 6F,G). After the neck cut, the leg is brought back in a resting position on top of the contralateral leg in order to begin acetabular preparation.
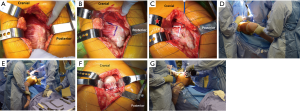
Exposure of the acetabulum begins by placing a wide, bent Hohmann retractor between the labrum and capsule at the 4 o’clock position for a right hip and the 8 o’clock position for a left hip. A curved, wide posterior retractor moves the femur posterior and is placed between the labrum and capsule at the 7 o’clock position for a right hip and at the 5 o’clock position for a left hip (Figure 7). To widen the exposure, tension is placed on the anterior and posterior retractors while the inferior capsule is divided down to the pectineus muscle. Following removal of the labrum, pulvinar, and rim osteophytes, acetabular reaming and component placement is carried out in the standard fashion.
Exposure of the proximal femur begins with removal of the Charnley retractor. The assistant should then hold the operative extremity in flexion, external rotation, and adduction. The foot is placed into the sterile bag opposite the surgeon. A cobra retractor is placed lateral at the greater trochanter and is used to retract the iliotibial band and fascia lata. A femoral neck retractor is placed along the medial calcar, proximal to the lesser trochanter. Finally, a wide, bent retractor is placed posterior to the proximal femur in order retract the intact, posterior two-thirds of the gluteus medius muscle (Figure 8A). Femoral preparation and component placement are then carried out in the standard fashion (Figure 8B). To reduce the reconstructed hip joint, the assistant should apply gentle traction while extending, internally rotating and abduction the hip back to a neutral position. During reduction, the surgeon should guide the head into the acetabulum while ensuring the tenotomized gluteus medius and minimus muscles do not become entrapped.
After reconstruction and a thorough irrigation of the joint, the gluteus minimus and medius tendons are repaired back to anatomic position with several interrupted absorbable stitches (Figure 9A). The split in the gluteus medius muscle is loosely closed with a running braided, absorbable suture while ensuring not to strangle the muscle (Figure 9B). Finally, the fascia lata, iliotibial band, subcutaneous tissue, and skin are closed according to surgeon preference.
Direct anterior approach
The DAA was described by Smith-Peterson (46) in the early 20th century and was subsequently modified in the 1950s by Heuter (47). This approach has gained considerable popularity in the last decade (31,48). Proponents of the DAA cite its intermuscular, internervous plane, low dislocation rate, and earlier functional recovery compared to other popular approaches (15,23,30,48-51). With the approach performed in the supine positioned either on a standard or specialized orthopaedic table, intraoperative fluoroscopy can be used for optimal component positioning (52,53).
A DAA THA begins with positioning the patient supine on either a radiolucent OR table or a specialized traction table, the former of which is preferred at our institution. The pubic symphysis is positioned at the break in the table to allow for lowering of the distal half of the table during femoral exposure. An arm board is attached distally to the OR table on the contralateral side to facilitate abduction of the contralateral leg and abduction of the operative extremity during femoral preparation. Both extremities are prepped and draped using a double leg drape.
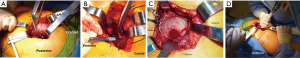
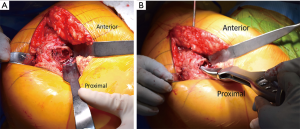
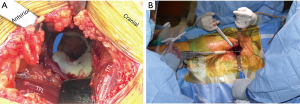
The anterior superior iliac spine (ASIS) is used as reference to mark the DAA incision. Beginning 1-inch lateral to the distal aspect of the ASIS, a 4–5 inch skin incision is carried distally towards the ipsilateral fibular head (Figure 10A). The obliquity of the incision is helpful in not only protecting the tensor fascia lata (TFL) throughout the procedure but also protective of the proximal aspect of the incision during femoral preparation. The incision is carried down to the fascia overlying the TFL (Figure 10B). A fasciotomy is sharply carried out in line with the TFL fibers. Using two Alice clamps on the medial aspect of the fasciotomy, the fascia is the sharply elevated from the TFL. Then using blunt finger dissection, the interval is found between the TFL and sartorius muscles is developed. The lateral femoral cutaneous nerve (LFCN) travels in the facia overlying the sartorius muscle and is protected by dissecting deep to the fascia overlying the TFL. This interval is marked by a “yellow fat-stripe” (Figure 10C). Using two Meyerding retractors, the perforating vessels of the ascending branch of the lateral femoral circumflex artery are identified and centered within the exposure by adjusting the Meyerding retractors. These vessels are the cauterized. The superior aspect of the femoral neck is identified using blunt finger dissection. A cobra retractor is placed along the superior femoral neck in an extracapsular fashion (Figure 10D). A second cobra is placed in an extracapsular fashion along the inferior neck by sweeping away the overlying, pericapsular fat (Figure 10E). Next, the interval between the capsule and the rectus femoris muscle is developed with a Cobb elevator. Once the interval is developed, a sharp, bent Hohmann retractor is placed over the anterior column of the pelvis, in line with femoral neck. Next, an anterior capsulotomy or capsulectomy is carried out followed by moving the cobra retractors to an intracapsular position on either side of the neck (Figure 10F). Using an oscillating or reciprocating saw, a femoral neck osteotomy is performed according to pre-operative templating. With gentle traction and 45° of external rotation of the leg, the femoral head is removed using a corkscrew.
