
Combined anterior cruciate ligament and anterolateral ligament lesions: from anatomy to clinical results
Introduction
Anterior cruciate ligament (ACL) tears are among the most common knee injuries and the number of ACL reconstructions (ACLR) has increased over the last few decades reaching approximately 130,000 procedures performed every year in the United States (1).
Isolated single-bundle ACLR is currently the gold standard surgical procedure for patients presenting with an ACL tear. The reconstruction is associated with superior quality of life, sports function, and a decrease in knee symptoms when compared with non-operative treatment (2). However, high graft failure rates and residual postoperative rotational instability has been reported in up to 25% of patients after ACLR (3). To improve postoperative outcomes many different strategies have been developed including double-bundle ACLR or a lateral extra-articular tenodesis (LET). To date, there has been no clinical or biomechanical evidence to show superiority of a double-bundle ACLR over a single-bundle reconstruction (4). With regards to a LET, the reason for adding this concomitant procedure was based on its ability to provide an increased lever arm for controlling rotation (5). However, it was abandoned in 1990’s because of a potentially increased morbidity and higher risk of complications and late osteoarthritis (OA) (5,6). Fortunately, the higher risk of OA has recently been disproven in a study performed by Ferretti et al. with a minimum follow-up of 10 years (7).
A renewed interest in anterolateral structures and their biomechanical properties has grown in the orthopeadic community after the publication by Claes et al. in 2013 offering a detailed description of the anterolateral ligament (ALL) of the knee (8). The “rediscovery” of this ligament, originally described by Segond in 1879, has recently inflamed passions and become the source of vigorous debates among surgeons. While some authors demonstrated its anatomy and its important contribution in knee stability others have questioned its role as knee stabilizer and even its existence (9-13). Difficulty to clearly identify the ALL could be due to the type of specimen available (embalmed or fresh) and the dissection technique utilized. However in a consensus meeting last year, the ALL was identified as a clear anatomical structure within the anterolateral complex (14). Additionally its reconstruction in patients with ACLR showed promising clinical results (15-18).
The purpose of this review is to highlight the actual understanding of the ALL anatomy and function and the impact of its reconstruction in patients with ACLR.
Anatomy
After many years of vigorous debate in literature, a panel of international researchers and clinicians who are amongst the most influent in ACL surgery have finally come to a consensus: the ALL exists (14).
The ALL was first described in 1879 by Dr. Paul Segond as a “pearly, resistant, fibrous band” that could result in an avulsion fracture of the tibial plateau when the knee was forcefully internally rotated: the Segond Fracture (19). At the beginning of the 19th century, French anatomists Vallois and then Jost took an interest in the anterolateral structures of the knee. Afterwards, it was not until 1976 when Hughston et al. described a “middle third of the lateral capsular ligament “that interest renewed in the anterolateral structures of the knee (20,21). Numerous studies followed and the ALL was named in a multitude of different ways resulting in high confusion surrounding the anterolateral anatomy of the knee (9,21).
The term “anterolateral ligament” was first used in literature in 1986 by Terry et al., but its existence was popularized beyond medical journals by Claes et al. in 2013 who gave a precise description of this structure (8,22).
Anatomical characteristics of the ALL have been investigated by various authors who reported some conflicting findings (9,11,23). Although the tibial insertion was consistently described halfway between anterior border of the fibular head and the posterior border of Gerdy’s Tubercle, the femoral insertion reported in literature varied (9,23,24). It wasn’t until recent precise dissection protocols that a consensus was found localizing its femoral insertion posterior and proximal to the lateral epicondyle (14,25).
At this location it lies superficially to the lateral collateral ligament and then runs in an anteroinferior direction to the proximal tibia, inserting an average 9.5 mm distal to the joint line (Figure 1) (23,26).
