Balloon arthroplasty: indications, technique, and European outcomes
Introduction
Massive irreparable rotator cuff tears remain a challenge in orthopaedic shoulder surgery. The optimal treatment method for addressing such case presentations is still unknown even with expanding surgical techniques and technologies. For many years, surgeons only had the option of treating patients with partial rotator cuff repair and debridement. Results varied but could be less than successful (1-3). In recent years, techniques such as superior capsular reconstruction, posterosuperior tendon transfers (e.g., latissimus dorsi or lower trapezius), and reverse shoulder arthroplasty have provided patients with more options. However, these options continue to remain limited by their complexity of surgical technique, durability of outcomes, and appropriate patient criteria. The interpositional subacromial balloon spacer (InSpace balloon, Orthospace, Caesarea, Israel) was developed in 2010 for patients with irreparable posterosuperior rotator cuff tears and has demonstrated significant success throughout its utilization in Europe. The device recently underwent an FDA Investigational Device Exemption trial in the United States.
Proximal humeral migration in the setting of cuff deficiency
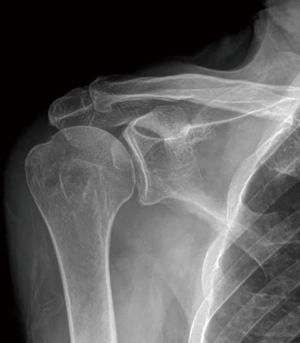
In addition to providing functional movement of the glenohumeral joint in various planes, the rotator cuff serves an important role in providing counterforce to the deltoid muscle for proper elevation of the arm. As the deltoid muscle contracts, it provides a superior vector force on the humerus. Without a counterforce at the glenohumeral joint, this superiorly directed vector creates a sheer effect of the humeral head against the glenoid face. As dynamic stabilizers, the tendons of the rotator cuff work in a concerted effort to compress the humeral head against the face of the glenoid during deltoid contraction which creates a fulcrum at the glenohumeral joint. This allows the contraction of the deltoid to raise the humerus around the center of rotation about the glenohumeral interface. In the setting of a massive posterosuperior rotator cuff tear, this compressive force is lost and the head of the humerus can begin to migrate proximally as the unrivaled force of the deltoid pulls superiorly. Over time, this can manifest as proximal humeral migration as seen on radiographic imaging (Figure 1). Eventually, the proximal migration of the humerus is halted as the humeral head abuts the undersurface of the overlying acromion. Repetitive contact between these two bones can result in the acetabularization pattern of wear seen in the most extreme forms of cuff tear arthropathy.
Indications for use
Patients who demonstrate proximal humeral migration in the setting of an irreparable rotator cuff tears are the primary indicated population for the subacromial balloon spacer. The primary purpose of the subacromial balloon is to help restore the missing counterforce to the deltoid and recreate the proper biomechanics of a cuff-deficient shoulder. In certain instances, patients who demonstrate proximal humeral migration can still have a rotator cuff tear that is repairable. All patients should be evaluated for such repairability in the preoperative and intraoperative setting. Advanced imaging such as MRI, ultrasound, or a CT arthrogram can often give clues toward cuff tendon length, retraction, and muscle atrophy that may indicate whether a repair is possible. Intraoperatively, the quality and mobility of the tendon tissue can be assessed. If adequate excursion of the tendon is achievable and it appears amenable to repair, a primary repair is always recommended. In those patients where a repair is unlikely, the subacromial spacer balloon is a viable option.