Acetabular preparation begins by placement of the sharp, bent Hohmann retractor in the soft-spot between the labrum and capsule at the 4 o’clock position for a right hip and at the 8 o’clock position for a left hip. The sharp, bent Hohmann retractor remains intact at the 2 o’clock position for a right hip and at the 10 o’clock position for a left hip. Finally, a posterior retractor is placed between the labrum and capsule around the posterior-inferior acetabular wall at the 8 o’clock position for a right hip and 4 o’clock position for the left hip (Figure 11A). The labrum and rim osteophytes are removed circumferentially, followed by the pulvinar. Acetabular reaming and component placement are then carried out with or without the use of intraoperative fluoroscopy (Figure 11B).
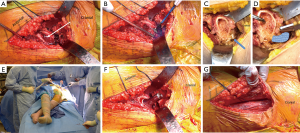
After completing the acetabular reconstruction and removal of acetabular retractors, the assistant controlling the leg will externally rotate the extremity to 90°. The pubofemoral ligament should be released along the medial calcar of the remaining femoral neck. An intramedullary bone hook is placed along the medial calcar. The surgeon should first pull laterally to clear the greater trochanteric from the posterior wall of the acetabulum followed by a lateral-anterior pull vector. The superior capsule is episiotomized from its insertion on the anterior greater trochanter (Figure 12A). If more elevation of the proximal femur is required, the legs may be lowered at the break in the table. Additionally, a rolled stack of sterile towels may be placed underneath the proximal thigh to aid in elevating the femur. If yet more elevation is required, the conjoined tendon, comprised of the obturator internus, superior gemellus, and inferior gemellus tendons, may be released (Figure 12B-D). The obturator externus tendon insertion, which is located more posterior and medial in the piriformis fossa, is critical to posterior hip stability and should be kept intact. As a general rule, elevation of the proximal femur within the wound is considered sufficient once the medial calcar is at the level of the anterior margin of the acetabulum. At this point, still with the bone hook in hand pulling lateral and anterior, the contralateral leg is abducted (or elevated on a padded, sterile Mayo stand), while the operative extremity is adducted and held in 90° of external rotation (Figure 12E). A femoral neck retractor is placed along the medial aspect of the proximal femur, proximal to the lesser, which helps lateralize the proximal femur within the wound. A pronged, greater trochanteric retractor is placed over the lateral greater trochanter between the capsule and gluteus minimus tendon. Routine femoral preparation and component placement is then performed (Figure 12F). After final component placement, the fascia overlying the TFL is closed in an interrupted or running fashion (Figure 12G). Routine subcutaneous tissue and skin closure should be performed according to surgeon preference.
Outcomes
The success of THA is found in consistent, long-term survivorship of 89–94% (4-9,54) and excellent patient satisfaction ranging from 87% to 95% (55-58). In recent years, the optimal approach has been hotly debated. Proponents of the DAA tout its intermuscular and internervous planes, and report evidence for a faster recovery (15,30,49,59), earlier discontinuation of assistive devices (30,50), and more normal gait characteristics (60). However, there is also a plethora of literature detailing a steep learning curve associated with DAA (61-63), higher complication rates (16,64,65), and early failure (17,66,67). While the DAA for THA is strongly marketed as superior to the other approaches (68,69), there is no evidence demonstrating the superiority of any approach beyond 3 months following the procedure (15,20-22,70,71).
Infection is a rare but devastating complication in THA (72). In several large studies, the incidence of prosthetic joint infection (PJI) following THA ranges from 0.2–1.2% (18,73,74). Retrospective studies have shown that there is no difference in rates of PJI between approaches (18,71,75). However, a few retrospective studies have cited greater wound complications with the DAA especially in patients with BMIs over 28 kg/m2 (27,67,76).
