Review of an exploratory phase II FDA regulated clinical trial of a novel surgical innovation: completion of a prospective, randomized, controlled trial to compare NeoCart with the standard-of-care, microfracture, for articular cartilage repair
Introduction
Cartilage defects represent a clinical and therapeutic challenge as full thickness injury cannot intrinsically heal, leading to significant pain and functional impairment. When left untreated, lesions further progress to degenerative joint conditions. The goal of treatment is to restore a congruent functional joint surface to prevent disease progression. Current primary treatment options for relatively small cartilage injuries include mechanical chondroplasty and/or microfracture (1,2). Microfracture involves drilling or tapping into the subchondral bone to facilitate transposition of marrow cells to fill the defect with a fibrocartilaginous tissue (3,4). However, clinical improvement after microfracture is inconsistently observed, with about 25% of patients reporting minimal relief within the first 12–24 months of treatment (5,6), and a peak in clinical improvement at 24 months (7,8). Despite varied outcomes and short-term efficacy, the FDA established microfracture as the standard in cartilage repair to which any new therapy for cartilage repair is compared in the United States (9).
Alternatives to microfracture, including autologous chondrocyte implantation (ACI), have historically been secondary procedures performed after chondroplasty and microfracture fail. ACI represents the first generation of “regenerative techniques” in which healthy cartilage is used to provide an autogenous cell therapy to produce hyaline-like cartilage tissue within the defect. Chondrocytes are isolated and expanded in a laboratory before surgical application to the defect. Reoperation has been reported in approximately 1/3 of first generation ACI cases; although, second and third generation techniques have improved reoperation rates (10). Failure rates of about 16% after ACI treatment have been reported, with favorable results in 83% of patients after 5–11 years (11), or 69% after 9 years (12). In addition, Zaslav et al. reported a 24% failure rate and significant improvements in all clinical outcome measures in patients receiving ACI implantation for failed articular cartilage treatments (13). Comparing ACI treatment with microfracture, Knutsen et al. reported a 23% failure rate in each cohort and significant, but equal, clinical improvements in both cohorts after 5 years (14). Saris et al. reported equal improvements at 3 years post-operative with second generation characterized chondrocyte implantation or microfracture (15), while others have reported superior clinical outcomes with microfracture (16).
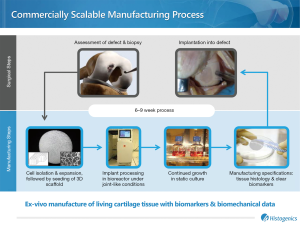
NeoCart® (Histogenics, Waltham, MA), an autologous cartilage tissue-engineered implant, represents a novel approach for primary treatment of articular knee cartilage defects. NeoCart is a laboratory-generated tissue derived from autogenous chondrocytes obtained through arthroscopic biopsy. In NeoCart manufacture, chondrocytes are isolated, embedded in a type I collagen matrix, and incubated in a bioreactor prior to implantation. The bioreactor recreates physiologic pressure and oxygen tension to favor a chondrocyte cell phenotype, which is crucial to proper tissue development (17). At implantation, the approximately 2 mm thick disc consists of autogenous chondrocytes and an average of 10 mg/mL of sulfated glycosaminoglycans—a critical component of hyaline cartilage extracellular matrix. Neither microfracture nor currently available ACI treatments provide both chondrocytes and hyaline specific extracellular matrix molecules at the time of surgery, which may contribute to relatively high failure rates.
The objective of the current study was to assess clinical and patient-reported outcomes acquired at final follow-up from an FDA-regulated exploratory phase II randomized, controlled study comparing NeoCart with microfracture for primary treatment of grade 3 International Cartilage Repair Society (ICRS) cartilage injuries of the distal femoral condyle in adults (18–55 years). We have previously reported short-term results from the phase I and phase II trials that demonstrated safety of the NeoCart implant with decreased pain and improved functional outcomes at 6–24 months post-treatment (18,19). The phase II randomized clinical trial (Clinicaltrials.gov, NCT00548119) is now complete with mean final follow-up of 51 months. To further evaluate the safety and efficacy of NeoCart for primary repair of femoral condyle cartilage defects, we evaluated temporal changes in patient reported outcomes in NeoCart-treated patients compared with microfracture controls. As an exploratory study, we used this data to establish parameters for a phase III confirmatory study protocol.
