Chondral and osteochondral lesions in the patellofemoral joint: when and how to manage
Introduction
Successful treatment of chondral and osteochondral injury in the patellofemoral (PF) joint remains a clinical challenge. The etiology of symptomatic chondral/osteochondral pathology is complex and often multifactorial. Diverse causes include, but are not limited to, traumatic impaction, PF instability events, repetitive microtrauma and/or chronic overload in the setting of malalignment and/or obesity, and osteochondritis dissecans (OCD) lesions. To select an appropriate non-operative or operative treatment strategy, the surgeon must have a comprehensive understanding of all patient specific, lesion specific, and joint/limb specific variables. If cartilage restoration is indicated, optimization of joint biomechanics through concomitant bony and/or soft tissue procedures will maximize the opportunity for a good outcome. This paper will focus on the evaluation and treatment of symptomatic chondral/osteochondral lesions in the PF joint. Our goal is to aid the surgeon in their decision making regarding when and how to manage these lesions.
Epidemiology
Chondral and osteochondral defects in the PF compartment are often encountered in clinical practice on advanced imaging studies and/or during arthroscopy. A review of a Polish registry found that more than half of patients undergoing knee arthroscopy had chondral defects, with 5.2% having Outerbridge Grade III or IV lesions. Of these, 37.5% were in the patella alone (1). Curl et al., in a review of 31,516 knee arthroscopies, found over 53,000 hyaline cartilage lesions in over 19,000 patients; most of the lesions found were actually grade III defects in the patella (2). In professional athletes, two series of knee magnetic resonance imaging (MRI) in asymptomatic professional basketball players revealed an incidence of abnormal chondral signal in 57% of all players, with 35% having high grade patella signal and 25% with high grade trochlea signal (3,4). The key point to recognize is that the vast majority of chondral/osteochondral lesions are asymptomatic and should be observed, but not aggressively treated. While indications continue to evolve, there is no clear role for prophylactic cartilage restoration in the setting of asymptomatic lesions. In contrast, large and/or full thickness lesions with localizing symptoms (that have failed non-surgical optimization), especially in the setting of obvious pathoanatomy and aberrant biomechanics, require treatment of both the lesion and underlying co-morbidity.
Clinical evaluation
Patients do not initially present complaining of a chondral lesion. Their chief complaints are pain with PF loading, swelling, and a subset have patellar instability. It is the physician who must be suspicious that the symptoms may be in part due to PF chondral lesion(s). As with all patient problems, the first step is a comprehensive history. Care should be taken to elucidate whether complaints are primarily pain or instability, and if pain, localization is critical. Global pain is a red flag for debilitation, overuse, and variants of complex regional pain syndrome or secondary gain. Cartilage restoration is not part of these patients’ treatment. Activities and their relation to pain should also be investigated. Patients should be queried for the presence of activity related effusions and/or mechanical symptoms of catching or locking. The overall mental state of the patient should also be assessed. It has been shown that patients with a positive outlook and mindset about life as measured by Short Form 36 (SF-36) tend to have better outcomes (5). Documenting prior treatments and the response to them is essential to understanding the underlying pathology. Documenting family history of ligamentous laxity or any musculoskeletal disorders is important.
The physical exam should begin with observation of the patient’s gait and limb alignment, followed by stepping up and down evaluating both the arc of pain and any pelvic drop (indicative of hip/pelvis weakness) or dynamic valgus positioning. Seated evaluation is useful for patella height, Q-angle estimate, assessment of patella tracking via J-sign, and crepitus through a ROM arc. Quadriceps lag based on pain, apprehension, or weakness should be noted. Supine evaluation can localize tenderness to palpation, effusion, ROM, muscle bulk and tone, and soft tissue balance/ligament integrity. Patella mobility, as described by the quadrant method, should be documented and compared to the contralateral side (6,7). Medial to lateral mobility is tested at 0, 30, and 60 degrees in Fairbank’s position (the patient is in the supine position with the leg abducted; lateral pressure is applied to the patella as the knee is flexed) to assess the competence of medial soft tissue restraints (i.e., MPFL, MPTL). Lateral retinacular tightness, fixed patella tilt, or iatrogenic or idiopathic lateral retinacular incompetence (i.e., hyperlaxity, prior lateral release) are assessed on the lateral side of the knee. Prone examination allows for evaluation of femoral/hip anteversion, tibial torsion, and thigh-foot axis.
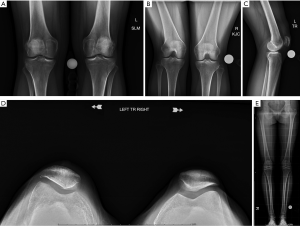
Standard initial radiographs include bilateral comparison weight bearing anteroposterior, PA flexion (45° PA), 30° flexion true lateral, and low flexion angle axial views such as Merchants (Figure 1) (8). A hip-to-ankle view allows measurement of coronal plane mechanical axis alignment (i.e., varus, valgus deformity), which may play a role in PF tracking. AP and PA flexion views assess the tibiofemoral joint space. Axial and lateral views help assess patellar height and morphologic features such as trochlear dysplasia (i.e., crossing sign, supratrochlear spur, double contour) and patella tilt. Caton-Deschamps or Blackburne-Peel ratios are the current patellar height measurements of choice as they change with tuberosity surgical movement unlike the Insall-Salvati (Figure 2).