Instability following THA is another complication of concern for patients and surgeons. A large evaluation of Medicare patients following elective THA in the United States reports a dislocation of 3.9% (73). However, the current literature suggests that dislocation rates may be related to surgical approach. Masonis and Bourne performed a systematic review and found a dislocation rate of 0.55% and 3.23% for the DLA and PA, respectively (44). In a meta-analysis, Kwon et al. reported dislocation rates 0.43% and 1.01% for the DLA and PA, respectively (45). Higgins et al. performed a recent meta-analysis demonstrating dislocation rates of the DAA (0.3%) compared to the PA (1.2%) (25). In a recent, multi-institution study, Meneghini et al. evaluated the etiologies of 342 revision THAs (17). They reported a revision rate for instability of 11.6% (40/342). They found that the majority of these revisions had the primary procedure performed via the PA (47.5%) or DAA (37.5%) compared to the DLA (15.0%) (P<0.001). A critique of this study is the lack of reporting of primary THAs performed during the study period. Therefore, the authors could not report a true incidence of dislocation stratified by approach. Angerame et al. recently performed a single institution study of nearly 7,000 primary THAs (2,431 DAA; 4,463 PA) and evaluated for early failure as deemed by revision surgery within 5 years of the index procedure (18). The authors reported a rate of THA failure by means of instability of 0.25% (6/2,431) for the DAA and 0.49% (20/4,463) for the PA (P=0.04, OR =2.78, 95% CI: 1.01–7.68). Registry studies have demonstrated similar findings suggesting lower dislocation rates with the DAA and DLA compared to the PA (28,77). Single cohort studies have reported overall dislocations rates of 0.6–1.0% for DAA, 0.3–0.6% for the DLA, and 1.7–5.3% for the PA (29,48,78-84). Despite convincing reports, the literature is not entirely clear on superiority of a single approach as there are reports of insignificant differences in instability rates between approaches (26,71).
The learning curve (50–100 cases) for the DAA has been clearly established in the literature (61-63). Perhaps, the most challenging part of the DAA is the femoral exposure. Difficulties with femoral exposure may result in varus malalignment and, therefore, undersizing of the FC. Furthermore, a difficult exposure and inability to elevate the femur may lead to intraoperative fracture which has been shown to be more prevalent in the DAA (1.0–5.7%) (48,64,71,85-87) but still have been reported the DLA (4.0%) and PA (1.0%) (88,89). Unlike acetabular component aseptic loosening or failed osseointegration, the FC loosening with the DAA has been found to be a significant cause of failure in recent years (17,73,86,90). Meneghini et al. found a higher risk of revision surgery for FC aseptic loosening with the DAA (26.4%, 34/112) and DLA (23.8%, 31/130) compared to the PA (8.4%, 7/83) (P=0.005) (17). Both Angerame et al. and Eto et al. found an increased risk of revision surgery for FC loosening with the DAA compared the PA (18,90).
Abductor muscle insufficiency is common in the immediate post-operative period following the DLA. As the gluteus medius and minimus are partially incised and repaired during the procedure, abductor insufficiency may result and is manifested as muscle weakness, a Trendelenburg gait or sign, pain, or abnormal gait mechanics (44). At the authors’ institution, patients are able to weight-bear as tolerated following a DLA THA but are restricted for the first six weeks with no active abduction. Abductor insufficiency, however, may persist in 4–20% according to a systemic review by Masonis and Bourne (44). A meticulous, anatomic repair of the abductors at the end of the procedure is critical to avoiding abductor insufficiency. While more prevalent with the DLA, abductor insufficiency may be seen with either DAA or PAs (91).
Nerve injury is a devastating complication following THA, and, although infrequent, may be debilitating for patients. Depending on the surgical exposure, the LFCN, the superior gluteal nerve (SGN), the femoral nerve, and the sciatic nerve are the nerves most at risk during THA. The LFCN is at highest risk for injury with superficial dissection during the DAA given that it runs on top of the fascia overlying the sartorius muscle (92,93). The femoral nerve and SGN are may also be injured during the DAA, however, given their proximities to the exposure, at a less frequent rate (94). The SGN is most often injured during the DLA with reported rates ranging from 2.2% to 42.5% (95-98). Jacobs and Buxton defined a “safe-area” of 5 cm proximal to the tip of the greater trochanter where dissection through the gluteus medius muscle is considered safe from SGN injury (99). With its proximity to the posterior aspect of the hip joint, sciatic nerve injury has been shown to be higher in the PA (100). While more debilitating than LFCN or SGN injuries, femoral and sciatic nerve injuries are less common and occur at rates of 0.0–2.3% and 0.1–0.7%, respectively (98,100-102).