There are, however, elements of rotator cuff function that must be intact in order for the balloon to be most effective. A functional subscapularis and teres minor tendon are essential for the use of the subacromial balloon. These two tendons are responsible for restoring the force couple of internal and external rotation of the humeral head against the glenoid and must be repaired if they are torn prior to insertion of the balloon. If these tendons remain torn, a patient is unlikely to achieve partial or full benefit of the balloon as the missing rotational force couple will inhibit the ability to actively rotate and even perform forward elevation. Additionally, patients indicated for the procedure must have a functional deltoid muscle as this will be the primary elevator of the arm like it is in the setting of a reverse shoulder arthroplasty. Patients with active forward elevation to at least 90 degrees are best indicated as those with true pseudoparalysis of the arm are likely beyond the limits the subacromial balloon’s capabilities. This criterion is critical when considering patients for subacromial balloon spacer placement. Patients and surgeons alike must realize that the balloon is not a universal solution to those with deltoid dysfunction or true pseudoparalysis and careful patient selection is critical to a successful outcome.
There are two other major contraindications to the use of the subacromial balloon spacer in addition to deltoid dysfunction and true pseudoparalysis. Patients with advanced glenohumeral arthritis are not recommended to undergo balloon placement as they will likely continue to have residual pain from their arthritic joint surface or even limited motion secondary to osteophyte formation. Lastly, the balloon, like any orthopaedic device, should not be implanted in the setting of an active infection. This could lead to the formation of a biofilm on the device and results in propagating a septic joint.
Surgical technique
After general endotracheal anesthesia is administered, the patient is seated upright in the beach chair position with all areas of bony prominences and possible sites of nerve compression well-padded and offloaded. The beach chair position is preferred over lateral decubitus as it allows for gravitational distraction of the humeral head. This is more consistent with the in vivo position of the humerus seen on standing plain radiographs and allows for a more accurate assessment of the acromiohumeral interval distance. This is important for correct selection of the appropriately sized balloon as there are currently three available sizes for the subacromial balloon (InSpace, OrthoSpace, Caesarea, Israel): small (40 mm ×50 mm), medium (50 mm ×60 mm), and large (60 mm ×70 mm).
A standard posterosuperior arthroscopic portal is created for initial diagnostic arthroscopy of the intraarticular joint space. The articular surfaces of the glenoid and humeral head are carefully assessed for any cartilage damage. Patients with moderate to severe arthritic surfaces may continue to have pain and dysfunction even after balloon placement and it is important to ensure that no such findings are present. The undersurface of the posterosuperior rotator cuff can also be examined. In order to have a properly balanced force couple, the subscapularis must be intact or repairable. This can be performed at this time prior to subacromial positioning of the balloon. A standard anterior portal can be created in the rotator interval just above the superior border of the subscapularis for instrumentation. Once the integrity of the subscapularis is confirmed, the surgeon can perform a labral debridement and biceps tenotomy/tenodesis if it is deemed appropriate.
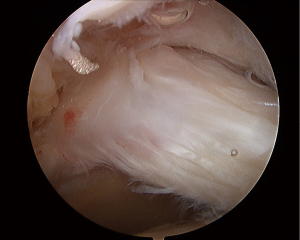
Attention is then turned to the subacromial space to begin preparation of balloon insertion. The arthroscope is introduced into the subacromial space through the initial posterior portal. A spinal needle is used to determine the placement of a lateral portal in line with the posterior border of the clavicle. Prior to creating the lateral portal with the scalpel, it is important to ensure that the spinal needle rests parallel with the floor and is not impeded by the acromion or humeral head. This will help decrease the risk of these structures obstructing any instrumentation used through the portal. A combination of an arthroscopic shaver and electrocautery can be used through the lateral portal to perform a bursectomy so that visualization of the humeral head and rotator cuff is achieved. However, a bursectomy is kept to a minimum. Extensive bursectomy is avoided as it can lead to increased dead space which can result in balloon migration into the supraspinatus or infraspinatus fossa (Figure 2). Prior to the decision for balloon placement, the superior and posterior cuff should be assessed and mobilized as best as possible for repair. Restoration of anatomy should always take precedence over placement of the balloon and repair of the cuff should be performed whenever possible. If only a partial repair of the posterior rotator cuff is possible, then this should be performed to assist in restoring the force couple similar to the repair of the subscapularis anteriorly.