Methods
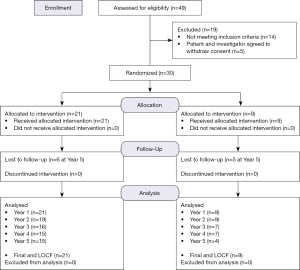
The study was approved by the Institutional Review Board. Inclusion and exclusion criteria for enrollment are in Table S1, and details for randomization and treatment are included in Figure 1.
Forty-nine subjects were consented. Pre-operative MR images were obtained, and patients with one (n=28) or two (n=2) isolated contained articular cartilage lesions of the femoral condyle, confirmed by arthroscopy, were enrolled. Patients were randomized 2:1; two NeoCart per microfracture for the following reasons: (I) accommodating patient preference for a novel therapeutic versus a well-known standard; (II) establishing experience for surgeons learning a new technical procedure; and (III) meeting the defined statistical power for a novel therapeutic in comparison with a procedure of established efficacy (20). Microfracture patients underwent treatment at the index arthroscopy. NeoCart patients underwent arthroscopic cartilage biopsy and implantation approximately 6 weeks later (Figure 2). Neocart was surgically placed during an “ambulatory” procedure via a mini-arthrotomy and secured with a proprietary collagen-based bio-adhesive (21). The rehabilitation protocol was the same for both study cohorts. Additional operative and rehabilitative details were reported previously (18).
All patients were assessed for range of motion (ROM) and completed the following patient-reported outcome questionnaires at baseline, 3 months, 6 months, 1 year, and annually thereafter through 5 years: International Knee Documentation Committee (IKDC; subjective), Knee injury and Osteoarthritis Outcome Score (KOOS) Pain, KOOS Activity of Daily Living (ADL), KOOS Quality of Life (QOL), KOOS Symptoms, KOOS Sports & Recreation, Short Form 36 (SF-36) and Visual Analog Scale (VAS) Pain. IDKC and KOOS Pain were established as the primary end points by the FDA. One NeoCart patient was lost to follow-up at 2 years, one withdrew at 3 years, two were lost at 3 years, two were lost at 5 years, and 15 (71%) patients were considered long-term follow-up at 5 years. One microfracture patient was lost to follow-up at 3 years, one withdrew at 5 years, three were lost at 5 years, leaving four (44%) patients considered long-term follow-up at 5 years.
Statistical analyses
Objective data was collected and managed by the study sponsor data management services (Synteract, Carlsbad, CA, USA). Descriptive statistics and responder rate were calculated at each time for the intent to treat population with significance set at P<0.05 (two sided). Patients were classified as responders if they achieved a 12-point improvement in the KOOS Pain score and a 20-point improvement in the IKDC subjective score (18). Mean change from baseline was calculated for all outcome measures and time points, in addition to scores at final follow-up for all patients (last observation carried forward) (22). A paired t-test was applied comparing score change from baseline between cohorts. Primary outcomes, IKDC and KOOS scores, and change from baseline to final follow-up were evaluated by an analysis of covariance (ANCOVA), using the baseline scores as the covariate.
Results
Safety
AEs were captured by principal investigators and categorized using the definition of the US Department of Health and Human Services Office for Human Research Protections (23). Ninety-nine AEs were reported in the NeoCart cohort and 31 in the microfracture cohort during the study period. There were 21 AEs related specifically to the procedure in the 21 NeoCart patients [moderate procedural pain (n=5), mild arthralgia (n=7), moderate hypoesthesia (n=1), moderate joint effusion (n=1), mild joint effusion (n=1), mild joint stiffness (n=1), mild limb injury (n=1), mild wound secretion (n=1), mild muscle atrophy (n=1), and mild neuralgia (n=1)] and eight AEs related specifically to the procedure in the nine microfracture patients [mild arthralgia (n=6), mild joint swelling (n=1), severe meniscus lesion (n=1)]; these rates did not differ significantly between treatment arms.