Conclusions
A number of surgical approaches may be used to perform THA. The DAA, DLA, and PA are the most common approaches used today. As discussed in this review, there is not a consensus on the most optimal approach as each exposure has a unique set of advantages, disadvantages, and risks. Currently, there is a paucity of high-quality comparisons of these approaches. Surgeons should select their optimal surgical exposure based on their comfort, anatomical familiarity, and experience with a given approach.
Acknowledgments
The authors would like to acknowledge Rose Johnson for her contributions in the preparation of this article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Charlie C. Yang) for the series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.04.08). The series “Direct Anterior Approach (DAA) for Total Hip Arthroplasty (THA)” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:1508-19. [Crossref] [PubMed]
- Charnley J. The Long-Term Results of Low-Friction Arthroplasty of the Hip Performed as a Primary Intervention. Clin Orthop Relat Res 2005;3-11; discussion 12. [PubMed]
- Charnley J. Total hip replacement by low-friction arthroplasty. Clin Orthop Relat Res 1970;7-21. [PubMed]
- Berger RA, Kull LR, Rosenberg AG, et al. Hybrid total hip arthroplasty: 7- to 10-year results. Clin Orthop Relat Res 1996;134-46. [PubMed]
- Engh CA Jr, Culpepper WJ 2nd, Engh CA. Long-term results of use of the anatomic medullary locking prosthesis in total hip arthroplasty. J Bone Joint Surg Am 1997;79:177-84. [Crossref] [PubMed]
- Engh CA, Hopper RH Jr. The odyssey of porous-coated fixation. J Arthroplasty 2002;17:102-7. [Crossref] [PubMed]
- Madey SM, Callaghan JJ, Olejniczak JP, et al. Charnley total hip arthroplasty with use of improved techniques of cementing. The results after a minimum of fifteen years of follow-up. J Bone Joint Surg Am 1997;79:53-64. [Crossref] [PubMed]
- Espehaug B, Furnes O, Engesæter LB, et al. 18 years of results with cemented primary hip prostheses in the Norwegian Arthroplasty Register. Acta Orthop 2009;80:402-12. [Crossref] [PubMed]
- Mäkelä KT, Matilainen M, Pulkkinen P, et al. Countrywise results of total hip replacement. Acta Orthop 2014;85:107-16. [Crossref] [PubMed]
- Hailer NP, Garellick G, Kärrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop 2010;81:34-41. [Crossref] [PubMed]
- Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA 1996;275:858-65. [Crossref] [PubMed]
- Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: preparing for an epidemic. J Bone Joint Surg Am 2008;90:1598-605. [Crossref] [PubMed]
- Kurtz SM, Ong KL, Schmier J, et al. Future Clinical and Economic Impact of Revision Total Hip and Knee Arthroplasty. J Bone Joint Surg Am 2007;89:144-51. [PubMed]
- Johnsen SP, Sørensen HT, Lucht U, et al. Patient-related predictors of implant failure after primary total hip replacement in the initial, short- and long-terms. A nationwide Danish follow-up study including 36,984 patients. J Bone Joint Surg Br 2006;88:1303-8. [Crossref] [PubMed]
- Taunton MJ, Trousdale RT, Sierra RJ, et al. John Charnley Award: Randomized Clinical Trial of Direct Anterior and Miniposterior Approach THA: Which Provides Better Functional Recovery? Clin Orthop Relat Res 2018;476:216-29. [Crossref] [PubMed]
- Poehling-Monaghan KL, Kamath AF, Taunton MJ, et al. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res 2015;473:623-31. [Crossref] [PubMed]