With all fixable portions of the rotator cuff tear repaired, area for balloon placement is measured. A graduated probe is used to measure the deficient cuff space in both the sagittal and coronal planes. Measurements from anterior to posterior are taken from the subscapularis to the posterior intact cuff. The medial to lateral distance is measured from the edge of the glenoid rim to the greater tuberosity footprint. These measurements are then used to choose from the three available balloon sizes to determine the best fit. Placement of the balloon can be performed through either the posterior or lateral portal based on surgeon preference. The authors have found posterior placement to offer better visualization and theoretically less likelihood of balloon migration. Therefore, the arthroscope is placed into the lateral portal.
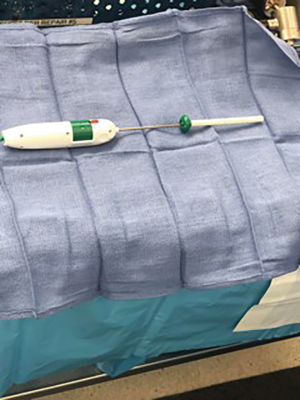
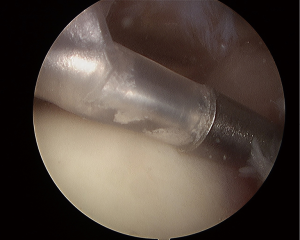
Once the proper balloon size is selected, it is prepared on the back table. A 60-cc syringe is filled with sterile saline warmed to roughly 40 degrees Celsius and connected to the extension tubing of the insertional device (Figure 3). All air bubbles are carefully removed from the syringe and tubing. The insertion device is placed into the posterior portal with positioning over the glenoid rim. The protective sheath around the balloon is withdrawn from the uninflated balloon by pulling back on the green handle of the inserter (Figure 4). The syringe is then used to fill the saline into the balloon as it is visualized in the subacromial space. The translucent balloon allows its proper placement to be maintained as the humeral head, acromion, and intact portions of the cuff are seen arthroscopically. The balloon is initially filled with 40 cc of saline to fully expand it. Once expanded, any excess fluid is removed to the recommended amount necessary for each of the three balloon sizes. The balloon is then sealed and detached from the inserter using the proper buttons on the device.
With the balloon now inflated, the arm is taken through a range of motion (ROM) to ensure no migration of the balloon (Figure 5). If there is subluxation of the balloon, it may be pierced with a spinal needle and removed for replacement. Once the balloon is confirmed stable, the arthroscope is removed from the shoulder and the portal incisions are closed with simple interrupted non-absorbable monofilament suture. Sterile dressings are applied and the arm is placed into a standard sling. Rehabilitation consists of three 4-week block phases. For the first 4 weeks, the patient wears a sling for comfort and remains non-weightbearing with the operative arm. The arm is allowed to be used for light activities of daily living (i.e., feeding, dressing, writing). Formal physical therapy is begun the following 4 weeks with a focus on phase 1 and 2 stretching with early strengthening. The following 4 weeks consists of full strengthening with the development of a home exercise program. After a total of 12 weeks, all restrictions are lifted.
European outcomes
Although the results of the recently completed FDA investigational trial have yet to be revealed, much of what has been published on the use of the subacromial balloon spacer comes from the European experience where the device has been used for nearly a decade. Over 10,000 cases have been performed in Europe using the InSpace balloon. The first reported outcomes were published by Senekovic et al. in 2013 looking at a cohort of twenty patients with minimum of 3 years of follow up (4). Within 1 week of surgery, patients demonstrated significant pain relief (4). The authors found that patients gained a rapid improvement in Constant scores as well as their ROM as early as 6 weeks following surgery (4). The improvement in Constant scores continued for up to 3 years with average scores increasing from 33.4 preoperatively to 65.4 at the most recent follow-up visit (4). The authors followed up with these patients at 5 years and found that patients continued to demonstrate satisfactory outcomes. The majority of the patients maintained a considerable improvement of their Constant scores (85% with minimum 15-point improvement; 62% with minimum 25-point improvement) and ROM (75%) (5). In addition, 95% of the patients showed complete degradation of the device at 3 years out from surgery (5).