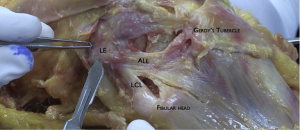
According to a reward-winning study published by Claes et al. in 2014, this location corresponds to the same location of Segond avulsion fractures (27). There are other structures that also attach on this region though and consensus could not be reached about which of these structures is strictly responsible for this lesion (14).
Histologically, the ALL is a ligamentous structure composed of dense organized collagen fibers distinct from the lateral capsular tissue that possesses attachment to the lateral meniscus (11,26,28,29).
Dimension of the ALL
Numerous studies have analyzed the dimensions of the ALL. On average, it measures 35 to 40 mm in length, 7 mm in width and 1 to 3 mm in thickness (11,13,23). It does not follow isometric behavior and the results of cadaveric studies about its length change properties during flexion are inconsistent. While some authors reported that the length of the ligament increased with knee flexion, others demonstrated that it decreased (26,28,30-32). A possible explanation for this disagreement could be related to the previously misidentified origin of the ALL on the femur. With a femoral origin close to or anterior and distal to the lateral epicondyle center, Helito et al. and Zens et al. reported an increase in the ALL length with knee flexion (31,32). On the other hand, Dodds et al. demonstrated that the ALL slackened with knee flexion if it originated proximal and posterior to the lateral femoral epicondyle. This loosening of ALL would be a condition inherently necessary to allow physiological internal rotation of the tibia during knee flexion (30,33). Both results are in accordance with the study of Imbert et al. who showed an identical behavior of the ALL contingent on these two different femoral insertions (34).
Biomechanics
The maximal load to failure and stiffness of the ALL reported in literature vary from 175 to 205 N and 20 N/mm to 42 N/mm, respectively (26,35,36). These results confirm that a semitendinosus graft (1,216 N) or a gracilis graft (838 N) are appropriate for ALL reconstruction (26).
The ALL is a stabilizer of the knee. While results about its contribution in an ACL intact knee remains controversial in literature, it is actually well documented that the ALL is an important restraint for internal rotation and pivot shift in ACL deficient knees (35,37-39). Several studies have shown that an isolated ACLR in ACL and ALL deficient knees did not restore the normal kinematics of the knee unlike combined ACLR + ALLR (40,41). In a cadaveric study, Schon et al. warned of a possible risk of over-constraint of the internal rotation of the knee after ALLR (42). This finding was recently disproven by Nielsen et al. and Inderhaug et al. who reported that ALLR was not found to over-constrain the knee joint (40,43).
Injury
Injuries to the anterolateral structures of the knee can occur at the time of an ACL tear or can be a result of overloading or subsequent giving-way episodes in chronic cases (44). The trauma mechanism for a combined ACL and ALL lesion is similar to the one for isolated ACL injury: early flexion, dynamic valgus, and internal rotation (9). According to the results of Ferretti et al., these concomitant injuries occurred in 90% of patients with apparently isolated ACL tears (44). These results are in accordance with previous studies reporting incidence of concomitant injuries to ACL and anterolateral structures from 80% to 100% (44).
Clinical diagnosis of an ALL tear remains a challenge for orthopaedic surgeons (9). The pivot-shift test remains the most reliable test to evaluate its integrity. Monaco et al demonstrated that a grade III pivot shift could be seen only in the absence of both ALL and ACL in vitro (45). This finding was not confirmed in literature though, as other authors showed that a high-grade pivot shift could be caused by injuries to the lateral meniscus, the iliotibial band, an increased tibial slope, or a general hyperlaxity (9,46).
With regards to radiology, two modalities are commonly reported on for evaluation of the ALL: ultrasound (US) and magnetic resonance imaging (MRI) (Figure 2).
