Raman spectroscopy for the assessment of osteoarthritis
Introduction
Joints hold the cartilage-covered ends of the bones together and depending on the type of the joint, allow for different degrees and types of movement. The most common type is the synovial joint, where the surfaces of the bones do not come in direct contact with each other. This allows for a variety of actions such as gliding, rotational, angular, and other special movements. The synovial joint is surrounded by the articular capsule, a two-layer tissue consisting of an outer fibrous layer of connective tissue (fibrous membrane) that keeps bones together, and an inner layer (synovial membrane) which secretes the synovial fluid (SF) into the cavity created by the articular capsule. The synovial membrane (synovium) is a specialized connective tissue around the joint and apart from containing and secreting the SF, it serves as a semi-permeable membrane through which solutes are exchanged (1). It contains two types of cells: macrophage-like synovial cells (type A cells), which play an important role in phagocytosis, and fibroblastic synovial cells (type B cells), which secrete hyaluronic acid (HA), lubricin and other proteins into the SF (2). The SF is a non-Newtonian fluid with high viscosity that fills up the joint cavity (3). In normal conditions, SF is thick, clear, and colorless though various pathological conditions can affect its appearance (4). SF is mainly composed of water, proteins, proteoglycans, glycosaminoglycans (GAGs) and lipids. It has lubricating properties, due to lubricin, and shock absorbance properties, due to HA, an unsulfated GAG, which reduces the friction and wear of the articular cartilage surfaces. Additionally, SF has the important role of supplying the cartilage with nutrients and removing the waste products of metabolism (5,6). The articular cartilage is a viscoelastic tissue that covers the ends of the bones of synovial joints. It provides bone with a lubricative surface that minimizes friction during movement and helps to distribute external loads on joint. The cartilage tissue is avascular and has a slow turnover rate (7). Hence, in case of injury, the cartilage can repair itself very slowly, if at all. The cartilage is composed of chondrocytes, the only type of cells found in it, and an extracellular matrix (ECM), which primarily consists of water (up to 80% by weight), type II collagen fibrils (two-thirds of the dry cartilage weight), proteoglycans (mainly aggrecan attached on chondroitin sulfate molecules) and HA (8). The role of the chondrocytes is to produce and maintain ECM and they are located in the cartilage lacunae. Three main zones make up the articular cartilage, namely the superficial zone (10–20% of total cartilage thickness), the middle zone (40–60% of total cartilage thickness) and the deep zone (30–40% of total cartilage thickness) (8). In the molecular level, each zone is discrete from the others, having different collagen and proteoglycan content. Collagen content is high in the superficial zone, low in the middle zone and increases in the deep zone, while the proteoglycan content is low in the superficial zone, increases in the middle zone and gets its highest level in the deep zone. Moreover, in the superficial zone, collagen fibrils are oriented parallel to the cartilage surface, randomly in the middle zone, and perpendicularly on the articular surface in the deep zone (9). Beneath the deep zone lies a zone of mineralized tissue, the calcified zone. The calcified cartilage is responsible for the fixation of the collagen fibrils onto the bone adjacent to the cartilage that is called the subchondral bone. The composition of the subchondral bone is the same as that of regular cortical bones. Osteoblasts, osteoclasts and osteocytes are embedded on a mineralized organic matrix composed of water, bone mineral (mainly hydroxyapatite crystals) and organic content (mainly type I collagen) (10). Among the joint-affecting diseases (arthritic diseases), osteoarthritis (OA) is the most common, estimated to affect approximately 15% of the population, according to recent studies (11). It is a chronic, degenerative disease that primarily affects the knee and hip joints of elder people, causing pain and mobility impairment. OA was considered as a degenerative disease of the cartilage. Yet, literature suggests that OA is a disease that affects the joint as a whole, i.e., the synovial membrane, SF, articular, and calcified cartilage and subchondral bone, both in macroscopic and molecular level (12). At the first stages of OA, biochemical and/or mechanical factors cause minor alterations in the superficial layer of the articular cartilage (e.g., collagen degeneration) and/or the SF (e.g., decrease in lubricin content). These changes increase the friction coefficient between the cartilages and they begin to wear out. As the disease progresses, the cartilage gets thinner leading to increased pressure on bones. As a countermeasure, more SF is secreted which can lead to the swelling of the joint, pain, and inflammation. The subchondral bone also responds to these changes by thickening, producing irregularities (osteophytes) and roughening the cartilage surface. At this stage, cartilage degeneration causes mechanical decay with further increase of the friction coefficient. At the final disease stages, the cartilage is severely damaged, having cracks and fissures down to the subchondral bone. Diagnosis of OA relies on the overall clinical impression of the patient (pain, joint swelling, difficulty in movement, patient history, etc.) and on clinical imaging. These diagnostic methods are crucial for the clinical assessment of OA; however, they cannot provide information on the molecular changes induced by the disease and thus, on possible biomarkers.