Another series looked at 37 patients with at least 1 year of follow-up (mean, 33 months) with a similar demonstration of Constant score and ROM improvement (6). Average Constant scores improved from 44.8 to 76.0 and ROM improved in multiple planes including forward elevation (30-degree improvement), abduction (60-degree improvement), and external rotation (15-degree improvement) (6). The authors also found that the majority of patients (32/37) remained stable with the degree of arthritis in their glenohumeral joint (6). Piekaar et al. of the Netherlands also examined 1-year outcomes in 46 shoulders of 44 patients with improvements demonstrated (7). Patients improved significantly for both Constant scores (mean increase of 21.58 points) and Oxford shoulder scores (mean increase of 10.46 points) (7). The majority of patients (80%) reported satisfaction with their outcomes at 1 year out from surgery (7). A smaller series out of the UK looked at 14 patients for a mean of 1.5 years out from surgery and also found significant improvements in Constant scores and all ROM planes (8). Even Oxford shoulder scores were found to improve with an average score of 26 preoperatively increasing to 48.2 postoperatively at the latest follow up visit (8). None of the patients in this study experienced night pain following surgery and they demonstrated an average 40% increase in activities of daily living (8). Most impressive is that most of the patients (12/14) in this study had active forward elevation below 90 degrees in the preoperative setting with only one patient unable to achieve that ROM goal following surgery (8).
More recent studies have begun to look beyond a cohort of patients undergoing implantation of the balloon device and have started to compare groups. In Israel, Maman et al. compared 42 patients who underwent spacer placement with half undergoing biceps tenotomy and half without tenotomy (9). All patients demonstrated improved Constant scores with no significant difference noted between those with and without a concurrent biceps tenotomy performed (9). In Germany, Holschen et al. compared 23 patients who underwent balloon placement to 23 patients who underwent debridement and partial rotator cuff repair (10). Preoperative ASES and Constant scores were lower for the balloon spacer group as were the subsequent postoperative scores, but the patients who underwent spacer placement had a higher absolute improvement of both scores compared to the more conventionally treated patients (10). Both groups reported pain improvement and were overall satisfied with their outcomes (10).
Taking this notion that the use of the subacromial spacer can result in similar improvement to more conventional methods, Castagna et al. looked at the use of the spacer in the setting of cost-effectiveness. They compared the subacromial spacer to conservative treatment, rotator cuff repair, and reverse shoulder arthroplasty (11). The authors found that the use of the subacromial spacer was favorable over cuff repair and arthroplasty with a cheaper cost and increased effectiveness when accounting for quality-adjusted life years (11). And while conservative treatment was certainly the least costly option, the subacromial spacer resulted in a greater improvement in quality-adjusted life years per cost in what the authors deemed the incremental cost-effectiveness ratio (11). Collectively, these studies present the use of the subacromial spacer as an effective and promising alternative to treating patients with irreparable cuff tears.
Only one recently published study has demonstrated less than satisfactory outcomes with the use of the device. Ruiz Ibán et al. from Spain followed 15 patients for 2 years after placement of the subacromial spacer and evaluated the patients using the Constant score, Simple Shoulder Test, and QuickDash questionnaire (12). One-third of the patients required conversion to a reverse shoulder arthroplasty with only 60% of the remaining patients experiencing an improvement in Constant scores greater than 10 points (12). The authors concluded that only 40% of patients in the study seemed to clearly benefit from the use of the subacromial spacer (12). Two of the five patients that required conversion to a reverse total shoulder arthroplasty were pseudoparalytic at preoperative presentation (12).