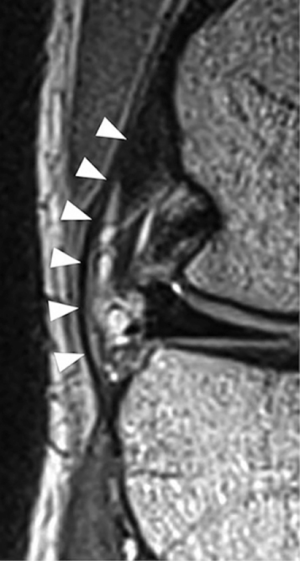
In a recent systematic review, Puzzitiello et al. identified 13 articles published between 2013 and 2017 and evaluating for ALL injury in the setting of ACL rupture using either MRI or US (46). On MRI, the ALL could be seen in its entirety or at least one portion in 76% to 100% of the knees. However, tears of the ligament remain difficult to identify with studies reporting an injured ligament in 10.8% to 62.5% of the knees. These rates are far below those reported by Ferretti et al. (90%), which suggests that false negative rate of MRI for diagnosing ALL injury remains high (44,46). However using a three dimensional (3D) MRI, Muramatsu et al. identified a higher rate of ALL injury in patients with acute ACL tear (87.5%) than previous authors using standard MRI. This rate was significantly higher when the 3D MRI was performed sooner after the trauma (<1 month) rather than later (47).
In a cadaveric study performed by Cavaignac et al., the ALL could be identified with US in all specimens and the findings corresponded precisely to the anatomical dissection (48). The same group compared then pathological appearance of ALL on US and MRI in 30 patients (49). They showed that the ALL was visible in all patients using an US and there was a significant correlation between the US and MRI findings. The ALL was found to be injured in 63% of patients with ACL tear.
ALL reconstruction and clinical outcomes
Based on our recent advances in understanding of the anatomic and biomechanical characteristics of the ALL, surgical techniques for anatomic ALL reconstruction have been described (50).
Most techniques used a single or double Gracilis graft with a femoral fixation proximal and posterior to the epicondyle (51-53). The graft was then passed deep to the ITB and fixed on the tibia equidistant between the Gerdy tubercle and the fibular head, 10 mm distal to the joint line. Sonnery-Cottet et al. proposed a distal fixation of the graft through a bony tunnel but others have used anchors or interference screw as well (50,53). The graft tension angle remains a source of debate, but it has been biomechanically demonstrated that an ALL reconstruction fixed proximal and posterior to the epicondyle and tightened in full knee extension can restore normal kinematics of the knee without applying any over-constraint of the articulation (40,53).
Despite the resurgence of interest in the ALL since 2013, studies reporting clinical outcomes of combined ACLR + ALLR with a minimum follow-up of 2 years remain scarce in literature (Table 1).

Full table
The first clinical series including 92 patients with combined ACLR + ALLR was published by Sonnery-Cottet et al. in 2015. With a mean follow-up of 32±4 months, Lysholm score and objective and subjective International Knee Documentation Committee (IKDC) scores were all significantly increased (P<0.0001) (17). 91.6% of patients were graded A on the IKDC objective, IKDC subjective score was 86.7±12.3, and Lysholm score was 92±9.8. These excellent postoperative results have subsequently been confirmed by all clinical studies published since then and were similar or even better than those reported after ACLR (16,52,55).
Graft rupture is a major concern after ACLR occurring in up to 18% of high-risk patients (56). In a comparative study including 502 patients, Sonnery-Cottet et al. demonstrated that ACLR + ALLR in a high-risk population was associated with significantly decreased graft rupture rates when compared with ACLR. The graft rupture was 10.77% (range, 6.60–17.32%) for quadrupled hamstring tendon grafts, 16.77% (9.99–27.40%) for bone-patellar tendon-bone grafts and 4.13% (2.17–7.80%) for hamstring tendon graft combined with ALLR at a mean follow-up of 38.4 months (Figure 3) (16). The rate of graft failure in ACLR + ALLR was 3.1 times lower than the quadrupled hamstring tendon grafts group and 2.5 times lower than the bone-patellar tendon-bone grafts group.