Vibrational spectroscopy techniques, like Fourier-transformed infrared (FT-IR), near-infrared (NIR) and Raman spectroscopy are well-suited for the diagnosis and evaluation of OA even at the early disease stages. Raman spectroscopy, in particular, is a label-free, non-invasive technique with imaging capabilities and high spatial resolution. It is non-destructive, requires minimal to no sample preparation, and shows minimal sensitivity to the presence of water. That makes Raman spectroscopy an excellent technique for measurements of ex vivo biological samples that have high water content, like articular cartilage, without the requirement of dehydration. As a result, the sample remains as close to the in vivo state as possible. Here, we review the recent advances in the diagnosis and evaluation of OA by means of Raman spectroscopy.
Raman spectroscopy
Raman spectroscopy is a vibrational spectroscopic technique that relies on the inelastic scattering (Raman scattering) of monochromatic light, usually produced by a laser. The interaction of the laser radiation with the vibrational states of a material’s molecules shifts the energy of the scattered photons up (anti-Stokes shift) or down (Stokes shift) and this energy difference is eventually measured as the Raman spectrum. However, not all materials are Raman active. For Raman scattering to occur, a change in a molecule’s polarizability due to a molecular vibration has to take place (13). Each molecule has its own characteristic vibrational features, resulting from the different vibrational energy and modes between its atoms. Neighboring molecules can also influence these variables. Therefore, Raman spectra can provide information about a specific molecule along with its molecular interactions. The characteristics of a vibrational band (position, intensity, integrated area, width etc.) can be used to identify and monitor a particular functional group or regions of a specific chemical compound (14).
Due to the aforementioned factors, Raman spectra are usually complicated, having broad vibrational bands with underlying peaks belonging to both molecular and intermolecular vibrations. First- and second-derivative spectroscopy, along with peak-detection and curve-fitting algorithms, are applied to determine the exact position, number, and relative contribution of each peak. Other pre-processing techniques, like fluorescence background estimation, are typically required for the successful spectra analysis (15,16). Moreover, Raman spectra are often analyzed by univariate or multivariate statistical analysis techniques that can be used for clustering and discrimination of spectra using unsupervised and supervised algorithms (17). Raman spectroscopy has been successfully used to study diverse biological tissues providing qualitative and quantitative information of biomolecules, molecular imaging of cells and tissues, and medical diagnosis (18).
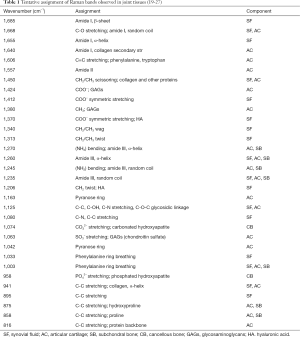
Raman bands
The most prominent Raman bands in joint tissues are shown in Table 1.