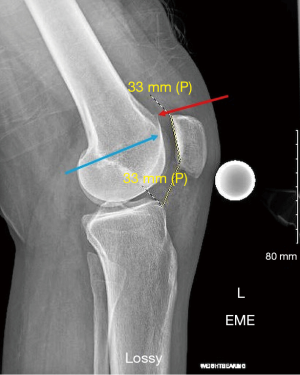
Advanced imaging typically includes MRI and/or CT scans. Standard sequences on 1.5T or 3T MRI are typically sufficient; however, cartilage specific sequences or specialized techniques may be useful adjuncts in specific challenging situations. MR arthrogram is rarely additive, but CT arthrogram can be useful in the assessment of OCD lesion stability. It is important to recognize that measurement of key alignment parameters vary from MRI to CT (9). In addition to comprehensive evaluation of the PF joint, MRI gives knowledge on the status of the medial/lateral compartments, other ligaments, menisci, and helps rule out other pathology (i.e., tumors, avascular necrosis, tendinopathy). MRI is useful to investigate the status of the underlying subchondral bone. The presence or absence of bone marrow lesions (BMLs) suggests bone overload through malalignment and/or loss of chondral protection. Specific to the PF joint, MRI gives a plethora of information including soft tissue competence, chondral status, trochlear morphology, presence of loose bodies, acuity of injury (i.e., bone bruise pattern), and alignment parameters in multiple planes. A portion of each image read is the tibial tubercle-trochlear groove (TT-TG) distance (which is not possible with a flat or convex trochlea) and the tibial tubercle-posterior cruciate ligament (TT-PCL), which is possible even with trochlear dysplasia (Figure 3). Noyes reported the method for using MRI cuts through the hip, knee, and ankle for measuring femoral anteversion and tibial torsion while avoiding the ionizing radiation of CT.
While knowledge of the average and recommended threshold values for each imaging measurement is a useful “starting place,” no one value should be relied upon for clinical decision making. For example, several studies have shown that the TT-TG measurement has significant variation and several limitations, especially in the setting of trochlear dysplasia (10,11). Also, MRI typically underestimates the TT-TG compared to computerized tomography (CT) (9). Applying the patient’s specific parameters to published normative and pathologic values will aid the surgeon in understanding the pathology and formulating a rational plan. That is, the treatment plan is made for an individual patient, not specific numbers.
Conservative treatment
Non-operative management is preferred for the vast majority of chondral/osteochondral lesions in the PF joint. Following acute injury or in the presence of chronic, insidious symptoms, activity modification is important to rest the joint and to avoid further harm from overload. Medical and/or biologic injections may be helpful, when indicated, to reduce pain so that the patient may rehabilitate successfully. Nonsteroidal anti-inflammatory drugs medications, Tramadol, and/or Tylenol can be useful agents; narcotic medications are not indicated for the non-operative management of PF chondral/osteochondral disease in most cases although tramadol can be used in certain cases for short periods of time. Alternative modalities may be considered for a multi-modal approach to pain management (i.e., transcutaneous electrical nerve stimulation, localized creams, patches, injections). If corticosteroid injections are indicated for acute/subacute flair (i.e., painful effusion), they should be used sparingly, especially in younger patients, due to concerns over deleterious effects on articular cartilage over time (12). Several studies have shown that local anesthetics (i.e., 1% Lidocaine) are chondrotoxic and should be avoided. A corticosteroid such as Kenalog, however, has demonstrated no significant negative effect on chondrocyte viability, and may be utilized in a saline vehicle. Efficacy of viscosupplementation remains controversial, but it is safe for articular cartilage and can be considered (13,14). It is critical to recognize that not all viscosupplements are the same. In general, we prefer higher molecular weight hyaluronic acid preparations formed through bacterial fermentation, which have demonstrated efficacy versus other agents. Injection of platelet rich plasma has gained interest with a growing body of literature support. This has shown promise, especially with leukocyte poor formulations, but definitive evidence is still lacking (15-19). Other injection agents (i.e., amniotic fluid/cells) are similarly promising, but expensive and continue to be under clinical investigation.
Rehabilitation is the key component to any plan for the PF joint. A comprehensive “core-to-floor” rehabilitation plan should be undertaken to correct any underlying muscular weakness and/or neuromuscular imbalance (20). Physical therapy, focusing not only on quadriceps strengthening, but also paying attention to the core and posterior chain musculature (i.e., gluteus, hip external rotators, hamstring), should be the first line treatment. Hamstring:quadriceps ratio should be optimized to reduce loads on the knee joint during joint activity. Proprioception and flexibility training can also help to improve symptoms. There is limited evidence for or against patellar stabilization braces or compression sleeves. Braces may be useful after acute patella dislocation and/or surgery and sleeves may help with proprioception and swelling during the rehabilitation process (21).