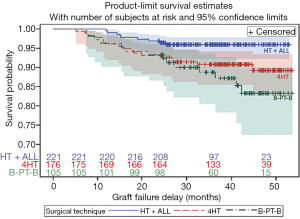
This improvement in graft failure rate after combined ACLR + ALLR was also reported by Helito et al. In their cohort of patients with a minimum 2-year follow-up, graft failure rate was 0% and 7.3% in patients with ACLR + ALLR and ACLR, respectively (P>0.05) (52).
According to Sonnery-Cottet et al., ACLR + ALLR protects the ACL graft but it also protects medial meniscus repairs. In another comparative study including 383 patients, the survival rate of a meniscal repair at 36 month follow-up in ACLR + ALLR group was 91.2% [95% confidence interval (CI), 85.4–94.8] compared to 83.8% (95% CI, 77.1–88.7%) (P=0.033) in the ACLR group (Figure 4) (15).
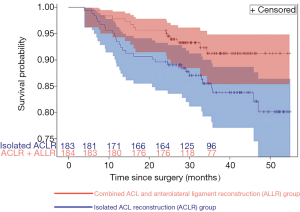
The probability of failure of a medial meniscal repair was more than two times lower in patients with ACLR + ALLR compared to patients with ACLR (hazard ratio, 0.443; 95% CI, 0.218–0.866). No other prognostic factors (e.g., age, type of sport, BMI) significantly influenced medial meniscus repair failure. This protective effect on medial meniscus repair could play an important role in long-term preservation of the articulation since it has been demonstrated that patients who underwent a medial meniscectomy at the time of ACL reconstruction had a higher risk of developing OA (57).
It is also important to note that adding an extra-articular reconstruction to an ACL reconstruction did not increase the risk of post-operative complications. In a large series of 548 patients, Thaunat et al. reported an ipsilateral knee reoperation rate of 14% at a mean of 20.4±8.0 months after the surgery. This rate is comparable to those reported after isolated ACLR (6.5% to 26.7%) (18). Additionally, among all reoperations, only 3 were specifically related to the ALL procedure and all required removal of the femoral screw. Helito et al. also reported one patient who had loosening of a femoral anchor after ACLR + ALLR that needed to be removed because of an irritation of the lateral soft part of the knee (52). Lastly, high rates of knee stiffness reported in historical series of LET were not observed in the recent series after anatomic ALLR (17,18,52).
Despite promising clinical results with ACLR + ALLR and evidence that the addition of an extra-articular reconstruction to the ACLR improves rotational laxity control, indications for a combined ACLR + ALLR remains source of debate in literature (14,58). An expert group proposed criteria to identify patients eligible for such surgical procedure (Table 2) (9).
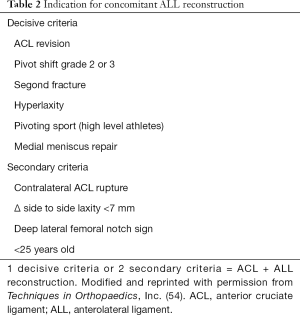
Full table
In a recently published consensus paper about ALL, Getgood et al. reported that appropriate indication for combined ACLR + ALLR may include revision ACL, high grade pivot-shift, generalized ligamentous laxity/genu recurvatum and young patients returning to pivoting activities (14).
Future
In recent years, our knowledge about anatomy and biomechanical properties of the ALL has vastly improved. It has been well demonstrated that ACLR + ALLR restores the normal kinematics of the knee unlike isolated ACLR. This improvement in knee stability is likely responsible for the promising clinical results reported in literature. However, more randomized controlled trials (RCTs) with long term follow-up are needed to confirm these results. Indeed, except for one RCT, all clinical studies included in this review are retrospective and have a nonrandomized design. In such situations, the risk of selection bias could not be excluded although multivariate analysis was performed in some studies to mitigate demographic differences between patients (15,16). Additionally, no long term follow-up studies are available in literature that could minimize the reoperation rates, which is known to increase with time elapsed from the surgery.