- Meneghini RM, Elston AS, Chen AF, et al. Direct Anterior Approach: Risk Factor for Early Femoral Failure of Cementless Total Hip Arthroplasty. J Bone Joint Surg Am 2017;99:99-105. [Crossref] [PubMed]
- Angerame MR, Fehring TK, Masonis JL, et al. Early Failure of Primary Total Hip Arthroplasty: Is Surgical Approach a Risk Factor? J Arthroplasty 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Abe H, Sakai T, Takao M, et al. Difference in Stem Alignment Between the Direct Anterior Approach and the Posterolateral Approach in Total Hip Arthroplasty. J Arthroplasty 2015;30:1761-6. [Crossref] [PubMed]
- Alecci V, Valente M, Crucil M, et al. Comparison of primary total hip replacements performed with a direct anterior approach versus the standard lateral approach: perioperative findings. J Orthop Traumatol 2011;12:123-9. [Crossref] [PubMed]
- Barrett WP, Turner SE, Leopold JP. Prospective Randomized Study of Direct Anterior vs Postero-Lateral Approach for Total Hip Arthroplasty. J Arthroplasty 2013;28:1634-8. [Crossref] [PubMed]
- Cheng TE, Wallis JA, Taylor NF, et al. A Prospective Randomized Clinical Trial in Total Hip Arthroplasty-Comparing Early Results Between the Direct Anterior Approach and the Posterior Approach. J Arthroplasty 2017;32:883-90. [Crossref] [PubMed]
- Graves SC, Dropkin BM, Keeney BJ, et al. Does Surgical Approach Affect Patient-reported Function After Primary THA? Clin Orthop Relat Res 2016;474:971-81. [Crossref] [PubMed]
- Hamilton WG, Parks NL, Huynh C. Comparison of Cup Alignment, Jump Distance, and Complications in Consecutive Series of Anterior Approach and Posterior Approach Total Hip Arthroplasty. J Arthroplasty 2015;30:1959-62. [Crossref] [PubMed]
- Higgins BT, Barlow DR, Heagerty NE, et al. Anterior vs. Posterior Approach for Total Hip Arthroplasty, a Systematic Review and Meta-analysis. J Arthroplasty 2015;30:419-34. [Crossref] [PubMed]
- Maratt JD, Gagnier JJ, Butler PD, et al. No Difference in Dislocation Seen in Anterior Vs Posterior Approach Total Hip Arthroplasty. J Arthroplasty 2016;31:127-30. [Crossref] [PubMed]
- Purcell RL, Parks NL, Cody JP, et al. Comparison of Wound Complications and Deep Infections With Direct Anterior and Posterior Approaches in Obese Hip Arthroplasty Patients. J Arthroplasty 2018;33:220-3. [Crossref] [PubMed]
- Sheth D, Cafri G, Inacio MC, et al. Anterior and Anterolateral Approaches for THA Are Associated With Lower Dislocation Risk Without Higher Revision Risk. Clin Orthop Relat Res 2015;473:3401-8. [Crossref] [PubMed]
- Tripuraneni KR, Munson NR, Archibeck MJ, et al. Acetabular Abduction and Dislocations in Direct Anterior vs Posterior Total Hip Arthroplasty: A Retrospective, Matched Cohort Study. J Arthroplasty 2016;31:2299-302. [Crossref] [PubMed]
- Zawadsky MW, Paulus MC, Murray PJ, et al. Early Outcome Comparison Between the Direct Anterior Approach and the Mini-Incision Posterior Approach for Primary Total Hip Arthroplasty: 150 Consecutive Cases. J Arthroplasty 2014;29:1256-60. [Crossref] [PubMed]
- Chechik O, Khashan M, Lador R, et al. Surgical approach and prosthesis fixation in hip arthroplasty world wide. Arch Orthop Trauma Surg 2013;133:1595-600. [Crossref] [PubMed]
- Moretti VM, Post ZD. Surgical approaches for total hip arthroplasty. Indian J Orthop 2017;51:368-76. [Crossref] [PubMed]
- Moore AT. The self-locking metal hip prosthesis. J Bone Joint Surg Am 1957;39-A:811-27. [Crossref] [PubMed]
- Hoppenfeld S, DeBoer P, Buckley R. Surgical exposures in orthopaedics: the anatomic approach, Fourth edition. Philadelphia, PA: Lippincott Williams and Wilkins, 2009.