Surgical indications
In general, non-surgical treatment is the rule for the majority of PF conditions, including chondral/osteochondral lesions. Surgery should only be considered if patients have persistent or worsening symptoms despite improvements in dynamic strength, flexibility, and neuromuscular status (8). Early surgical intervention is recommended for acute chondral/osteochondral injury with displaced fragment, which often occurs following patella dislocation or traumatic impaction injury (21,22). Early surgery is similarly warranted for patients with symptomatic, unstable OCD lesions. Good results have been shown even in fixation of purely chondral fragments (23). Surgical indications for patella instability follow current recommended guidelines (i.e., risk factor stratification for first time dislocators, surgery for recurrent dislocators, or patients with subluxation events or habitual/fixed dislocation that have failed conservative treatment). The need for treatment of concomitant cartilage lesions during patella instability surgery will be discussed in a later section.
Otherwise, for the majority of cases of PF chondral/osteochondral lesion(s) and/or underlying malalignment, failure of up to 6 months of conservative treatment is warranted. In these patients with persistent painful effusion and localizing mechanical symptoms effecting daily life and quality of life, surgery may be considered. A thorough pre-operative conversation should be undertaken with the patient as to realistic expectations for the final outcome possibilities as there is always a range from excellent to frank failure. Unlike PF conditions that may likely lead to a “normal” outcome with return to unrestricted activity (i.e., isolated MPFL reconstruction), cartilage repair patients often have complex and multifactorial presentation. Success for cartilage restoration patients often includes reduction of pain, improvement in ADLs, ability to return to occupation, and low impact sporting activities. Secondary goals over time may include return to full sports, but this is often not expected in the majority of patients and never promised. Nonetheless, the majority of well-selected surgical patients improve with treatment, but likely have some sort of permanent activity restriction (24).
Risk factor modification is critical prior to surgery. Patient specific factors such as body mass index (BMI), tobacco use, chronic pain management, and diabetes may affect the overall outcome and should be addressed prior to recommending surgery. Smoking certainly is detrimental to any surgical procedure; studies to date have been unclear as to the exact outcome of nicotine on chondral restorative procedures. However, many surgeons view the use of tobacco as a contraindication to cartilage restoration (25). BMIs above 35 may also result in early failures (26).
Concomitant procedures
When addressing chondral pathology, all underlying abnormal biomechanical factors need to be evaluated for correction either in a staged fashion or concomitantly. That is, a TT-PCL of 26 and patellar height of 1.3 are out of the normal envelope, but a risk/reward assessment for a particular patient may or may not suggest these should be treated surgically at the time of cartilage restoration. Regardless of a one- or two-step approach, diagnostic arthroscopy should be performed to assess chondral surfaces and patella tracking. Examination under anesthesia should be performed to assess tibiofemoral ligamentous stability as well as that of the patella soft tissue envelope. The affected side should be compared to the contralateral limb. During arthroscopy, the location, dimension, and chondrosis grade is documented along with the tibiofemoral compartments. The intra-articular tracking of the PF joint should also be assessed under low inflow or air-only conditions.
The combination of pathology location and tracking will allow titration of the treatment algorithm. Maltracking and instability will need to be fully assessed and a comprehensive plan developed prior to or simultaneously with any chondral procedure. This could include even femoral or tibial derotational osteotomies. Tuberosity realignment may aid in normalizing abnormal loading parameters, but one should be aware of the effects of shifting loads, as studied by Pidoriano et al. (27). For example, a standard anteromedialization (AMZ) of the tibial tubercle moves loading forces proximal and medial on the patella. Patients with malalignment and distal/lateral patella or trochlea chondral lesion(s) may be successfully treated with isolated AMZ, negating the need for cartilage restoration. Similarly, patients with medial or pan-patella chondral disease do poorly with isolated AMZ, as it overloads the lesion. When performing realignment for these patients, concomitant cartilage restoration is indicated to optimize outcome. Lastly, realignment may be deferred altogether when treating impaction type chondral injuries of the proximal pole or medial patella lesions resulting from patella dislocation. Soft tissue stabilization (i.e., MPFL, MPTL reconstruction) is indicated for patients with patella instability. Other procedures, such as lateral releases vs. retinacular lengthenings (or even retinacular reconstructions), should be undertaken as dictated by physical examination parameters in conjunction with chondral pathology. Trochleoplasty for trochlear dysplasia is reserved for advanced trochlear dysplasia and even advocates state it is contraindicated if significant chondrosis is present.
Chondral procedures
A summary of the outcomes of cartilage restoration procedures for the PF joint may be found in Table S1.
Debridement
Debridement as an isolated treatment has become more difficult to apply as insurance medical policies cite studies primarily investigating arthritic conditions in the tibiofemoral compartment that show no improvement over non-operative management. However, these conditions are not equivalent to unstable chondral lesions in a non-arthritic joint. Chondral lesions often have unstable tissue, which may be conservatively stabilized with an oscillating shaver; unstable flaps can be resected and stable vertical walls of healthy adjacent cartilage can be created with a curette when the lesion is full thickness. Until there is universally accepted evidence that electrical debridement is full chondral safe in all hands, it is not recommended. Chondroplasty affords the quickest recovery, as there are typically no weight bearing restrictions, no bracing, and patients can return to activities without limitations during recovery. Debridement is the surgical treatment of choice for unstable, small (<2 cm2), partial or full thickness lesions in low demand patients or in patients who are not good candidates for more complex cartilage restoration pathways (i.e., obesity, non-compliant) (28). For larger, unstable lesions in high demand patients, debridement may be performed for mechanical symptoms at the time of staging arthroscopy alongside biopsy for future cell based cartilage transplantation.