Another point that merits further considerations is the indication for performing a combined ACLR + ALLR. So far, no concrete consensus could be reached about who should be eligible for this combined surgical procedure. Due to lack of clinical exam maneuvers to diagnose a concomitant ALL injury in patients with ACL tear, ACLR + ALLR are currently performed in patients who are high-risk for ACL graft rupture or those presenting with signs of high rotational instability suggesting a concomitant injury of the ALL. In the future, radiologist and surgeons should increase their expertise in the evaluation of ALL on MRI or US as it could improve the accuracy of these modalities in identifying ALL injuries (46). Additionally, the diagnosis of an ALL tear could be improved by new imaging procedures like 3D MRI.
Conclusions
The ALL is an important stabilizing structure in the knee. It works to restrain internal rotation of the tibia and minimizes the pivot shift in ACL deficient knees. Its anatomy and course have been well described and it is now agreed that the ligament originates proximal and posterior to the lateral epicondyle and inserts halfway between the fibular head and Gerdy’s tubercle, 10 mm distal to the joint line. Biomechanical and clinical studies have demonstrated that the addition of an ALLR to an ACLR normalizes the kinematics of the knee, decreases graft rupture rates, and has a protective effect on medial meniscus repairs. Despite promising clinical results, the indications for ALLR continue to be debated in the literature. Future RCTs, continued technological progress in radiology, and increasing the level of expertise and familiarity with ALL evaluation by radiologists and surgeons could help identify patients who would benefit from its reconstruction in the near future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Camilo Partezani Helito and Jorge Chahla) for the series “The Multiligament Injured Knee” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The series “The Multiligament Injured Knee” was commissioned by the editorial office without any funding or sponsorship. B Sonnery-Cottet receives royalties from, and is a paid consultant for Arthrex Inc. PP Koch is consultant by Medacta Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med 2014;42:2363-70. [Crossref] [PubMed]
- Ardern CL, Sonesson S, Forssblad M, et al. Comparison of patient-reported outcomes among those who chose ACL reconstruction or non-surgical treatment. Scand J Med Sci Sports 2017;27:535-44. [Crossref] [PubMed]
- Chambat P, Guier C, Sonnery-Cottet B, et al. The evolution of ACL reconstruction over the last fifty years. Int Orthop 2013;37:181-6. [Crossref] [PubMed]
- Misonoo G, Kanamori A, Ida H, et al. Evaluation of tibial rotational stability of single-bundle vs. anatomical double-bundle anterior cruciate ligament reconstruction during a high-demand activity - a quasi-randomized trial. Knee 2012;19:87-93. [Crossref] [PubMed]
- Sonnery-Cottet B, Barbosa NC, Vieira TD, et al. Clinical outcomes of extra-articular tenodesis/anterolateral reconstruction in the ACL injured knee. Knee Surg Sports Traumatol Arthrosc 2018;26:596-604. [Crossref] [PubMed]
- Ferretti A. Extra-articular reconstruction in the anterior cruciate ligament deficient knee: a commentary. Joints 2014;2:41-7. [PubMed]
- Ferretti A, Monaco E, Ponzo A, et al. Combined Intra-articular and Extra-articular Reconstruction in Anterior Cruciate Ligament-Deficient Knee: 25 Years Later. Arthroscopy 2016;32:2039-47. [Crossref] [PubMed]