Preclinical and clinical applications
Raman spectroscopy (spontaneous Raman scattering)
Raman spectroscopy in its simplest form as spontaneous scattering, was used for the evaluation of two models of induced OA in rats (23). For the first model, OA was induced by collagenase (28), while for the second model OA was induced by treadmill exercise (29). For the experiments, the right hind knees from 8 intra-articularly saline-injected rats (used as control), 8 rats with collagenase-induced OA and 8 rats with treadmill-exercise-induced OA were obtained. Raman spectra from different points on the articular cartilage of the medial tibial plateau of each specimen were recorded using a dispersive Raman spectrometer with an excitation wavelength of 830 nm. The following ratios of integrated intensities (areas), corresponding to characteristic Raman bands observed in the mean spectra of each group, were used to assess their overall joint quality: (I) the chondroitin sulfate to phosphated HA ratio (1,063/962 cm−1), which is an indicator of the relative amount of cartilage to bone (30); (II) the phosphated HA to amide III ratio (962/1,280 cm−1), which depends on the mineralization of the tissue (31); (III) the carbonated HA to amide III ratio (1,071/1,280 cm−1), that is a marker of the remodeling of the tissue (32); (IV) the proline to hydroxyproline ratio (856/881 cm−1), which is an indicator of the collagen cross-linking (i.e., the stability of the collagen helix) (27); (V) the carbonated HA to phosphated HA ratio (1,071/962 cm−1), that is representative of mineral crystallinity (33). When considering individual integrated intensities, one-way analysis of variance (ANOVA) did not show any significant differences, except for the phenylalanine peak at 1,003 cm−1, which was found to be significantly different (P<0.05) among OA-induced rats. Furthermore, results showed that mineralization (962/1,280 cm−1) and tissue remodeling (1,071/1,280 cm−1) ratios were significantly higher in the OA-induced groups. The crystallinity ratio (1,071/962 cm−1) was also significantly different among the OA-induced samples. Takahashi et al. used Raman spectroscopy to examine human knee articular cartilages (21). The cartilage specimens came from the knees of five female patients (82–87 years old) diagnosed with OA who underwent total knee replacement surgery. A sample from the knee of a female cadaveric donor without OA was used as control. For each cartilage specimen, the degree of OA was evaluated using the Collins (0–IV) scale (34), which is based on the assessment of the excised cartilage morphology. Raman spectra were collected from each sample using a Raman microprobe spectrometer in back-scattering geometry at an excitation wavelength of 647.1 nm. The spectra were obtained from six different tibial plateau regions (3 on the medial and 3 on the lateral zones) with various degrees of OA for each sample. The ratio of disordered (random coil) to ordered (α-helix) collagen, represented by the intensity ratio 1,241/1,269 cm−1, confirmed that the medial side of the osteoarthritic cartilages has suffered more damaged than the lateral side. Furthermore, differences observed between grade I and grade II OA, using the same intensity ratio, indicated that major biochemical changes occur in cartilage as OA progresses from early (grade I) to more advanced (grade II–IV) stages. The transition from grade 0 to grade I OA did not show a significant difference, which in part could be explained by the fact that other factors than biochemical play a bigger role in OA at this stage. The authors also successfully correlated the molecular information obtained by Raman spectroscopy to the morphology-based Collins classification.
Raman microspectroscopy
Raman microspectroscopy has been employed for evaluating biochemical and structural changes that occur in the ECM of subchondral and spongy bone due to OA (35). Samples were collected from three different regions from the femoral heads of 10 patients with idiopathic hip OA and 10 patients who had suffered a fracture of the femoral neck, without, however, being diagnosed with OA prior to the fracture (control group). Samples from the central region of the femoral head were used to obtain information about the spongy bone, while samples from the superolateral (most weight-bearing) and inferomedial (least weight-bearing) regions were used to assess both subchondral and spongy bone. The cartilage was not removed from the samples. The severity of OA for each patient was evaluated by radiological inspection using the K/L (0–IV) scale (36). Raman measurements were conducted using a Raman microspectrometer, equipped with a 785 nm diode laser. For each sample, seven 50×50 µm2 Raman maps were acquired and summed up to produce the spectra that were used for analysis. Hydroxyapatite to collagen ratio (960/1,244 and 1,268 cm−1) is a measure of the degree of mineralization of the tissues, the carbonate apatite to hydroxyapatite ratio (1,071/960 cm−1), contains information about the carbonate ion substitution into the apatite, while the amide III ratio (1,268/1,244 cm−1) determines the relative amount of ordered (α-helix) to disordered (random) collagen. A two-way ANOVA did not yield any significant changes between OA and non-OA spongy bones. The situation is different, however, for subchondral bone; the mineralization ratio was significantly lower (P<0.0001) in the OA samples and was attributed to the greater remodeling of the subchondral bone under OA. Additionally, the carbonate ion substitution ratio was found to be significantly higher (P<0.0001) in the osteoarthritic subchondral bone and was considered as the counterbalancing effect to the decreased mineralization of the tissue. A significant difference was also noticed in the ordered to disordered collagen ratio (P<0.0001), with the latter being higher in the diseased samples. For all ratios examined, no significant difference was observed between the most and least weight-bearing surfaces. This suggests that, in the case of OA, biochemical factors have a bigger impact in the biochemical composition and molecular structure of subchondral bone than mechanical ones, like increased weight load. Similar conclusions were derived by another study that utilized Raman microspectroscopy (37). In their experiments, Kerns et al. used 10 tibial plateaus from patients diagnosed with grade IV [Outerbridge scale (38)] OA of the knee, that had undergone total knee replacement surgery, and 10 non-OA specimens, that were used as control. Raman spectra were collected directly from the subchondral bone of cylindrical cores, excised from the specimens, using a Raman micro spectrometer, at an excitation wavelength of 830 nm. From each sample, 4 cores were excised (2 from the