Internal fixation
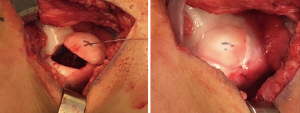
Traumatic patella instability episodes may result in chondral or osteochondral shear injuries to the medial patella, lateral trochlea, and/or lateral femoral condyle. Alternatively, unstable OCD lesions may similarly present with pain, swelling, and mechanical symptoms. A potentially fixable loose osteochondral body in the setting of a first-time PF dislocation or idiopathic OCD is an indication for early surgery (Figure 4). In skeletally immature patients, internal fixation of chondral only fragments may be attempted with sutures or suture anchors with reasonable success rates (29,30). Osteochondral or chondral loose bodies that result from recurrent patella instability or from longstanding OCD in skeletally mature patients may not be repairable. In these patients, excision is the preferred option, with careful determination of the size, depth, and location of the chondral injury for potential future cartilage restoration procedure.
Marrow stimulation techniques (MST)
These include abrasion arthroplasty, microfracture, drilling or nanofracture techniques. The premise of treatment is replacement of deficient articular hyaline cartilage (type II collagen) with fibrocartilage (type I collagen) formed after maturation of marrow elements released from beneath the subchondral plate. Abrasion arthroplasty removes a superficial layer of the subchondral bone exposing vasculature, whereas the other techniques make penetrations of varying size and depth deep to the subchondral plate to access marrow elements. In general, microfracture outcomes are best in small (<2 cm2), contained, acute or subacute surface lesions in young patients (31-33). Despite studies demonstrating deteriorating results over time, microfracture remains the standard by which other chondral restorative procedures are compared for femoral lesions in FDA approved clinical trials. There have been no FDA trials in the PF joint for cartilage restoration products. However, most studies show deterioration within 18–36 months and PF lesions did significantly worse (33). It may be reasonable to consider trochlea marrow stimulation arthroscopically, particularly for small and contained lesions in low demand patients. For patella lesions, the combination of poor results and requirement of at least a mini-arthrotomy for technical execution makes this a less desirable treatment option. Additionally, microfracture can damage the underlying subchondral bone and is associated with intra-lesional osteophyte formation in a percentage of treated patients. This plays a role in the 3× higher failure rate of cell based cartilage repair following microfracture when compared to primary treatment of a lesion (34,35). Evolving techniques utilize narrower and deeper instruments and/or augmentation of MST with scaffolds (autologous matrix induced chondrogenesis, AMIC) may help to address these underlying issues (36-39). However, to date, MST remains a fibrocartilaginous repair with persistent doubt regarding repair strength and durability for most PF lesions (40).
Cell based repair
Cell based cartilage repair is a time-honored treatment for symptomatic, large, contained lesions of the patella and/or trochlea without bone loss. Several long-term studies have demonstrated efficacy of cell based repair in the PF joint, making it a first-line workhorse for solitary, bipolar, or multifocal lesions on either side of the joint. Benefits include preservation of bone stock, relative technical ease, ability to match complex PF topology; drawbacks include typical need for 2-stage surgery, prolonged and complex rehabilitation, need for remodeling and maturation in vivo (18–24 months), and expense. Treatments discussed below include matrix autologous chondrocyte implantation (MACI®, Vericel, Cambridge, MA) and particulated juvenile allograft cartilage (PJAC) (DeNovo NT®, Zimmer Biomet, Warsaw, IN).
ACI/MACI
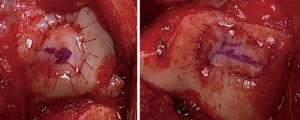
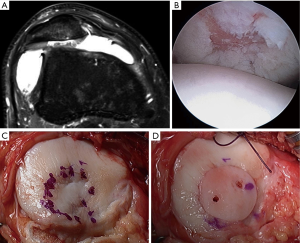
Histologic studies have shown that autologous chondrocyte implantation (ACI) (and by extension MACI) results in hyaline-like Type II collagen (41). ACI has the most data to support its use compared with the other techniques; however, use on the patella was considered off-label in the US based on the FDA conditional biologics license applications (BLA) issued in 1997. The BLA was issued based on the Brittberg et al. initial study of ACI (42). While femoral lesions had acceptable outcomes, the few patients with patellar lesions showed poor results (42). However, malalignment was not addressed in this initial patient cohort. When bony alignment and other co-morbidities were corrected concurrently or in a staged fashion, results by the same authors were shown to be similar to femoral lesions of the knee (43). Subsequent authors corroborated this (44,45). Poorer results were seen in ACI performed as revision surgery for failed microfracture compared to those performed as the initial index procedure (34,35). MACI was approved by the FDA in 2017 and is indicated for use in all compartments of the knee as a primary treatment option. Studies of MACI also show PF results which are comparable to the tibiofemoral results when appropriate concomitant procedures are performed (46,47). Filardo et al. have shown the difference in outcomes between patellar and trochlear lesions when treated by MACI (48). ACI is no longer available for commercial use as it has been replaced by MACI. Some studies have shown a tendency towards better results with MACI compared to ACI for the PF joint (Figures 5,6) (49,50).