- Claes S, Vereecke E, Maes M, et al. Anatomy of the anterolateral ligament of the knee. J Anat 2013;223:321-8. [Crossref] [PubMed]
- Sonnery-Cottet B, Daggett M, Fayard JM, et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament - deficient knee. J Orthop Traumatol 2017;18:91-106. [Crossref] [PubMed]
- Ingham SJM, de Carvalho RT, Martins CAQ, et al. Anterolateral ligament anatomy: a comparative anatomical study. Knee Surg Sports Traumatol Arthrosc 2017;25:1048-54. [Crossref] [PubMed]
- Kraeutler MJ, Welton KL, Chahla J, et al. Current Concepts of the Anterolateral Ligament of the Knee: Anatomy, Biomechanics, and Reconstruction. Am J Sports Med 2018;46:1235-42. [Crossref] [PubMed]
- Williams A. Editorial Commentary: The Anterolateral Ligament: The Emperor's New Clothes? Arthroscopy 2018;34:1015-21. [Crossref] [PubMed]
- Patel RM, Brophy RH. Anterolateral Ligament of the Knee: Anatomy, Function, Imaging, and Treatment. Am J Sports Med 2018;46:217-23. [Crossref] [PubMed]
- Getgood A, Brown C, Lording T, et al. The anterolateral complex of the knee: results from the International ALC Consensus Group Meeting. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Sonnery-Cottet B, Saithna A, Blakeney WG, et al. Anterolateral Ligament Reconstruction Protects the Repaired Medial Meniscus: A Comparative Study of 383 Anterior Cruciate Ligament Reconstructions From the SANTI Study Group With a Minimum Follow-up of 2 Years. Am J Sports Med 2018;46:1819-26. [Crossref] [PubMed]
- Sonnery-Cottet B, Saithna A, Cavalier M, et al. Anterolateral Ligament Reconstruction Is Associated With Significantly Reduced ACL Graft Rupture Rates at a Minimum Follow-up of 2 Years: A Prospective Comparative Study of 502 Patients From the SANTI Study Group. Am J Sports Med 2017;45:1547-57. [Crossref] [PubMed]
- Sonnery-Cottet B, Thaunat M, Freychet B, et al. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique With a Minimum 2-Year Follow-up. Am J Sports Med 2015;43:1598-605. [Crossref] [PubMed]
- Thaunat M, Clowez G, Saithna A, et al. Reoperation Rates After Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction: A Series of 548 Patients From the SANTI Study Group With a Minimum Follow-up of 2 Years. Am J Sports Med 2017;45:2569-77. [Crossref] [PubMed]
- Paul S. Recherches cliniques et experimentales sur les epanchements sanguins du genou par entorse. Progres Medical 1879;297-299:319-321, 340-341.
- Hughston JC, Andrews JR, Cross MJ, et al. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am 1976;58:173-9. [Crossref] [PubMed]
- Cavaignac E, Ancelin D, Chiron P, et al. Historical perspective on the "discovery" of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc 2017;25:991-6. [Crossref] [PubMed]
- Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med 1986;14:39-45. [Crossref] [PubMed]
- Van der Watt L, Khan M, Rothrauff BB, et al. The structure and function of the anterolateral ligament of the knee: a systematic review. Arthroscopy 2015;31:569-582.e3. [Crossref] [PubMed]
- Daggett M, Ockuly AC, Cullen M, et al. Femoral Origin of the Anterolateral Ligament: An Anatomic Analysis. Arthroscopy 2016;32:835-41. [Crossref] [PubMed]
- Daggett M, Busch K, Sonnery-Cottet B. Surgical Dissection of the Anterolateral Ligament. Arthrosc Tech 2016;5:e185-8. [Crossref] [PubMed]
- Kennedy MI, Claes S, Fuso FA, et al. The Anterolateral Ligament: An Anatomic, Radiographic, and Biomechanical Analysis. Am J Sports Med 2015;43:1606-15. [Crossref] [PubMed]