medial and 2 from the lateral zones) and from each core 5 spectra were recorded (400 spectra in total). The Raman spectra were subjected to multivariate analysis using principal components analysis (PCA) and PCA-linear discriminant analysis (PCA-LDA), as well as univariate analysis. PCA managed to successfully discriminate samples between OA and non-OA groups, without, however, being able to discriminate between samples from the medial and lateral zones in each group. PCA-LDA also separated the OA and non-OA groups, but was not effective in separating samples from the medial and lateral zones in each group. Detailed analysis revealed that the bands that provide the most discriminating capability primarily belong to phosphate, amide I and phenylalanine, which indicates that the secondary structure of collagen may be responsible for the alteration of the subchondral bone mineralization. Application of PCA separately on the medial zone of OA and non-OA samples, and on the lateral zone of OA and non-OA samples, showed a separation in both cases. In the first case, the main differences were attributed to phosphate and amide I, while in the second case they were associated with hydroxyproline/proline, amide I and phosphate, suggesting that changes in the collagen structure occur during OA. Analysis of the Raman spectra using univariate statistics showed significant differences between OA and non-OA specimens for the phosphate to amide ratio (960/1,660 cm−1) and the bioapatite to collagen ratio [960/(885 + 870) cm−1]. The phosphate to amide ratio was found to be in agreement with density measurements using peripheral quantitative tomography (pQCT), that were conducted on the samples in the same study. This result indicates that the subchondral bone not only thickens during OA progression, but also undergoes biochemical changes. The bioapatite to collagen ratio, that describes the degree of mineralization, suggests that the subchondral bone is richer in mineral than collagen in OA and can be used as an OA biomarker for Raman measurements.
Confocal Raman spectroscopy
Confocal Raman spectroscopy was used for the classification of human articular cartilage specimens with varying degrees of OA and the relative assessment of protein secondary structure and proteoglycan content during the disease progression (20). Using a standard confocal Raman microscope at 632.1 nm excitation wavelength, Raman spectra from 12 articular cartilage specimens from the femoral condyle of the knee of 3 patients undergoing total knee replacement surgery were collected. The specimens were fixed in formalin and evaluated as grade I (4 specimens), grade II (4 specimens) and grade III (4 specimens) using the ICRS grading system (39). No grade IV specimens were collected, since grade IV specimens contain almost no cartilage. To obtain a more representative view of the cartilage, accounting for tissue heterogeneity at the microscopic level, thus overcoming the small sampling volume “limitation” of confocal Raman spectroscopy, spectra were acquired from 27 different locations for each specimen, resulting in a total of 108 spectra for each grade. Application of PCA on the averaged spectra showed that the three different ICRS grades were effectively discriminated in three distinct clusters, when plotted against the first three PCs (98.5% of the total variance); 87.0%, 90.7%, 100% specificity and 81.4%, 85.1%, 88.8% sensitivity was found for ICRS grades I, II and III, respectively, while the overall predictive efficiency was around 85%. The intensity band ratio of amide III at 1,245/1,270 cm−1, which represents the relative amount of random coil to ordered coil of proteins in cartilage was found to increase as OA progresses. Using a non-parametric Kruskal-Wallis ANOVA, a statistically significant difference between ICRS grades I and II, as well as I and III of OA (P<0.001 for both) was observed. However, the difference between grades II and III was not statistically significant. This could be explained by the fact that during the early OA stages biochemical factors play a greater role than mechanical factors in the progression of the disease, while the latter are more significant in the later stages. The relative proteoglycan content ratio (1,064/1,004 cm−1) was also studied and a decrease was observed as OA progresses. A non-parametric ANOVA test showed significant differences between ICRS grades I and III, as well as II and III of OA (P<0.001 and P<0.01, respectively), but not between grades I and II. This result is in agreement with other studies (40-42) that suggest that in early OA the rate of proteoglycans synthesis is increased as a countermeasure of the loss of proteoglycans due to the disease, up to a plateau, which is reached at the later stages of the disease. Both amide III and proteglycan content ratios are potentially good biomarkers for early-stage OA detection. The same group applied confocal Raman spectroscopy on human osteoarthritic chondrocytes with the purpose of detecting biomolecular features related with OA progression at the cellular level. They also tested if chondrocytes isolated from formalin-fixed osteoarthritic cartilage with different ICRS grades could be discriminated using multivariate statistics (43). The chondrocytes were isolated from 15 human articular cartilage specimens of different ICRS grades (5 grade I, 5 grade II, 5 grade III), excised from the femoral condyle of patients older than 65 years with primary OA. Raman spectra focused on the cell’s nucleus, from 30 randomly selected cells from each cartilage sample (450 spectra in total) were obtained using a commercial 632.1 nm confocal Raman spectrometer. Normalized mean spectra for each ICRS grade were used to qualitatively assess the main differences observed between chondrocytes of different OA grades. The protein content of chondrocytes, represented by the amide I (1,612–1,696 cm−1), amide III (1,229–1,300 cm−1) and phenylalanine (1,001–1,007 cm−1) Raman bands, was found to decrease with the progression of OA. The nucleic acid content (780–794 cm−1 band) was also found to decrease with OA progression, which could be attributed to the increased cleavage of internucleosomal DNA that has been observed in the advanced stages of OA (44). On the other hand, the area of 1,302–1,307 cm−1, was attributed to either lipids or amide III/nucleobase, and was observed to increase with OA severity. The application of PCA on the chondrocyte spectra yielded three distinct clusters, which correspond to the three ICRS gradings, when the spectra where plotted against the first three PCs. Results showed 100.0%, 98.1%, 90.7% specificity and 98.6%, 82.8%, 97.5% sensitivity for ICRS grades I, II and III, respectively, while the overall predictive efficiency was 92.2%.