PJAC
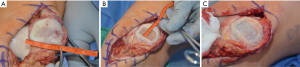
DeNovo NT has neither randomized controlled trial nor long-term data at present. However, preliminary outcomes are promising. The first report on DeNovo NT in patellar chondral lesions showed significant improvements in MRI scores, functional scores, and pain scores at over 2 years follow up (51). A recent MRI study out to two years showed that lesion fill at 6, 12, and 24 months was 82%, 85%, and 75%, respectively (52). Clinical outcome measures were not reported in this study. Other studies have shown that imaging results do not necessarily correlate with clinical outcomes (53). Buckwalter et al. did present clinical outcomes of DeNovo NT in patellar lesions at an average of 8.2 months; significant improvements in KOOS scores were seen with a trend towards improvement in KOOS subset scores (54). A 2-year prospective trial showed improvements in clinical scores, radiographic appearance, and even histology of tissue in the defect (55). Advantages of DeNovo NT include the ability to perform implantation in a single surgical setting as well as the use of immature chondrocytes, which have been shown to have increased metabolic and proliferative activity when compared to adult chondrocytes (Figure 7) (56).
Osteochondral treatments
The two main treatments in this category are osteochondral autograft transplantation (OAT) and osteochondral allograft (OCA) transplantation. Both of these share the benefit of the ability to replace diseased subchondral bone, making them useful in cases of compromised bone bed (i.e., OCD, prior microfracture, uncontained lesions, subchondral cystic changes, AVN) and in revision. Benefits include the transplantation of mature, hyaline cartilage at time zero; drawbacks include donor site limitations and morbidity for OAT and graft matching, availability/viability, technical difficulty, and expense for OCA.
OAT
OAT in the PF joint is challenging due to the difference in patellar cartilage thickness, typical donor sites (trochlea), and the complex nature of the anatomy. The size of the lesion also limits the use of this technique, as increasing the number of plugs harvested also increases the risk of creating donor site (trochlea) morbidity (57,58). That is, harvesting trochlea OAT plugs for a patella lesion in patients with PF symptoms is a concern. However, OAT is advantageous in rare situations for small, focal chondral or osteochondral lesions because it can be done in a single surgery. Use of autogenous tissue also has the advantage of minimizing the chance of bony junction integration failure, as well as minimizing costs. A 2-year outcome study of PF lesions between 1–2.5 cm2 demonstrated significant improvements in functional outcomes. Integration was reported at 83% 6 months after surgery, and 100% at 1-year post-implantation. It was also noted that patients receiving one plug fared better than patients that received two plugs, and that lateral plugs had significantly better outcomes than those with medial and lateral plugs. Central lesions were excluded from the study (59). Figueroa et al. conducted a prospective case series of OATS in the patella and saw improvements in clinical, functional, and radiographic parameters (60). Nho et al. also found good results with OAT in the patella, but found that the subset with concomitant maltracking (even though corrected) did not fare as well as those without maltracking (61).
OCA
There are numerous allograft types, ranging from fresh viable OCA, cryopreserved, and off-the-shelf non-viable options. Fresh stored OCA is the preferred technique. OCA may be used for large and uncontained lesions, as donor site morbidity is not an issue with these cases. However, drawbacks include size matching and availability of donors and the very low risk of disease transmission. Also, newer techniques for graft storage have the ability to expand the lifespan and cell viability of grafts, effectively increasing the donor pool (62,63). Another significant risk is failure of graft incorporation. The osteochondral unit is essentially an immune privileged entity, so rejection in the sense of solid organs is not an issue, obviating the need for anti-rejection medication; however, the donor bony tissue may not become fully integrated into the recipient osteology. This can be mitigated by thoroughly pressure irrigating the bone of the OCA to remove donor stromal elements. Additionally, modalities to augment osseous substitution and integration, such as with bone marrow aspirate concentrate, have shown early promise (64).
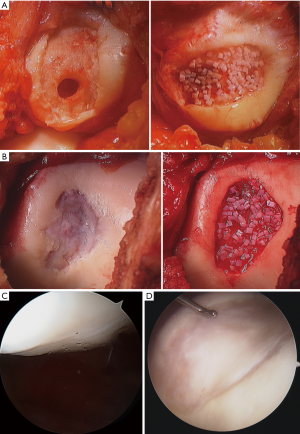
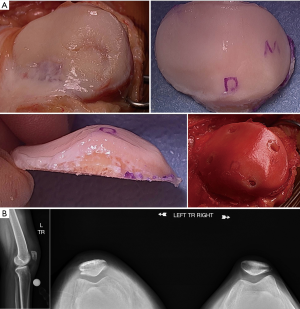
In the past, allograft transplantation to the PF joint has had poorer outcomes when compared to transplantation to the condyles due to the unique morphology (65); however, modern techniques have significantly improved outcomes. Bugbee’s group has shown over a 91% survivorship at 10 years for isolated trochlear OCA, with 89% satisfaction (66). The same group also showed 78% survivorship at 5 and 10 years for isolated patellar OCA, with 55.8% survivorship at 15 years, and satisfaction was reported at 89% (67). Additionally, it has also shown favorable results in the case of bipolar lesions, albeit with higher rates of failure than monopolar lesions (68). OCA can be particularly helpful in revision situations and in conditions where the underlying subchondral bone is compromised (69). It is also a preferred technique over cell based cartilage repair in patients with early osteophytes, early joint space narrowing, increasing age, and elevated BMI. Torga Spak and Teitge showed good results for OCAs performed for PF arthritis (70). Fixation for OCA is myriad; for the simple dowel technique, press fit is often all that is required whereas more complex geometries may utilize a shell allograft technique and thereby require fixation with a combination of non-absorbable and absorbable materials (Figures 8,9).