- Claes S, Luyckx T, Vereecke E, et al. The Segond fracture: a bony injury of the anterolateral ligament of the knee. Arthroscopy 2014;30:1475-82. [Crossref] [PubMed]
- Weber AE, Zuke W, Mayer EN, et al. Lateral Augmentation Procedures in Anterior Cruciate Ligament Reconstruction: Anatomic, Biomechanical, Imaging, and Clinical Evidence. Am J Sports Med 2018;363546517751140 [Epub ahead of print]. [PubMed]
- Helito CP, Demange MK, Bonadio MB, et al. Anatomy and Histology of the Knee Anterolateral Ligament. Orthop J Sports Med 2013;1:2325967113513546 [Crossref] [PubMed]
- Dodds AL, Halewood C, Gupte CM, et al. The anterolateral ligament: Anatomy, length changes and association with the Segond fracture. Bone Joint J 2014;96-B:325-31. [Crossref] [PubMed]
- Zens M, Niemeyer P, Ruhhammer J, et al. Length Changes of the Anterolateral Ligament During Passive Knee Motion: A Human Cadaveric Study. Am J Sports Med 2015;43:2545-52. [Crossref] [PubMed]
- Helito CP, do Prado Torres JA, Bonadio MB, et al. Anterolateral Ligament of the Fetal Knee: An Anatomic and Histological Study. Am J Sports Med 2017;45:91-6. [Crossref] [PubMed]
- Qi W, Hosseini A, Tsai TY, et al. In vivo kinematics of the knee during weight bearing high flexion. J Biomech 2013;46:1576-82. [Crossref] [PubMed]
- Imbert P, Lutz C, Daggett M, et al. Isometric Characteristics of the Anterolateral Ligament of the Knee: A Cadaveric Navigation Study. Arthroscopy 2016;32:2017-24. [Crossref] [PubMed]
- Sonnery-Cottet B, Lutz C, Daggett M, et al. The Involvement of the Anterolateral Ligament in Rotational Control of the Knee. Am J Sports Med 2016;44:1209-14. [Crossref] [PubMed]
- Helito CP, Bonadio MB, Rozas JS, et al. Biomechanical study of strength and stiffness of the knee anterolateral ligament. BMC Musculoskelet Disord 2016;17:193. [Crossref] [PubMed]
- Huser LE, Noyes FR, Jurgensmeier D, et al. Anterolateral Ligament and Iliotibial Band Control of Rotational Stability in the Anterior Cruciate Ligament-Intact Knee: Defined by Tibiofemoral Compartment Translations and Rotations. Arthroscopy 2017;33:595-604. [Crossref] [PubMed]
- Monaco E, Fabbri M, Mazza D, et al. The Effect of Sequential Tearing of the Anterior Cruciate and Anterolateral Ligament on Anterior Translation and the Pivot-Shift Phenomenon: A Cadaveric Study Using Navigation. Arthroscopy 2018;34:1009-14. [Crossref] [PubMed]
- Rasmussen MT, Nitri M, Williams BT, et al. An In Vitro Robotic Assessment of the Anterolateral Ligament, Part 1: Secondary Role of the Anterolateral Ligament in the Setting of an Anterior Cruciate Ligament Injury. Am J Sports Med 2016;44:585-92. [Crossref] [PubMed]
- Inderhaug E, Stephen JM, Williams A, et al. Anterolateral Tenodesis or Anterolateral Ligament Complex Reconstruction: Effect of Flexion Angle at Graft Fixation When Combined With ACL Reconstruction. Am J Sports Med 2017;45:3089-97. [Crossref] [PubMed]
- Geeslin AG, Moatshe G, Chahla J, et al. Anterolateral Knee Extra-articular Stabilizers: A Robotic Study Comparing Anterolateral Ligament Reconstruction and Modified Lemaire Lateral Extra-articular Tenodesis. Am J Sports Med 2018;46:607-16. [Crossref] [PubMed]
- Schon JM, Moatshe G, Brady AW, et al. Anatomic Anterolateral Ligament Reconstruction of the Knee Leads to Overconstraint at Any Fixation Angle. Am J Sports Med 2016;44:2546-56. [Crossref] [PubMed]
- Nielsen ET, Stentz-Olesen K, de Raedt S, et al. Influence of the Anterolateral Ligament on Knee Laxity: A Biomechanical Cadaveric Study Measuring Knee Kinematics in 6 Degrees of Freedom Using Dynamic Radiostereometric Analysis. Orthop J Sports Med 2018;6:2325967118789699 [Crossref] [PubMed]