- Asayama I, Akiyoshi Y, Naito M, et al. Intraoperative pelvic motion in total hip arthroplasty. J Arthroplasty 2004;19:992-7. [Crossref] [PubMed]
- Schwarzkopf R, Muir JM, Paprosky WG, et al. Quantifying Pelvic Motion During Total Hip Arthroplasty Using a New Surgical Navigation Device. J Arthroplasty 2017;32:3056-60. [Crossref] [PubMed]
- Hemani ML, Lepor H. Skin preparation for the prevention of surgical site infection: which agent is best? Rev Urol 2009;11:190-5. [PubMed]
- Jacobson C, Osmon DR, Hanssen A, et al. Prevention of wound contamination using DuraPrep solution plus Ioban 2 drapes. Clin Orthop Relat Res 2005;32-7. [Crossref] [PubMed]
- Grove GL, Eyberg CI. Comparison of Two Preoperative Skin Antiseptic Preparations and Resultant Surgical Incise Drape Adhesion to Skin in Healthy Volunteers. J Bone Joint Surg Am 2012;94:1187-92. [Crossref] [PubMed]
- Alexander JW, Aerni S, Plettner JP. Development of a safe and effective one-minute preoperative skin preparation. Arch Surg 1985;120:1357-61. [Crossref] [PubMed]
- Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop Relat Res 1998;224-8. [Crossref] [PubMed]
- Tsai SJ, Wang CT, Jiang CC. The effect of posterior capsule repair upon post-operative hip dislocation following primary total hip arthroplasty. BMC Musculoskelet Disord 2008;9:29. [Crossref] [PubMed]
- Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg Br 1982;64:17-9. [Crossref] [PubMed]
- Masonis JL, Bourne RB. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop Relat Res 2002;46-53. [Crossref] [PubMed]
- Kwon MS, Kuskowski M, Mulhall KJ, et al. Does Surgical Approach Affect Total Hip Arthroplasty Dislocation Rates? Clin Orthop Relat Res 2006;34-8. [Crossref] [PubMed]
- Smith-Peterson MN. A new supra-articular subper-iosteal approach to the hip joint. J Bone Joint Surg Am 1917;15:592-5.
- Light TR, Keggi KJ. Anterior approach to hip arthroplasty. Clin Orthop Relat Res 1980;255-60. [PubMed]
- Matta JM, Shahrdar C, Ferguson T. Single-incision Anterior Approach for Total Hip Arthroplasty on an Orthopaedic Table. Clin Orthop Relat Res 2005;115-24. [Crossref] [PubMed]
- Rodriguez JA, Deshmukh AJ, Rathod PA, et al. Does the direct anterior approach in THA offer faster rehabilitation and comparable safety to the posterior approach? Clin Orthop Relat Res 2014;472:455-63. [Crossref] [PubMed]
- Taunton MJ, Mason JB, Odum SM, et al. Direct Anterior Total Hip Arthroplasty Yields More Rapid Voluntary Cessation of All Walking Aids: A Prospective, Randomized Clinical Trial. J Arthroplasty 2014;29:169-72. [Crossref] [PubMed]
- De Geest T, Vansintjan P, De Loore G. Direct anterior total hip arthroplasty: complications and early outcome in a series of 300 cases. Acta Orthop Belg 2013;79:166-73. [PubMed]
- Goodman GP, Goyal N, Parks NL, et al. Intraoperative fluoroscopy with a direct anterior approach reduces variation in acetabular cup abduction angle. Hip Int 2017;27:573-77. [Crossref] [PubMed]
- Gosthe RG, Suarez JC, McNamara CA, et al. Fluoroscopically Guided Acetabular Component Positioning: Does It Reduce the Risk of Malpositioning in Obese Patients? J Arthroplasty 2017;32:3052-5. [Crossref] [PubMed]
- Hailer NP, Weiss RJ, Stark A, et al. The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis. Acta Orthop 2012;83:442-8. [Crossref] [PubMed]
- Mancuso CA, Jout J, Salvati EA, et al. Fulfillment of Patientsʼ Expectations for Total Hip Arthroplasty. J Bone Joint Surg Am 2009;91:2073-8. [Crossref] [PubMed]
- Naal FD, Impellizzeri FM, Lenze U, et al. Clinical improvement and satisfaction after total joint replacement: a prospective 12-month evaluation on the patients’ perspective. Qual Life Res 2015;24:2917-25. [Crossref] [PubMed]
- Mancuso CA. Impact of new guidelines on physicians' ordering of preoperative tests. J Gen Intern Med 1999;14:166-72. [Crossref] [PubMed]
- Specht K, Kjaersgaard-Andersen P, Kehlet H, et al. High patient satisfaction in 445 patients who underwent fast-track hip or knee replacement. Acta Orthop 2015;86:702-7. [Crossref] [PubMed]
