Reverse shoulder arthroplasty: diagnostic and treatment options for the infected reverse
Introduction
Modern reverse total shoulder arthroplasty (RTSA) as initially described by Grammont, has increased in use significantly over the past years, and was approved by the United States Food and Drug Administration in 2003 (1,2). Although RTSA is a reliable treatment option for cuff tear arthropathy (CTA) and osteoarthritis, it is not without complications. Reported rates of infection for RTSA are up to 5% (3,4). Reported rates of infection in patients with rheumatoid arthritis can be up to 9.5% (5). Periprosthetic joint infections (PJI) of the shoulder are the main cause of revisions within the first 2 years following arthroplasty (6).
The definition of a periprosthetic infection of shoulder arthroplasty is unclear, and there is no consensus for infection workup. A meta-analysis by Hsu et al. showed significant variability in the definition of infection and culture practices (7). The current literature on infection in RTSA is generally poor and lacking definitive conclusions.
The normal flora of the shoulder makes diagnosing PJI difficult (8). With increasing knowledge of certain bacteria such as Cutibacterium acnes (formerly Propionibacterium acnes) and coagulase negative Staphylococcus species (i.e., papers written after 2000 showing modern diagnostic techniques), our understanding of true infection versus deep tissue inoculation versus specimen contamination continues to evolve. Though diagnostic strategies are controversial, the present study will present a general diagnostic approach and review of surgical treatment options.
Diagnosis
Diagnosis of shoulder PJI is very challenging. According to the Musculoskeletal Infection Society (MSIS) criteria a diagnosis of infection is made with 1 or more major criteria (sinus tract communicating with the prosthesis; or a pathogen isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint), or 4 or more minor criteria [elevated serum erythrocyte sedimentation rate (ESR) and serum C-reactive protein (CRP) concentration; elevated synovial leukocyte count; elevated synovial neutrophil percentage; presence of purulence in the affected joint; isolation of a microorganism in one culture of periprosthetic tissue or fluid; and greater than five neutrophils per high-power field in five high-power fields observed from histological analysis of peri-prosthetic tissue at ×400 magnification] (9,10). While PJI caused by virulent organisms (e.g., Staphylococcus aureus) commonly manifests with clinical signs (erythema, swelling) and elevated laboratory markers (CRP, ESR), PJI with less virulent organisms (Cutibacterium acnes) uncommonly presents with obvious evidence of infection (11). Other techniques that have demonstrated efficacy in hip and knee PJI, such as leukocyte esterase and IL-6, have not been shown to be a reliable test for PJI (12,13). As a result, the diagnosis of PJI is commonly made several weeks after revision surgery and is heavily reliant on culture results. Cultures are unfortunately difficult to interpret because C. acnes is the most commonly cultured bacteria in cases of both primary shoulder arthroplasty and revision arthroplasty. As a result, its role as a commensal organism, deep tissue inoculant, or infecting agent remains uncertain.
Despite the limitations, the number of cultures is a variable but important determinant of infection, as serological markers such as WBC, CRP, and ESR may be falsely normal even in the setting of infection. Mook and Garrigues described the phenomena of “failed arthroplasty with positive cultures” (FAPC) (14). In this scenario they recommend a description of the number of positive cultures over the total number of cultures taken and stating the organism (e.g., “2/5 for P. acnes” would indicate 2 cultures out of 5 taken were positive for P. acnes).
Clinical evaluation
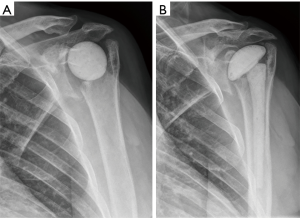
Clinical presentation of PJI can be extremely variable in appearance. Suppurative infections present similar to hip and knee periprosthetic infection, which generally involve pain, swelling, erythema, and purulence if a wound is present. Indolent infections require a high level of suspicion, as these do not present with the hallmarks of infection as described above. Patients may complain of poor function or degradation of function over time. As described in the following section in more detail, there may be signs of radiographic loosening or fracture which could represent infection (Figure 1).
Risk factors for periprosthetic infection include postoperative hematoma (15), arthroplasty for trauma (16), younger age (17), and male sex. Pain is the most common complaint, followed by stiffness, erythema, effusion, fever, and night sweats (18).
A thorough examination of the entire extremity is essential. The examiner must evaluate the skin completely for signs of skin breakdown, potential sinus tracts, erythema, swelling, and muscle bulk. A neurovascular examination is also critical to perform at this stage. The shoulder active and passive motion should be assessed. Pain with passive motion is a nonspecific sign of infection but is frequently present when there is an effusion.
Imaging
Initial imaging should include 4 views of the shoulder (AP, true-AP, scapular-Y, axillary views) to rule out other potential causes of shoulder pain. X-rays should be scrutinized for nonspecific changes including effusion, bony resorption, periosteal reaction, osteolysis, and contiguous radiolucent lines (Figure 1). Serial radiographs may show subtle changes over time, such as increase in size of radiolucent lines.
Computed tomography (CT) and/or magnetic resonance imaging (MRI) may be done for preoperative planning but is not necessary for diagnosis. If osteomyelitis is suspected, MRI may be beneficial for planning bony debridement and identifying pockets of purulence within the soft tissues prior to entering the operating room.
Laboratory testing
Although ESR and CRP are frequently performed in suspected cases of infection, these laboratory values have a low sensitivity, and thus are not helpful for ruling out PJI (19). One must bear in mind that normal inflammatory marker levels do not preclude deep joint infection in the shoulder as they do in the hip and knee. Though inflammatory markers have low sensitivity, specificity is relatively high (20); and so, our current practice includes obtaining ESR and CRP in cases with suspicion of infection.