- Ferretti A, Monaco E, Fabbri M, et al. Prevalence and Classification of Injuries of Anterolateral Complex in Acute Anterior Cruciate Ligament Tears. Arthroscopy 2017;33:147-54. [Crossref] [PubMed]
- Monaco E, Ferretti A, Labianca L, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc 2012;20:870-7. [Crossref] [PubMed]
- Puzzitiello RN, Agarwalla A, Zuke WA, et al. Imaging Diagnosis of Injury to the Anterolateral Ligament in Patients With Anterior Cruciate Ligaments: Association of Anterolateral Ligament Injury With Other Types of Knee Pathology and Grade of Pivot-Shift Examination: A Systematic Review. Arthroscopy 2018;34:2728-38. [Crossref] [PubMed]
- Muramatsu K, Saithna A, Watanabe H, et al. Three-dimensional Magnetic Resonance Imaging of the Anterolateral Ligament of the Knee: An Evaluation of Intact and Anterior Cruciate Ligament-Deficient Knees From the Scientific Anterior Cruciate Ligament Network International (SANTI) Study Group. Arthroscopy 2018;34:2207-17. [Crossref] [PubMed]
- Cavaignac E, Wytrykowsk K, Reina N, et al. Ultrasonographic Identification of the Anterolateral Ligament of the Knee. Arthroscopy 2016;32:120-6. [Crossref] [PubMed]
- Faruch Bilfeld M, Cavaignac E, Wytrykowski K, et al. Anterolateral ligament injuries in knees with an anterior cruciate ligament tear: Contribution of ultrasonography and MRI. Eur Radiol 2018;28:58-65. [Crossref] [PubMed]
- DePhillipo NN, Cinque ME, Chahla J, et al. Anterolateral Ligament Reconstruction Techniques, Biomechanics, and Clinical Outcomes: A Systematic Review. Arthroscopy 2017;33:1575-83. [Crossref] [PubMed]
- Ferreira MC, Zidan FF, Miduati FB, et al. Reconstruction of anterior cruciate ligament and anterolateral ligament using interlinked hamstrings - technical note. Rev Bras Ortop 2016;51:466-70. [Crossref] [PubMed]
- Helito CP, Camargo DB, Sobrado MF, et al. Combined reconstruction of the anterolateral ligament in chronic ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Sonnery-Cottet B, Barbosa NC, Tuteja S, et al. Minimally Invasive Anterolateral Ligament Reconstruction in the Setting of Anterior Cruciate Ligament Injury. Arthrosc Tech 2016;5:e211-5. [Crossref] [PubMed]
- Delaloye JR, Murar J, Gonzalez M, et al. Clinical Outcomes After Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction. Tech Orthop 2018; [Epub ahead of print]. [Crossref]
- Ibrahim SA, Shohdy EM, Marwan Y, et al. Anatomic Reconstruction of the Anterior Cruciate Ligament of the Knee With or Without Reconstruction of the Anterolateral Ligament: A Randomized Clinical Trial. Am J Sports Med 2017;45:1558-66. [Crossref] [PubMed]
- Webster KE, Feller JA. Exploring the High Reinjury Rate in Younger Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2016;44:2827-32. [Crossref] [PubMed]
- Shelbourne KD, Benner RW, Gray T. Results of Anterior Cruciate Ligament Reconstruction With Patellar Tendon Autografts: Objective Factors Associated With the Development of Osteoarthritis at 20 to 33 Years After Surgery. Am J Sports Med 2017;45:2730-8. [Crossref] [PubMed]
- Musahl V, Getgood A, Neyret P, et al. Contributions of the anterolateral complex and the anterolateral ligament to rotatory knee stability in the setting of ACL Injury: a roundtable discussion. Knee Surg Sports Traumatol Arthrosc 2017;25:997-1008. [Crossref] [PubMed]
Cite this article as: Delaloye JR, Murar J, Koch PP, Sonnery-Cottet B. Combined anterior cruciate ligament and anterolateral ligament lesions: from anatomy to clinical results. Ann Joint 2018;3:82.