Drop deposition Raman spectroscopy
Drop deposition is a technique based on the “coffee ring” effect (45), during which a fluid drop is left to dry on a solid substrate. While the drop dries, the fluid’s components get coarsely separated due to capillary flow. Drop deposition Raman spectroscopy (DDRS) is the application of Raman spectroscopy on drop deposits. A substantial benefit of DDRS is that signal collection from components that are weak Raman scatterers is typically enhanced, since components that because fluorescence tend to segregate to the dried drop center (46). DDRS has been applied on SF aspirates from the knee of 37 patients with varying degrees of OA for a “yes/no” classification of the disease and, also, to find possible correlation between SF Raman spectra and OA progression based on individual K/L scores (47). For the classification study, low K/L scores (K/L=0–1) were assigned to 14 patients and higher K/L scores (K/L=2–4) were assigned to 23 patients. The first were classified as belonging to a “no-damage” group, while the latter were classified as belonging to a “damage” group. SF droplets from each patient were left to dry overnight at room temperature on clean fused silica slides. Droplets were illuminated using a 785 nm laser and Raman spectra, which were obtained from the edges of the dried drops, were found to be primarily composed of proteins with diagnostic potential. To note that the interaction between proteins and other SF components, such as GAGs and lipids, can alter the overall appearance of protein Raman bands. However, studies on protein and biofluids solutions have shown that proteins retain their conformation in drop deposition experiments (25,48,49). The intensity band ratios at 1,080/1,001, 1,080/1,125, 1,235/1,260 cm−1 (amide III), 1,655/1,448 and 1,670/1,655 cm−1 (amide I), which were found to be potentially good markers of OA (24), were used. The 1,080/1,001 cm−1 ratio and the amide I ratio both were found to significantly differ (P<0.01) between the “no-damage” and “damage” groups. Unsupervised K-means cluster analysis using the same ratios also were able to discriminate the two groups with 74% sensitivity and 71% selectivity. When sorted by individual K/L scores, however, the same ratios showed only moderate correlation (0.31 and 0.35, respectively). Additionally, the ratios in the 1,010–1,150 cm−1 region provided information about the SF protein’s backbone chemical environment, and indicated that it is altered in patients with OA. Furthermore, increase of the amide I ratio in OA specimens indicated that the relative content of ordered protein secondary structure (α-helix or β-sheet) is reduced.
Surface-enhanced Raman spectroscopy
Surface-enhanced Raman spectroscopy (SERS) can be applied in cases where the signal from weak scattering biomolecules needs to be enhanced to be observed (50). A study has used SERS to measure the weak Raman signal from HA in SF, a component that could be used as a biomarker for OA (51). Using artificial and canine SF that were deposited and dried out on SERS substrates, they showed that HA could be effectively detected at low concentrations at the outer dried droplet ring in both cases. To reduce the interference of HA with other proteins (25), a trichloroacetic acid (TCA) treatment protocol was employed on the SFs. The limit of detection of HA was found at approximately 0.5 mg/mL which is close to the HA levels for pathological SF (52) but still with limited diagnostic value (53).