Arthroplasty
PF arthroplasty is reserved for older, lower demand individuals or for those that have failed all other restoration type procedures. Outcomes are fairly predictable and are typically good if mechanical alignment is corrected (71). Patients with history of post-traumatic PF arthritis typical fare better than patients with insidious onset of tricompartmental osteoarthritis, first presenting in the PF joint. Recovery is typically quicker than a chondral procedure, usually with weight bearing as tolerated, range of motion as tolerated, and no use of a brace.
Conclusions
The treatment of PF chondral lesions is complex and multifactorial. The vast majority of lesions are asymptomatic and require no specific treatment. Non-operative management is the rule for the majority of symptomatic chondral lesions. When surgery is indicated, treatment choice is dictated by lesion characteristic (i.e., size, location, depth) and by patient, joint, and limb specific parameters. Optimization of the joint environment by concomitant bony and/or soft tissue procedures is critical to success. With careful pre-surgical planning, meticulous technique, and compliance with a post-operative rehabilitation program, good to excellent outcomes may be achieved. Patient counseling is important to provide realistic goals and expectations.

Full table
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Vicente Sanchis-Alfonso and Scott F. Dye) for the series “The Patellofemoral Joint” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The series “The Patellofemoral Joint” was commissioned by the editorial office without any funding or sponsorship. VSA and SFD served as the unpaid Guest Editors of the series. SLS is also a paid consultant to Ceterix Orthopaedics, CONMED Linvatec, and Moximed. JF is also a paid consultant for Cartiheal, Exactech, Genzyme, Moximed, NuTech, Osiris Therapeutics, Inc., Regentis, Samumed and Zipline. All AAOS dislcosures were updated in May. SL Sherman reports paid consultant for Arthrex and Vericel; research support for Arthrex and Zimmer Biomet outside the submitted work. JF reports paid consultant for Allosource, Arthrex, RTI Biologics, Vericel, and Zimmer Biomet; non-financial support for RTI Biologics, Vericel, and Zimmer Biomet outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Widuchowski W, Lukasik P, Kwiatkowski G, et al. Isolated full thickness chondral injuries. Prevalance and outcome of treatment. A retrospective study of 5233 knee arthroscopies. Acta Chir Orthop Traumatol Cech 2008;75:382-6. [PubMed]
- Curl WW, Krome J, Gordon ES, et al. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456-60. [Crossref] [PubMed]
- Kaplan LD, Schurhoff MR, Selesnick H, et al. Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy 2005;21:557-61. [Crossref] [PubMed]
- Walczak BE, McCulloch PC, Kang RW, et al. Abnormal findings on knee magnetic resonance imaging in asymptomatic NBA players. J Knee Surg 2008;21:27-33. [Crossref] [PubMed]
- Minas T, Von Keudell A, Bryant T, et al. A minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res 2014;472:41-51. [Crossref] [PubMed]
- Hughston JC, Walsh WM, Puddu G. Patellar subluxation and dislocation, WB Saunders, 1984.
- Kolowich PA, Paulos LE, Rosenberg TD, et al. Lateral release of the patella: indications and contraindications. Am J Sports Med 1990;18:359-65. [Crossref] [PubMed]
- Pinkowsky GJ, Farr J. Considerations in evaluating treatment options for patellofemoral cartilage pathology. Sports Med Arthrosc Rev 2016;24:92-7. [Crossref] [PubMed]
- Camp CL, Stuart MJ, Krych AJ, et al. CT and MRI measurements of tibial tubercle-trochlear groove distances are not equivalent in patients with patellar instability. Am J Sports Med 2013;41:1835-40. [Crossref] [PubMed]
- Seitlinger G, Scheurecker G, Hogler R, et al. Tibial tubercle-posterior cruciate ligament distance: a new measurement to define the position of the tibial tubercle in patients with patellar dislocation. Am J Sports Med 2012;40:1119-25. [Crossref] [PubMed]
- Camp CL, Heidenreich MJ, Dahm DL, et al. Individualizing the tibial tubercle-trochlear groove distance: patellar instability ratios that predict recurrent instability. Am J Sports Med 2016;44:393-9. [Crossref] [PubMed]
- Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med 2015;3:2325967115581163 [Crossref] [PubMed]
- Evanich JD, Evanich CJ, Wright MB, et al. Efficacy of intraarticular hyaluronic acid injections in knee osteoarthritis. Clin Orthop Relat Res 2001;173-81. [Crossref] [PubMed]
- Masuko K, Murata M, Yudoh K, et al. Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med 2009;2:77-81. [Crossref] [PubMed]
- Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 2011;27:1490-501. [Crossref] [PubMed]
- Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 2015;43:1575-82. [Crossref] [PubMed]
- Laver L, Marom N, Dnyanesh L, et al. PRP for degenerative cartilage disease: a systematic review of clinical studies. Cartilage 2017;8:341-4. [Crossref] [PubMed]
- Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med 2017;45:339-46. [Crossref] [PubMed]
- Lisi C, Perotti C, Scudeller L, et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil 2018;32:330-9. [Crossref] [PubMed]
- Deppen R. From the CORE to the floor - interrelationships. In: Donatelli RA, editor. Sports-specific rehabilitation, St. Louis: Elsevier, 2007:145-73.
- Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am 2008;90:2751-62. [Crossref] [PubMed]
- Sherman SL, Erickson BJ, Cvetanovich GL, et al. Tibial tuberosity osteotomy: indications, techniques, and outcomes. Am J Sports Med 2014;42:2006-17. [Crossref] [PubMed]
- Anderson CN, Magnussen RA, Block JJ, et al. Operative fixation of chondral loose dodies in osteochondritis dissecans in the knee. Orthop J Sports Med 2013;1:2325967113496546 [Crossref] [PubMed]
- Krych AJ, Robertson CM, Williams RJ 3rd, et al. Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med 2012;40:1053-9. [Crossref] [PubMed]
- Kanneganti P, Harris JD, Brophy RH, et al. The effect of smoking on ligament and cartilage surgery in the knee. Am J Sports Med 2012;40:2872-8. [Crossref] [PubMed]
- Behery O, Siston RA, Harris JD, et al. Treatment of cartilage defects of the knee: expanding on the existing algorithm. Clin J Sport Med 2014;24:21-30. [Crossref] [PubMed]
- Pidoriano AJ, Weinstein RN, Buuck DA, et al. Correlation of patella articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med 1997;25:533-7. [Crossref] [PubMed]
- Anderson DE, Rose MB, Willie AJ, et al. Arthroscopic mechanical chondroplasty of the knee is beneficial for treatment of focal cartilage lesions in the absence of concurrent pathology. Orthop J Sports Med 2017;5:2325967117707213 [Crossref] [PubMed]
- Sherman SL, Nuelle CW, Farr II J. Issues Specific to Cartilage Restoration in the Patellofemoral Joint. In AAOS.
- Fabricant PD, Yen YM, Kramer DE, et al. Fixation of traumatic chondral-only fragments of the knee in pediatric and adolescent athletes:a retrospective multicenter report. Orthop J Sports Med 2018;6:2325967117753140 [Crossref] [PubMed]
- Mithoefer K, McAdams T, Williams RJ, et al. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 2009;37:2053-63. [Crossref] [PubMed]
- Gobbi A, Karnatzikos G, Kumar A A.. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc 2014;22:1986-96. [Crossref] [PubMed]
- Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 2006;14:1119-25. [Crossref] [PubMed]
- Minas T, Gomoll AH, Rosenberger R, et al. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med 2009;37:902-8. [Crossref] [PubMed]
- Pestka JM, Bode G, Salzmann G, et al. Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med 2012;40:325-31. [Crossref] [PubMed]
- Kusano T, Jakob RP, Gautier E, et al. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc 2012;20:2109-15. [Crossref] [PubMed]
- Gille J, Behrens P, Volpi P, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg 2013;133:87-93. [Crossref] [PubMed]
- Gille J, Schuseil E, Wimmer J, et al. Mid-term results of autologous matrix induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc 2010;18:1456-64. [Crossref] [PubMed]
- Schiavone Panni A, Del Regno C, Mazzitelli G, et al. Good clinical results with autologous matrix-induced chondrogenesis (Amic) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc 2018;26:1130-6. [PubMed]
- Orth P, Duffner J, Zurakowski D, et al. Small-diameter awls improve articular cartilage Repair after microfracture treatment in a translational animal model. Am J Sports Med 2016;44:209-19. [Crossref] [PubMed]
- Brittberg M, Nilsson A, Lindahl A, et al. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res 1996;270-83. [Crossref] [PubMed]
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889-95. [Crossref] [PubMed]
- Vasiliadis HS, Lindahl A, Georgoulis AD, et al. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc 2011;19:452-7. [Crossref] [PubMed]
- Gillogly SD, Arnold RM. Autologous chondrocyte implantation and anteromedialization for isolated patellar articular cartilage lesions: 5- to 11-year follow-up. Am J Sports Med 2014;42:912-20. [Crossref] [PubMed]
- Gomoll AH, Gillogly SD, Cole BJ, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med 2014;42:1074-81. [Crossref] [PubMed]
- Ebert JR, Schneider A, Fallon M, et al. A comparison of 2-year outcomes in patients undergoing tibiofemoral or patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med 2017;45:3243-53. [Crossref] [PubMed]
- Ebert JR, Fallon M, Smith A, et al. Prospective clinical and radiologic evaluation of patellofemoral matrix-induced autologous chondrocyte implantation. Am J Sports Med 2015;43:1362-72. [Crossref] [PubMed]
- Filardo G, Kon E, Andriolo L, et al. Treatment of ‘‘patellofemoral’’ cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med 2014;42:626-34. [Crossref] [PubMed]