- Parvizi J, Restrepo C, Maltenfort MG. Total Hip Arthroplasty Performed Through Direct Anterior Approach Provides Superior Early Outcome: Results of a Randomized, Prospective Study. Orthop Clin North Am 2016;47:497-504. [Crossref] [PubMed]
- Mayr E, Nogler M, Benedetti MG, et al. A prospective randomized assessment of earlier functional recovery in THA patients treated by minimally invasive direct anterior approach: a gait analysis study. Clin Biomech (Bristol, Avon) 2009;24:812-8. [Crossref] [PubMed]
- Goytia RN, Jones LC, Hungerford MW. Learning Curve for the Anterior Approach Total Hip Arthroplasty. J Surg Orthop Adv 2012;21:78-83. [Crossref] [PubMed]
- de Steiger RN, Lorimer M, Solomon M. What is the learning curve for the anterior approach for total hip arthroplasty? Clin Orthop Relat Res 2015;473:3860-6. [Crossref] [PubMed]
- Seng BE, Berend KR, Ajluni AF, et al. Anterior-supine minimally invasive total hip arthroplasty: defining the learning curve. Orthop Clin North Am 2009;40:343-50. [Crossref] [PubMed]
- Homma Y, Baba T, Ochi H, et al. Greater trochanter chip fractures in the direct anterior approach for total hip arthroplasty. Eur J Orthop Surg Traumatol 2016;26:605-11. [Crossref] [PubMed]
- Spaans AJ, van den Hout JA, Bolder SB. High complication rate in the early experience of minimally invasive total hip arthroplasty by the direct anterior approach. Acta Orthop 2012;83:342-6. [Crossref] [PubMed]
- Graw BP, Woolson ST, Huddleston HG, et al. Minimal Incision Surgery as a Risk Factor for Early Failure of Total Hip Arthroplasty. Clin Orthop Relat Res 2010;468:2372-6. [Crossref] [PubMed]
- Christensen CP, Karthikeyan T, Jacobs CA. Greater Prevalence of Wound Complications Requiring Reoperation With Direct Anterior Approach Total Hip Arthroplasty. J Arthroplasty 2014;29:1839-41. [Crossref] [PubMed]
- Mohan R, Yi PH, Hansen EN. Evaluating online information regarding the direct anterior approach for total hip arthroplasty. J Arthroplasty 2015;30:803-7. [Crossref] [PubMed]
- Trousdale WH, Taunton MJ, Mabry TM, et al. Patient Perceptions of the Direct Anterior Hip Arthroplasty. J Arthroplasty 2017;32:1164-70. [Crossref] [PubMed]
- Restrepo C, Parvizi J, Pour AE, et al. Prospective Randomized Study of Two Surgical Approaches for Total Hip Arthroplasty. J Arthroplasty 2010;25:671-9.e1. [Crossref] [PubMed]
- Malek IA, Royce G, Bhatti SU, et al. A comparison between the direct anterior and posterior approaches for total hip arthroplasty: the role of an “Enhanced Recovery” pathway. Bone Joint J 2016;98-B:754-60. [Crossref] [PubMed]
- Zmistowski B, Karam JA, Durinka JB, et al. Periprosthetic Joint Infection Increases the Risk of One-Year Mortality. J Bone Joint Surg Am 2013;95:2177-84. [Crossref] [PubMed]
- Phillips CB, Barrett JA, Losina E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am 2003;85-A:20-6. [Crossref] [PubMed]
- Pulido L, Ghanem E, Joshi A, et al. Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors. Clin Orthop Relat Res 2008;466:1710-5. [Crossref] [PubMed]
- Namba RS, Inacio MCS, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br 2012;94:1330-8. [Crossref] [PubMed]
- Jahng KH, Bas MA, Rodriguez JA, et al. Risk Factors for Wound Complications After Direct Anterior Approach Hip Arthroplasty. J Arthroplasty 2016;31:2583-7. [Crossref] [PubMed]
- Mjaaland KE, Svenningsen S, Fenstad AM, et al. Implant Survival After Minimally Invasive Anterior or Anterolateral Vs. Conventional Posterior or Direct Lateral Approach. J Bone Joint Surg Am 2017;99:840-7. [Crossref] [PubMed]
- Siguier T, Siguier M, Brumpt B. Mini-incision Anterior Approach Does Not Increase Dislocation Rate. Clin Orthop Relat Res 2004;164-73. [Crossref] [PubMed]
- Mulliken BD, Rorabeck CH, Bourne RB, et al. A modified direct lateral approach in total hip arthroplasty: a comprehensive review. Journal of Arthroplasty 1998;13:737-47. [Crossref] [PubMed]
- Demos HA, Rorabeck CH, Bourne RB, et al. Instability in primary total hip arthroplasty with the direct lateral approach. Clin Orthop Relat Res 2001;168-80. [Crossref] [PubMed]