There were 7 severe AEs in the NeoCart cohort; arthralgia (n=3) and subsequent joint locking (n=1) in the index knee, and ligamentous rupture (n=1) and septic arthritis (n=1, ultimately treated with knee arthroplasty) in the contralateral knee. Due to temporal relationships and exam findings, these were not considered related to either the NeoCart implant or to the procedure by treating enrolling investigators. There were two severe events in the microfracture cohort; a malignant pelvic neoplasm, and a meniscus lesion in the index knee (treated with debridement) after 4-year follow-up which was the sole severe AE deemed possibly related to the procedure.
The most common AEs in the NeoCart cohort included arthralgia, related to the implant (n=9, mild), and post-operative pain (n=5, moderate) and arthralgia (n=7, mild) related to the procedure itself. The most common AE in the microfracture cohort included arthralgia (n=6, mild). No patients were discontinued because of an AE, and the number, severity, and type of AE were not different in those that were lost to follow-up compared with those that were not.
Efficacy analysis
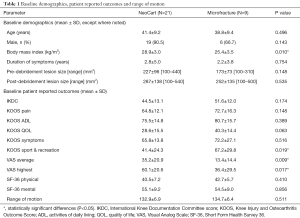
Demographics are summarized in Table 1; only body mass index (BMI) differed significantly between groups. Baseline patient reported outcome scores are also summarized in Table 1. VAS scores were significantly higher in the NeoCart cohort, KOOS Sports & Recreation was higher in the microfracture cohort and all other parameters were similar between cohorts.
Full table
Follow-up rates (defined as annually complete patient reported outcome scores) for IKDC measures in the NeoCart cohort were 95%, 86%, 71%, 62%, and 57% at years 1–5, and 100%, 90%, 76%, 71%, and 71% for KOOS measures. Follow-up rates for the IKDC and the KOOS measures were 100%, 100%, 78%, 78%, and 44% at years 1–5 in the microfracture cohort.
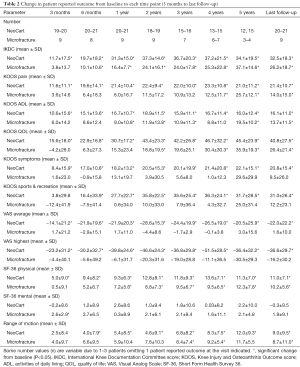
Change from baseline through final follow-up is summarized in Table 2. SF-36 Mental score did not change with either treatment at any time point, with the exception of the microfracture cohort at 3 months. Improvement with NeoCart compared to baseline was significant (P<0.05) for all other parameters at all time points, with the exception of ROM and KOOS Sports & Recreation at 3 months. Improvement for the microfracture cohort was limited to IKDC beginning at 6 months, ROM beginning at 3 years, KOOS Pain beginning at 4 years, KOOS ADL and QOL beginning at 1 and 2 years respectively, and SF-36 Physical beginning at 1 year.
Full table
Mean change from baseline for IKDC and KOOS Pain (the two primary study outcome measures) is represented graphically in Figure 3. Improvement in mean IKDC score at 1 and 2 years was greater (P<0.05) with NeoCart than with microfracture treatment (Figure 3A). The NeoCart cohort demonstrated greater improvement for KOOS Pain at 6 months and at 1 and 3–4 years (Figure 3B); for KOOS QOL at 3 months and at 1–2 years; for KOOS Symptoms at 6 months and at 3–4 years; and for KOOS Sports & Recreation at 1–4 years. Improvement in the VAS Highest score was greater in the NeoCart cohort at 1, 2, and 4 years, compared with microfracture (Figure 3C). Improvement in the VAS Average (usual) score was greater in the NeoCart cohort at 6 months, at 1–4 years, and at final follow-up, compared with microfracture (Figure 3D). At final follow-up, there were no other significant differences in change from baseline when the NeoCart and microfracture groups were compared.