Fiber-optic Raman spectroscopy
A custom-designed hand-held fiber-optic Raman probe was specifically built as a minimally invasive surgical tool for use in arthroscopy (30). Two types of human cadaveric specimens were used in that study: the first consisted of a non-diseased proximal radius specimen, from which Raman microspectroscopy spectra of cartilage, subchondral bone and cancellous bone were obtained to be used as negative controls using laser excitation at 785 nm. The diseased samples were one right and one left knee joint from a single donor with confirmed OA. Visual inspection of the articular cartilages showed regions with intact cartilage, focal lesions (partial erosions) (surface area <3 mm diameter with no visible subchondral bone) and full-thickness lesions (surface area >3 mm diameter with visible subchondral bone). Tissue phantoms resembling subchondral bone with no cartilage (no scattering), eroded cartilage (moderate scattering) and intact cartilage (high scattering) were prepared as described by the authors. The fiber-optic Raman spectra were collected using an 830 nm custom-designed hand-held fiber-optic probe, with illumination fibers located in a ring with a diameter of 3 mm and multiple collection fibers. Raman microspectroscopy measurements on the proximal radius specimen showed that the Raman band at ~1,063 cm−1, attributed to chondroitin sulfate, was unique in the cartilage and was used as a spectroscopic marker of the cartilage. Bands at 958 and 1,070 cm−1, which correspond to the phosphate and carbonate stretching modes, were used as markers of the subchondral bone, while bands at 1,301 and 1,744 cm−1 were assigned to lipids and used as cancellous bone markers. The fiber-optic Raman spectra of the knee tissues showed that in the region 850–1,100 cm−1, spectra of intact cartilage had contributions from both cartilage and subchondral bone. Spectra obtained at regions with focal lesions was mainly originated from the subchondral bone rather than intact cartilage while in full-thickness lesions sites, only subchondral bone Raman signal was observed, as expected. These qualitative results indicate that cartilage thickness plays an important role on the signal sampled from the subchondral bone. Band intensity ratios calculations showed that the cartilage to bone ratio (1,063/958 cm−1) was about 5 times higher at regions where the cartilage was intact, while the subchondral bone mineralization ratio (958/920 cm−1) was found to be about 4 times lower at the same regions. The latter was the result of the diffusion of light from multiple optical scattering sources in the cartilage (mainly collagen and aggrecan) downgrading the scattering detection efficiency from the subchondral bone. Measurements on tissue phantoms were in line with the observations for the knee joints and further supported the use of the 1,063/958 cm−1 ratio as a spectroscopic marker of the relative amount of cartilage to bone. These results suggest that tissue phantoms could be used effectively as joint tissue models for designing arthroscopic probes able to assess OA in vivo.
Conclusions
Raman spectroscopy is able to successfully discriminate between healthy and diseased joint tissues, even at early disease stages, providing information about the molecular changes that occur as OA progresses. Furthermore, the flexibility of the technique allows it to be used in vivo demonstrating its potential in a clinical setting.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.09.10). NK serves as an unpaid editorial board member of Annals of Joint from Sep 2018 to Aug 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Levick JR. An Analysis of the Interaction between Interstitial Plasma Protein, Interstitial Flow, and Fenestral Filtration and Its Application to Synovium. Microvasc Res 1994;47:90-125. [Crossref] [PubMed]
- Roelofs AJ, Zupan J, Riemen AHK, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun 2017;8:15040. [Crossref] [PubMed]
- Hron J, Málek J, Pustějovská P, et al. On the Modeling of the Synovial Fluid. Adv Tribol 2010. Available online: https://www.hindawi.com/journals/at/2010/104957/
- Brannan SR, Jerrard DA. Synovial fluid analysis. J Emerg Med 2006;30:331-9. [Crossref] [PubMed]
- Wang Y, Wei L, Zeng L, et al. Nutrition and degeneration of articular cartilage. Knee Surg Sports Traumatol Arthrosc 2013;21:1751-62. [Crossref] [PubMed]
- Sun EY, Fleck AKM, Abu-Hakmeh AE, et al. Cartilage Metabolism is modulated by synovial fluid through metalloproteinase activity. Ann Biomed Eng 2018;46:810-8. [Crossref] [PubMed]
- Tiku ML, Sabaawy HE. Cartilage regeneration for treatment of osteoarthritis: a paradigm for nonsurgical intervention. Ther Adv Musculoskelet Dis 2015;7:76-87. [Crossref] [PubMed]
- Ng HY, Lee KXA, Shen YF. Articular cartilage: structure, composition, injuries and repair. JSM Bone and Joint Dis 2017;1:1010.
- Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat 1977;123:437-57. [PubMed]
- Kourkoumelis N, Lani A, Tzaphlidou M. Infrared spectroscopic assessment of the inflammation-mediated osteoporosis (IMO) model applied to rabbit bone. J Biol Phys 2012;38:623-35. [Crossref] [PubMed]
- Plotnikoff R, Karunamuni N, Lytvyak E, et al. Osteoarthritis prevalence and modifiable factors: a population study. BMC Public Health 2015;15:1195. [Crossref] [PubMed]
- Man GS, Mologhianu G. Osteoarthritis pathogenesis - a complex process that involves the entire joint. J Med Life 2014;7:37-41. [PubMed]
- Colthup N. Introduction to infrared and Raman spectroscopy. Elsevier, 2012.
- Kourkoumelis N, Balatsoukas I, Moulia V, et al. Advances in the in vivo Raman spectroscopy of malignant skin tumors using portable instrumentation. Int J Mol Sci 2015;16:14554-70. [Crossref] [PubMed]
- Cadusch PJ, Hlaing MM, Wade SA, et al. Improved methods for fluorescence background subtraction from Raman spectra. J Raman Spectrosc 2013;44:1587-95. [Crossref]
- Kourkoumelis N. Continuum determination in spectroscopic data by means of topological concepts and Fourier filtering. Nucl Instrum Methods Phys Res A 2012;691:1-4. [Crossref]
- Kourkoumelis N, Gaitanis G, Velegraki A, et al. Nail Raman spectroscopy: A promising method for the diagnosis of onychomycosis. An ex vivo pilot study. Med Mycol 2018;56:551-8. [Crossref] [PubMed]
- Shipp DW, Sinjab F, Notingher I. Raman spectroscopy: techniques and applications in the life sciences. Adv Opt Photonics 2017;9:315-428. [Crossref]
- Mangueira NM, Xavier M, de Souza RA, et al. Effect of low-level laser therapy in an experimental model of osteoarthritis in rats evaluated through Raman spectroscopy. Photomed Laser Surg 2015;33:145-53. [Crossref] [PubMed]
- Kumar R, Grønhaug KM, Afseth NK, et al. Optical investigation of osteoarthritic human cartilage (ICRS grade) by confocal Raman spectroscopy: a pilot study. Anal Bioanal Chem 2015;407:8067-77. [Crossref] [PubMed]
- Takahashi Y, Sugano N, Takao M, et al. Raman spectroscopy investigation of load-assisted microstructural alterations in human knee cartilage: Preliminary study into diagnostic potential for osteoarthritis. J Mech Behav Biomed Mater 2014;31:77-85. [Crossref] [PubMed]
- Bonifacio A, Beleites C, Vittur F, et al. Chemical imaging of articular cartilage sections with Raman mapping, employing uni- and multi-variate methods for data analysis. Analyst 2010;135:3193-204. [Crossref] [PubMed]
- de Souza RA, Xavier M, Mangueira NM, et al. Raman spectroscopy detection of molecular changes associated with two experimental models of osteoarthritis in rats. Lasers Med Sci 2014;29:797-804. [Crossref] [PubMed]
- Esmonde-White KA, Mandair GS, Esmonde-White FWL, et al. Osteoarthritis screening using Raman spectroscopy of dried human synovial fluid drops. Opt Bone Biol Diagn 2009;7166:71660J [Crossref]
- Esmonde-White KA, Mandair GS, Raaii F, et al. Raman spectroscopy of dried synovial fluid droplets as a rapid diagnostic for knee joint damage. In: Mahadevan-Jansen A, Petrich W, Alfano RR, editors. San Jose, CA: SPIE BiOS, 2008:68530Y.