- Macmull S, Jaiswal PK, Bentley G, et al. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int Orthop 2012;36:1371-7. [Crossref] [PubMed]
- Nawaz SZ, Bentley G, Briggs TW, et al. Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am 2014;96:824-30. [Crossref] [PubMed]
- Tompkins M, Hamann JC, Diduch DR, et al. Preliminary results of a novel single-stage cartilage restoration technique: particulated juvenile articular cartilage allograft for chondral defects of the patella. Arthroscopy 2013;29:1661-70. [Crossref] [PubMed]
- Grawe B, Burge A, Nguyen J, et al. Cartilage regeneration in full-thickness patellar chondral defects treated with particulated juvenile articular allograft cartilage: an MRI analysis. Cartilage 2017;8:374-83. [Crossref] [PubMed]
- Wang T, Belkin NS, Burge AJ, et al. Patellofemoral cartilage lesions treated with particulated juvenile allograft cartilage: a prospective study with minimum 2-year clinical and magnetic resonance imaging outcomes. Arthroscopy 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Buckwalter JA, Bowman GN, Albright JP, et al. Clinical outcomes of patellar chondral lesions treated with juvenile particulated cartilage allografts. Iowa Orthop J 2014;34:44-9. [PubMed]
- Farr J, Tabet SK, Margerrison E, et al. Clinical, radiographic, and histological outcomes after cartilage repair with particulated juvenile articular cartilage: a 2-year prospective study. Am J Sports Med 2014;42:1417-25. [Crossref] [PubMed]
- Bonasia DE, Martin JA, Marmotti A, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med 2011;39:2355-61. [Crossref] [PubMed]
- Mithoefer K, Scopp JM, Mandelbaum BR. Articular cartilage repair in athletes. Instr Course Lect 2007;56:457-68. [PubMed]
- Miniaci A, Martineau PA. Technical aspects of osteochondral autograft transplantation. Instr Course Lect 2007;56:447-55. [PubMed]
- Astur DC, Arliani GG, Binz M, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg Am 2014;96:816-23. [Crossref] [PubMed]
- Figueroa D, Melean P, Calvo R, et al. Osteochondral autografts in full thickness patella cartilage lesions. Knee 2011;18:220-3. [Crossref] [PubMed]
- Nho SJ, Foo LF, Green DM, et al. Magnetic resonance imaging and clinical evaluation of patellar resurfacing with press-fit osteochondral autograft plugs. Am J Sports Med 2008;36:1101-9. [Crossref] [PubMed]
- Cook JL, Stoker AM, Stannard JP, et al. A novel system improves preservation of osteochondral allografts. Clin Orthop Relat Res 2014;472:3404-14. [Crossref] [PubMed]
- Stoker A, Garrity JT, Hung CT, et al. Improved preservation of fresh osteochondral allografts for clinical use. J Knee Surg 2012;25:117-25. [Crossref] [PubMed]
- Oladeji LO, Stannard JP, Cook CR, et al. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med 2017;45:2797-803. [Crossref] [PubMed]
- Jamali AA, Emmerson BC, Chung C, et al. Fresh osteochondral allografts results in the patellofemoral joint. Clin Orthop Relat Res 2005;176-85. [Crossref] [PubMed]
- Cameron JI, Pulido PA, McCauley JC, et al. Osteochondral allograft transplantation of the femoral trochlea. Am J Sports Med 2016;44:633-8. [Crossref] [PubMed]
- Gracitelli GC, Meric G, Pulido PA, et al. Fresh osteochondral allograft transplantation for isolated patellar cartilage injury. Am J Sports Med 2015;43:879-84. [Crossref] [PubMed]
- Meric G, Gracitelli GC, Gortz S, et al. Fresh osteochondral allograft transplantation for bipolar reciprocal osteochondral lesions of the knee. Am J Sports Med 2015;43:709-14. [Crossref] [PubMed]
- Gracitelli GC, Meric G, Briggs DT, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med 2015;43:885-91. [Crossref] [PubMed]
- Torga Spak R, Teitge RA. Fresh osteochondral allografts for patellofemoral arthritis: long-term followup. Clin Orthop Relat Res 2006;193-200. [Crossref] [PubMed]
- van der List JP, Chawla H, Zuiderbaan HA, et al. Survivorship and functional outcomes of patellofemoral arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2017;25:2622-31. [Crossref] [PubMed]
- Shubin Stein BE, Brady JM, Grawe B, et al. Return to activites after patellofemoral arthroplasty. Am J Orthop 2017;46:E353-E357. [PubMed]
- Odgaard A, Madsen F, Kristensen W, et al. The Mark Coventry Award: patellofemoral arthroplasty results in better range of movement and early patient-reported outcomes than TKA. Clin Orthop Relat Res 2018;476:87-100. [Crossref] [PubMed]
Cite this article as: Sherman SL, Thomas DM, Farr J 2nd. Chondral and osteochondral lesions in the patellofemoral joint: when and how to manage. Ann Joint 2018;3:53.