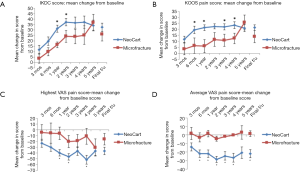
By 5 years, 100% of non-responders in the microfracture cohort were lost to follow-up (n=3 of 3), whereas only 50% of non-responders were lost to follow-up in the NeoCart cohort (n=2 of 4). Due to the relatively small numbers of patients and to address the potential impact of loss to follow-up, responder rates in each cohort were calculated using imputed data for all time points with the last observation carried forward method (19). Based on a change from baseline in IKDC score of >20 and a change in KOOS Pain score of >12, significantly more NeoCart patients responded to treatment at 1 year (16/21, 76.2%, P=0.046) compared with microfracture patients (1/9, 11.1%). At years 2–5, responder rates for the NeoCart group were 15/18 (83.3%), 12/16 (75.0%), 12/15 (80.0%), and 13/15 (86.7%), respectively. Responder rates for the control group in years 2–5 were 2/9 (22.2%), 1/7 (14.3%), 1/7 (14.3%), and 3/4 (75%), respectively. Responder rates at years 2-5 did not differ significantly between cohorts. At final follow-up (51.6±13.8 months NeoCart; 52.0±8.5 months microfracture), 67% (6/9) of microfracture patients were considered responders while 81% (17/21) of NeoCart patients were responders, although these did not differ significantly.
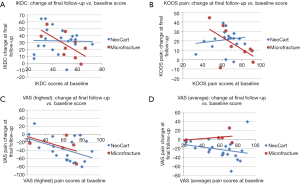
To adjust for variation in baseline IKDC, KOOS Pain and VAS scores, an ANCOVA was performed using the baseline score as co-variant. The adjusted IKDC and KOOS Pain score changes from baseline differed significantly between the NeoCart and microfracture cohorts at 1 year (P=0.028 and 0.016, respectively). Baseline scores did not disproportionately influence the change in IKDC and KOOS Pain scores at 1 year. However, by final follow-up, IKDC (Figure 4A) and KOOS Pain (Figure 4B) scores in the NeoCart cohort improved regardless of baseline values, whereas with microfracture, there was less treatment efficacy in those with higher baseline function or pain scores. Of note, this temporal phenomenon for the microfracture cohort was not seen in VAS (Highest and Average/usual) scores at either 1 year (18,19) or final follow-up (Figure 4C,D).
Discussion
NeoCart implantation appears safe and efficacious over 5 years, supporting further study as a primary treatment of focal cartilage injuries in the knee. While the NeoCart cohort had significantly improved patient reported outcomes and higher follow-up rate over the 5-year study period, there were more overall AEs compared with microfracture. The procedure-related AE’s, however, were equivalent in the two groups. Only one severe AE was considered possibly related—a meniscus lesion in the microfracture cohort. All other events were mild or moderate and were consistent with the minimally invasive nature of these procedures. Thus, NeoCart appears to be as safe as microfracture, the standard primary surgical treatment for isolated cartilage lesions in the United States.
Both cohorts experienced significantly improved patient reported outcome scores during the 5-year study. Improvements were generally greater, in comparison with baseline, and occurred sooner (from 3–48 months earlier) in the NeoCart cohort. Unlike with microfracture, the NeoCart cohort reported significant improvements in KOOS Symptoms, KOOS Sports & Recreation, and VAS scores. In addition, both baseline VAS scores and average BMI were higher than in the microfracture cohort. This potentially provides for greater likelihood of improved outcomes in patients with lower BMI in the microfracture cohort (8). However, we observed the opposite; consistently strong positive outcomes were observed in change from baseline data with NeoCart compared to microfracture. The NeoCart cohort also included a significantly higher incidence of positive responders, based on both 20-point improvements in IKDC score and 12-point improvements in KOOS Pain score at 1 year compared with microfracture. By 5 years, all non-responders were lost in the microfracture cohort, while 50% of non-responders in the NeoCart cohort remained. This attrition amongst the microfracture non-responders may well explain the idiosyncratic improvement in outcomes at 5 years in the small cohort reporting at study conclusion.