Combining all of these studies, the most impressive finding is the reported safety and ease of the surgical technique. There has only been two device-related complication reported in the aforementioned studies (4-12). That complication, in both instances, was migration of the implant (4,8). Only in the study by Senekovic et al. was action required with removal and replacement (4). Of the studies that examined operative time, placement of the device required only 2 to 30 minutes with two studies claiming an average of 10 minutes. These studies also demonstrated that as the learning curve improves, the required operative time becomes less (4-6).
Conclusions
The subacromial interpositional balloon spacer has great potential in the treatment of the difficult condition known as an irreparable rotator cuff tear. Serving to restore the humeral head in its native position and reverse proximal humeral migration, the device works by counteracting the unopposed pull of the deltoid muscle that is seen in cuff tear arthropathy. Since its release in Europe in 2010, the device has had very promising results. It has been demonstrated to improve outcome scores, reduce pain, and increase shoulder ROM while remaining relatively devoid of complications and significant costs. Experience in a recent FDA trial in the United States has been met with similar encouraging findings. The subacromial spacer is relatively safe and easy to use and has not been found to prevent any potential future surgeries should it fail to provide adequate outcomes. When indications are appropriately followed and patients are properly selected, the subacromial balloon spacer offers surgeons a very powerful tool in helping to treat the irreparable rotator cuff tear.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Evolving Trends in Reverse Shoulder Arthroplasty”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.10.02). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. JAA reports other from OrthoSpace, during the conduct of the study, and received financial support from OrthoSpace for the IDE trial of the arthroplasty balloon. JAA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from Apr 2018 to May 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shon MS, Koh KH, Lim TK, et al. Arthroscopic Partial Repair of Irreparable Rotator Cuff Tears: Preoperative Factors Associated With Outcome Deterioration Over 2 Years. Am J Sports Med 2015;43:1965-75. [Crossref] [PubMed]
- Iagulli ND, Field LD, Hobgood ER, et al. Comparison of partial versus complete arthroscopic repair of massive rotator cuff tears. Am J Sports Med 2012;40:1022-6. [Crossref] [PubMed]
- Jeong JY, Yoon YC, Lee SM, et al. Arthroscopic Incomplete Repair Using a "Hybrid Technique" for Large to Massive Rotator Cuff Tears: Clinical Results and Structural Integrity. Arthroscopy 2018;34:2063-73. [Crossref] [PubMed]
- Senekovic V, Poberaj B, Kovacic L, et al. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol 2013;23:311-6. [Crossref] [PubMed]
- Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg 2017;137:95-103. [Crossref] [PubMed]
- Deranlot J, Herisson O, Nourissat G, et al. Arthroscopic Subacromial Spacer Implantation in Patients With Massive Irreparable Rotator Cuff Tears: Clinical and Radiographic Results of 39 Retrospectives Cases. Arthroscopy 2017;33:1639-44. [Crossref] [PubMed]
- Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskelet Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Yallapragada RK, Apostolopoulos A, Katsougrakis I, et al. The use of a subacromial spacer-inspace balloon in managing patients with irreparable rotator cuff tears. J Orthop 2018;15:862-8. [Crossref] [PubMed]
- Maman E, Safran O, Beyth S, et al. Biceps tenotomy does not affect the functional outcomes of patients treated with spacer implantation due to massive irreparable rotator cuff tears. Open Orthop J 2017;11:1577-84. [Crossref] [PubMed]
- Holschen M, Brand F, Agneskirchner JD. Subacromial spacer implantation for massive rotator cuff tears: clinical outcome of arthroscopically treated patients. Obere Extrem 2017;12:38-45. [Crossref] [PubMed]
- Castagna A, Garofalo R, Maman E, et al. Comparative cost-effectiveness analysis of the subacromial spacer for irreparable and massive rotator cuff tears. Int Orthop 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Ruiz Ibán MA, Lorente Moreno R, Ruiz Díaz R, et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Horneff JG 3rd, Abboud JA. Balloon arthroplasty: indications, technique, and European outcomes. Ann Joint 2018;3:85.