- White RE, Forness TJ, Allman JK, et al. Effect of posterior capsular repair on early dislocation in primary total hip replacement. Clin Orthop Relat Res 2001;163-7. [Crossref] [PubMed]
- Sierra RJ, Raposo JM, Trousdale RT, et al. Dislocation of Primary THA Done through a Posterolateral Approach in the Elderly. Clin Orthop Relat Res 2005;262-7. [Crossref] [PubMed]
- Khan RJK, Yao F, Li M, et al. Capsular-Enhanced Repair of the Short External Rotators After Total Hip Arthroplasty. J Arthroplasty 2007;22:840-3. [Crossref] [PubMed]
- Goldstein WM, Gleason TF, Kopplin M, et al. Prevalence of dislocation after total hip arthroplasty through a posterolateral approach with partial capsulotomy and capsulorrhaphy. J Bone Joint Surg Am 2001;83-A:2-7. [Crossref] [PubMed]
- Berend KR, Mirza AJ, Morris MJ, et al. Risk of Periprosthetic Fractures With Direct Anterior Primary Total Hip Arthroplasty. J Arthroplasty 2016;31:2295-8. [Crossref] [PubMed]
- Woolson ST, Pouliot MA, Huddleston JI. Primary total hip arthroplasty using an anterior approach and a fracture table: short-term results from a community hospital. J Arthroplasty 2009;24:999-1005. [Crossref] [PubMed]
- Jewett BA, Collis DK. High Complication Rate With Anterior Total Hip Arthroplasties on a Fracture Table. Clin Orthop Relat Res 2011;469:503-7. [Crossref] [PubMed]
- Nakata K, Nishikawa M, Yamamoto K, et al. A clinical comparative study of the direct anterior with mini-posterior approach: two consecutive series. J Arthroplasty 2009;24:698-704. [Crossref] [PubMed]
- Hendel D, Yasin M, Garti A, et al. Fracture of the greater trochanter during hip replacement. Acta Orthop Scand 2002;73:295-7. [Crossref] [PubMed]
- Eto S, Hwang K, Huddleston JI, et al. The Direct Anterior Approach is Associated With Early Revision Total Hip Arthroplasty. J Arthroplasty 2017;32:1001-5. [Crossref] [PubMed]
- Kiyama T, Naito M, Shinoda T, et al. Hip abductor strengths after total hip arthroplasty via the lateral and posterolateral approaches. J Arthroplasty 2010;25:76-80. [Crossref] [PubMed]
- Goulding K, Beaulé PE, Kim PR, et al. Incidence of Lateral Femoral Cutaneous Nerve Neuropraxia After Anterior Approach Hip Arthroplasty. Clin Orthop Relat Res 2010;468:2397-404. [Crossref] [PubMed]
- Bhargava T, Goytia RN, Jones LC, et al. Lateral Femoral Cutaneous Nerve Impairment After Direct Anterior Approach for Total Hip Arthroplasty. Orthopedics 2010;33:472. [PubMed]
- Grob K, Manestar M, Ackland T, et al. Potential Risk to the Superior Gluteal Nerve During the Anterior Approach to the Hip Joint: An Anatomical Study. J Bone Joint Surg Am 2015;97:1426-31. [Crossref] [PubMed]
- Khan T, Knowles D. Damage to the Superior Gluteal Nerve During the Direct Lateral Approach to the Hip. J Arthroplasty 2007;22:1198-200. [Crossref] [PubMed]
- Picado CH, Garcia FV, Marques W Jr. Damage to the Superior Gluteal Nerve after Direct Lateral Approach to the Hip. Clin Orthop Relat Res 2007;209-11. [Crossref] [PubMed]
- Ramesh M, O'Byrne JM, McCarthy N, et al. Damage to the superior gluteal nerve after the Hardinge approach to the hip. J Bone Joint Surg Br 1996;78:903-6. [Crossref] [PubMed]
- Oldenburg M, Müller RT. The frequency, prognosis and significance of nerve injuries in total hip arthroplasty. Int Orthop 1997;21:1-3. [Crossref] [PubMed]
- Jacobs LG, Buxton RA. The course of the superior gluteal nerve in the lateral approach to the hip. J Bone Joint Surg Am 1989;71:1239-43. [Crossref] [PubMed]
- Farrell CM, Springer BD, Haidukewych GJ, et al. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am 2005;87:2619-25. [Crossref] [PubMed]
- Simmons C, Izant TH, Rothman RH, et al. Femoral neuropathy following total hip arthroplasty. Anatomic study, case reports, and literature review. J Arthroplasty 1991;6:S57-66. [Crossref] [PubMed]
- Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. J Bone Joint Surg Am 1991;73:1074-80. [Crossref] [PubMed]
Cite this article as: Angerame MR, Dennis DA. Surgical approaches for total hip arthroplasty. Ann Joint 2018;3:43.