We previously reported that the adjusted change in outcome scores at 1 year, based on adjusting for differences in baseline values, was significantly higher for the NeoCart group; by 11.6 points for IKDC score and 12.1 points for KOOS Pain score (18). By final follow-up, it became clear that microfracture treatment mostly benefited those who reported the lowest baseline IKDC and KOOS Pain scores, while the outcome for the NeoCart cohort was independent of baseline scores. Consequently, NeoCart patients experienced similar improvements in outcome across the spectrum of baseline pain and function values, but the microfracture cohort outcomes appeared dependent on the baseline values. Microfracture patients improved less than NeoCart patients if their baseline IKDC or KOOS pain scores were higher, and this difference between cohorts was magnified at later time points. Analysis of baseline-adjusted VAS scores did not demonstrate this distinction between the cohorts. This is of interest, as it may be that an element of the effect of NeoCart in patients with higher scores (higher functioning and potentially earlier disease) reflects improvements in functional capacity to a greater degree than simply reduction in VAS pain score. Thus, NeoCart may represent a better initial treatment option for patients with higher functional capacity in earlier disease states.
Chondroplasty and microfracture are primary therapeutic options for the repair of focal chondral injuries. Microfracture is associated with production of a fibrocartilage fill that is less durable, resilient, and able to withstand biomechanical forces in comparison to native hyaline cartilage. Bony overgrowth has been described in 25% (8), to nearly 50% (24), of microfracture patents, and the benefits plateau after 1–2 years (7,8). The hyaline-like cartilage associated with ACI is a promising alternative to microfracture for these injuries (11). However, in direct comparisons with microfracture, ACI showed no statistically significant improvements at 5 years (14), or 15-year follow-up (25). In contrast to current therapies, NeoCart is implanted with chondrocyte functional matrix, which likely explains earlier efficacy. In a comparison with microfracture, NeoCart led to significantly more positive responders at 1 year (18). With sustained benefit through 5 years, this data confirms the benefits of NeoCart persist and are superior to microfracture. In a separate evaluation of MRI follow-up of the NeoCart group, we found that radiographic measures improved longitudinally until 2 years, with maintenance of the improvement until final follow-up at 5 years (26).
Strengths of our study include its prospective randomized nature, comparison to control current standard, and 5-year follow-up period (27), as a minimum of 2–3-year follow-up is recommended for comparing clinical outcomes after knee cartilage treatment (28,29). We used two common validated measures as “primary” outcomes, IKDC and KOOS, which reliably score knee symptoms and function in patients with articular cartilage lesions (30). We also reported the proportion of responders and AEs based on FDA guidelines (23). For responder analysis using IKDC and KOOS Pain scales, we used more stringent criteria than recommended; a minimum value of 8–10 points in the KOOS score has been suggested to represent a clinically significant difference (31) and a score of 11.5 or more indicates meaningful improvement in IKDC (29).
A principal study limitation is the low sample size. This is a function of “exploratory” trial design, to gain and establish parameters for larger confirmatory trials and calculate power (32,33). Thus, we were not able analyze subgroups to better define variables that may influence outcomes, such as BMI and age. Similarly, low numbers, especially in the microfracture cohort, are particularly troubling as loss to follow-up of a few patients potentially affects the statistical power and a type I or II error is at risk (22). The phase II trial was initially statistically powered in favor of the NeoCart cohort through 2:1 unequal randomization because microfracture had already been well defined in the literature, patients expressed a strong preference toward a novel therapeutic over the standard-of-care, and the surgeons performing the operation needed to gain experience with NeoCart implantation in order to facilitate the learning curve necessary for a larger phase III study.