- Rieppo L, Töyräs J, Saarakkala S. Vibrational spectroscopy of articular cartilage. Appl Spectrosc Rev 2017;52:249-66. [Crossref]
- Lim NSJ, Hamed Z, Yeow CH, et al. Early detection of biomolecular changes in disrupted porcine cartilage using polarized Raman spectroscopy. J Biomed Opt 2011;16:017003 [Crossref] [PubMed]
- Adães S, Mendonça M, Santos TN, et al. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res Ther 2014;16:R10. [Crossref] [PubMed]
- Beckett J, Jin W, Schultz M, et al. Excessive running induces cartilage degeneration in knee joints and alters gait of rats. J Orthop Res 2012;30:1604-10. [Crossref] [PubMed]
- Esmonde-White KA, Esmonde-White FWL, Morris MD, et al. Fiber-optic Raman spectroscopy of joint tissues. The Analyst 2011;136:1675-85. [Crossref] [PubMed]
- McCreadie BR, Morris MD, Chen T, et al. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006;39:1190-5. [Crossref] [PubMed]
- de Souza RA, Xavier M, da Silva FF, et al. Influence of creatine supplementation on bone quality in the ovariectomized rat model: an FT-Raman spectroscopy study. Lasers Med Sci 2012;27:487-95. [Crossref] [PubMed]
- Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop 2011;469:2160-9. [Crossref] [PubMed]
- Collins DH, McElligott TF. Sulphate (35SO4) Uptake by chondrocytes in relation to histological changes in osteo-arthritic human articular cartilage. Ann Rheum Dis 1960;19:318-30. [Crossref] [PubMed]
- Buchwald T, Niciejewski K, Kozielski M, et al. Identifying compositional and structural changes in spongy and subchondral bone from the hip joints of patients with osteoarthritis using Raman spectroscopy. J Biomed Opt 2012;17:017007 [Crossref] [PubMed]
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502. [Crossref] [PubMed]
- Kerns JG, Gikas PD, Buckley K, et al. Evidence from Raman spectroscopy of a putative link between inherent bone matrix chemistry and degenerative joint disease. Arthritis Rheumatol 2014;66:1237-46. [Crossref] [PubMed]
- Wright RW, Ross JR, Haas AK, et al. Osteoarthritis classification scales: Interobserver reliability and arthroscopic correlation. J Bone Joint Surg Am 2014;96:1145-51. [Crossref] [PubMed]
- ICRS SCORE/GRADE|ICRS Main Site 2018. Available online: https://cartilage.org/society/publications/icrs-score/
- Rizkalla G, Reiner A, Bogoch E, et al. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest 1992;90:2268-77. [Crossref] [PubMed]
- Thompson RC, Oegema TR. Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am 1979;61:407-16. [Crossref] [PubMed]
- Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am 1971;53:523-37. [Crossref] [PubMed]
- Kumar R, Singh GP, Grønhaug KM, et al. Single cell confocal Raman spectroscopy of human osteoarthritic chondrocytes: A preliminary study. Int J Mol Sci 2015;16:9341-53. [Crossref] [PubMed]
- Verrier S, Notingher I, Polak JM, et al. In situ monitoring of cell death using Raman microspectroscopy. Biopolymers 2004;74:157-62. [Crossref] [PubMed]
- Deegan RD, Bakajin O, Dupont TF, et al. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997;389:827-9. [Crossref]
- Zhang D, Mrozek MF, Xie Y, et al. Chemical segregation and reduction of Raman background interference using drop coating deposition. Appl Spectrosc 2004;58:929-33. [Crossref] [PubMed]
- Esmonde-White KA, Mandair GS, Raaii F, et al. Raman spectroscopy of synovial fluid as a tool for diagnosing osteoarthritis. J Biomed Opt 2009;14:034013 [Crossref] [PubMed]
- Ortiz C, Zhang D, Xie Y, et al. Validation of the drop coating deposition Raman method for protein analysis. Anal Biochem 2006;353:157-66. [Crossref] [PubMed]
- Kopecký V Jr, Baumruk V. Structure of the ring in drop coating deposited proteins and its implication for Raman spectroscopy of biomolecules. Vib Spectrosc 2006;42:184-7. [Crossref]
- Bonifacio A, Cervo S, Sergo V. Label-free surface-enhanced Raman spectroscopy of biofluids: fundamental aspects and diagnostic applications. Anal Bioanal Chem 2015;407:8265-77. [Crossref] [PubMed]
- Dehring KA, Mandair GS, Roessler BJ, et al. Surface-enhanced Raman spectroscopy detection of hyaluronic acid: A potential biomarker for osteoarthritis. In: Kneipp K, Aroca R, Kneipp H, editors. New Approaches in Biomedical Spectroscopy. Washington: American Chemical Society, 2006:123.
- Kvam C, Granese D, Flaibani A, et al. Purification and characterization of hyaluronan from synovial fluid. Anal Biochem 1993;211:44-9. [Crossref] [PubMed]
- Pavelka K, Forejtová Š, Olejárová M, et al. Hyaluronic acid levels may have predictive value for the progression of knee osteoarthritis. Osteoarthritis Cartilage 2004;12:277-83. [Crossref] [PubMed]
Cite this article as: Pavlou E, Zhang X, Wang J, Kourkoumelis N. Raman spectroscopy for the assessment of osteoarthritis. Ann Joint 2018;3:83.