A second limitation was the number of dropouts in the control group during the study period. Our follow-up rate for most outcome measures was generally high through year 4, although the 5-year follow-up for the microfracture cohort dropped to 44%. This high dropout at the final follow-up prompted us to use imputation for final statistical analysis, which has clear and known limitations (34). Specifically, the observations that were carried forward were likely an overestimate of patient response, given that the therapeutic effect of microfracture peaks at 24 months. The data reporting the efficacy of microfracture, however, has accumulated since the original trial design, in which the FDA set the standard-of-care for the control group (9). Aside from these limitations in the longevity of the control procedure through final follow-up, microfracture is not necessarily a fixed standard because its popularity among surgeons and the prevalence of cases have been declining throughout the entire study duration (35). Thus, not coincidently, we had a large loss to follow-up after the known peak of microfracture longevity with only the control group in this study. This reflects the conundrum of recruiting for long term studies, for which comparison to standard-of-care controls have shorter term clinical benefits than the study duration. A solution to this problem may be a cross-over study design, whereby a patient leaves the control for the treatment group after failure. However, the concern of bias in early clinical response prohibited this method because neither patients nor surgeons were blinded to treatment, so there might have been incentive to seek further intervention in favor of the treatment group. Without the opportunity to cross over, those patients who were lost to follow up may have sought treatment elsewhere after failure.
In this exploratory phase II trial, the outcomes were statistically in favor of NeoCart; however, definitive statements require the statistical power of a phase III trial, which was designed and powered based on this exploratory phase. This process of graduated sample size in trial design methodology is critical to successful FDA-regulated Investigational New Drug (IND) evaluations for surgical procedures and biologic therapeutics. Unfortunately, randomized, controlled trials and the required phases for novel surgical devices are not well known to the orthopaedic audience. On submission of this data to leading orthopaedic journals, reviewers rejected the manuscript, primarily citing the low sample size and loss to follow-up in the control group despite the presentation of this data as long-term follow-up of an exploratory, not a confirmatory, clinical trial. We argue that these limitations provide basis for further investigation into the superiority of NeoCart in a confirmatory trial, and presentation of phase II data elucidates both the importance and the limitations of the exploratory phase of the FDA approval process for a novel surgical therapy.
Conclusions
Now completed, data from this FDA-regulated phase II exploratory clinical trial are allowed for parameter setting and fine tuning of the phase III multicenter confirmatory trial (NCT01066702, clinicaltrials.gov). We established that patients with excessively high baseline IKDC and KOOS Pain scores should be eliminated from inclusion to eliminate the ceiling effect, and that failures in each cohort remain failures over time and will likely leave the study. Using this preliminary data for the power analysis, 245 patients are required to confirm the efficacy of NeoCart treatment over that of microfracture treatment—a study that is currently underway, with a primary end point of one year, and evaluation through 3-years based on these experiences.

Full table
Acknowledgments
Funding: Histogenics (Waltham, MA, USA) funded Phase II clinical trial (to RJ Williams 3rd, TM DeBerardino, D Taylor, CB Ma, DC Crawford).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.06.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board. Informed consent was taken from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med 2005;33:443-60. [Crossref] [PubMed]
- Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am 2005;87-10:2232-9.
- Anderson DE, Rose MB, Wille AJ, et al. Arthroscopic mechanical chondroplasty of the knee is beneficial for treatment of focal cartilage lesions in the absence of concurrent pathology. Orthop J Sports Med 2017;5:2325967117707213 [Crossref] [PubMed]
- Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 2001;S362-9. [Crossref] [PubMed]
- Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 2004;86-A:455-464. [Crossref] [PubMed]
- Saris DB, Vanlauwe J, Victor J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 2008;36:235-46. [Crossref] [PubMed]
- Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 2006;14:1119-25. [Crossref] [PubMed]
- Mithoefer K, Williams RJ 3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am 2005;87:1911-20. [Crossref] [PubMed]
- Yanke AB, Cole BJ. Microfracture: dead or the future? Orthopedics 2014;37:798-800. [Crossref] [PubMed]
- Harris JD, Siston RA, Brophy RH, et al. Failures, re-operations, and complications after autologous chondrocyte implantation--a systematic review. Osteoarthritis Cartilage 2011;19:779-91. [Crossref] [PubMed]
- Peterson L, Minas T, Brittberg M, et al. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2003;85-A:17-24. [Crossref] [PubMed]
- Moseley JB Jr, Anderson AF, Browne JE, et al. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in US patients. Am J Sports Med 2010;38:238-46. [Crossref] [PubMed]
- Zaslav K, Cole B, Brewster R, et al. A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med 2009;37:42-55. [Crossref] [PubMed]
- Knutsen G, Drogset JO, Engebretsen L, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 2007;89:2105-12. [PubMed]
- Saris DB, Vanlauwe J, Victor J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 2009;37:10S-19S. [Crossref] [PubMed]
- Kon E, Gobbi A, Filardo G, et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 2009;37:33-41. [Crossref] [PubMed]
- Saini S, Wick TM. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng 2004;10:825-32. [Crossref] [PubMed]
- Crawford DC, DeBerardino TM, Williams RJ 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: an FDA phase-II prospective, randomized clinical trial after two years. J Bone Joint Surg Am 2012;94:979-89. [Crossref] [PubMed]
- Crawford DC, Heveran CM, Cannon WD Jr, et al. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med 2009;37:1334-43. [Crossref] [PubMed]
- Peckham E, Brabyn S, Cook L, et al. The use of unequal randomisation in clinical trials – An update. Contemp Clin Trials 2015;45:113-22. [Crossref] [PubMed]
- Jones HR, Crawford DC. An autologous tissue implant, NeoCart, for treatment of hyaline cartilage injury in the knee. Oper Tech Orthop 2014;24:264-70. [Crossref]
- Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012;367:1355-60. [Crossref] [PubMed]
- Administration FaD. Guidance for Industry on Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; Availability. US Food and Drug Administration. Available online: https://www.federalregister.gov/documents/2009/12/09/E9-29273/guidance-for-industry-on-patient-reported-outcome-measures-use-in-medical-product-development-to-support-labeling-claims
- Brown WE, Potter HG, Marx RG, et al. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res 2004;214-23. [Crossref] [PubMed]
- Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 Years. J Bone Joint Surg Am 2016;98:1332-9. [Crossref] [PubMed]
- Anderson DE, Williams RJ III, DeBerardino TM, et al. Magnetic resonance imaging characterization and clinical outcomes after NeoCart surgical therapy as a primary reparative treatment for knee cartilage injuries. Am J Sports Med 2017;45:875-83. [Crossref] [PubMed]
- Mithoefer K, Saris DB, Farr J, et al. Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: International Cartilage Repair Society recommendations based on current scientific evidence and standards of clinical care. Cartilage 2011;2:100-21. [Crossref] [PubMed]
- Administration FaD. Guidance for industry: preparation of IDEs and INDs for products intended to repair or replace knee cartilage. US Food and Drug Administration, 2011. Available online: https://www.fda.gov/downloads/ucm288011.pdf
- Vanlauwe J, Saris DB, Victor J, et al. Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 2011;39:2566-74. [Crossref] [PubMed]
- Roos EM, Engelhart L, Ranstam J, et al. ICRS Recommendation Document: Patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage 2011;2:122-36. [Crossref] [PubMed]
- Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee subjective knee form. Am J Sports Med 2006;34:1567-73. [Crossref] [PubMed]
- Kianifard F, Islam MZ. A guide to the design and analysis of small clinical studies. Pharm Stat 2011;10:363-8. [Crossref] [PubMed]
- Moore CG, Carter RE, Nietert PJ, et al. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci 2011;4:332-7. [Crossref] [PubMed]
- Lachin JM. Fallacies of last observation carried forward. Clin Trials 2016;13:161-8. [Crossref] [PubMed]
- Hancock KJ, Westermann RR, Shamrock AG, et al. Trends in knee articular cartilage treatments: an American Board of Orthopaedic Surgery database study. J Knee Surg 2018; [Epub ahead of print]. [PubMed]
Cite this article as: Kane MS, Williams RJ 3rd, DeBerardino TM, Taylor D, Ma CB, Anderson DE, Crawford DC. Review of an exploratory phase II FDA regulated clinical trial of a novel surgical innovation: completion of a prospective, randomized, controlled trial to compare NeoCart with the standard-of-care, microfracture, for articular cartilage repair. Ann Joint 2018;3:69.