Aspiration and cultures
There is no clear evidence to guide the decision to aspirate in the case of suspected infection. According the American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines for periprosthetic hip and knee infections, aspiration should be performed if the ESR or CRP is elevated (21). No thresholds for intraarticular WBC count have been reported in shoulder PJI and attempted aspirations are commonly “dry”. Dilisio et al. reported fluoroscopically guided glenohumeral aspiration yielded a sensitivity of 16.7%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 58.3% (22). Because of the high specificity and positive predictive value, we commonly obtain a preoperative aspiration in cases with suspicion of PJI.
Intraoperative cultures which grow an organism early, with multiple positive bottles may be a more reliable indication for a “true infection”. One study showed positive cultures with a median of 5 days in the probable true-positive group compared to a median of 9 days in the probable contaminant group (23). Our current practice involves taking deep cultures at 5 sites at the time of revision, including the anterior and posterior capsule, behind the glenoid component, under the explanted humeral head, and from within the humeral canal, and holding cultures for 13 days. Both aerobic and anaerobic media are utilized.
Surgical management
General approach to the infected RTSA
Once the decision has been made to perform surgical intervention for infection, several factors must be considered. The surgeon must assess the likely etiology of infection (e.g., direct inoculation from prior surgery, latent infection, osteomyelitis, systemic infection etc.), comorbidities, and patient functional level. Antibiotic suppression alone has shown high failure rates with 60% symptomatic shoulders (24). Irrigation and debridement with component retention has also shown high recurrence rates of 12–50% and is not routinely recommended (18,24). While certain clinical situations (infirm patients, acute hematogenous infections, etc.) may warrant an attempted irrigation and debridement with component retention, patients and surgeons must understand the high recurrence rate relative to alternative treatments.
Two-stage revision (25)
The traditional treatment of PJI has been a two-stage revision, consisting of removal of all components and cement, followed by a period of systemic culture-specific antibiotics and typically placement of an antibiotic cement spacer. There is no literature to support the time needed prior to definitive replantation, however a 6-week course is utilized by many clinicians.
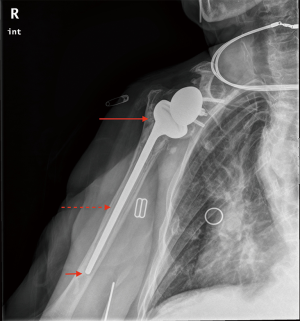
An antibiotic-loaded cement spacer is used at the time of explant to maintain soft tissue tension and to create an envelope for later implant. The spacer also provides local antibiotic efflux. Vancomycin and an aminoglycoside antibiotic are most commonly used in combination; however, there is no clear consensus for antibiotic choice. Antibiotic spacers can be fashioned in multiple ways including stemmed and stemless designs (Figure 2). Neither design has been demonstrated to be more effective for treatment of PJI (26). Another method described by Levy et al., used a hemiarthroplasty coated with antibiotic-loaded cement (27). Nine of 14 patients in their series had satisfactory outcomes with the functional spacer and did not go on to a second stage reimplantation (27).
RTSA is the treatment of choice for the second-stage reimplantation. As RTSA does not rely on a functional rotator cuff, the initial debridement may be more aggressive so as to clear any potential sites of contamination which may include the rotator cuff. In a recent series of infected RTSA, two-stage revision was performed in 14/32 cases (44%). The authors report a 36% complication rate, most of which were attributed to hematoma (28). Ortmaier et al. reported on their series of 20 patients with infected RTSA, 60% of which were treated with two-stage revision. The authors reported a success rate of 75% with two-stage revision, however no significant differences in Constant score or VAS pain scores were found when compared to debridement, but functional outcomes were significantly improved when compared to resection (29). One retrospective multicenter study showed similar treatment success, defined by eradication of infection, in two-stage revision, resection arthroplasty, or permanent spacer placement. However, resection arthroplasty had significantly worse function (30).
One-stage revision
Single-stage revision is an attractive treatment modality as patients requiring revision typically have medical comorbidities, are of advanced age, and may have significant bone loss. A single curative procedure offers benefits for cost savings and avoids multiple procedures for the patient. Several studies have shown equivalent rates of eradication of infection in single-stage compared to two-stage revision. In a recent systematic review, Nelson et al. found no statistically significant differences for treatment success between one-stage, two-stage, or resection arthroplasty, except for the highest Constant scores being in the one-stage revision patients (31). Jacquot et al. had 100% eradication of infection with a single-stage revision compared to 64% in the two-stage revision group, although this difference was not statistically significant (28). In a recent study, Hsu et al. described their single-stage treatment protocol in patients with 2 or more positive cultures for P. acnes with significant improvement in functional outcomes at 45 months from time of revision. This protocol does involve 6 months of antibiotics including IV and oral and resulted in 42% rate of antibiotic-related adverse events. This study involved mostly revision of hemiarthroplasty and anatomic total shoulder arthroplasty, which makes the results difficult to extrapolate to RTSA (32). Grosso et al. found a 5.9% recurrence rate in shoulders treated with a single-stage revision without prolonged postoperative antibiotics in those shoulders with a single positive culture at the time of revision. These patients were identified preoperatively and intraoperatively with a low suspicion for infection (33).
Frequently cultures are taken intraoperatively even when the suspicion for infection is low. Padegimas et al. showed a 24% positive culture rate at time of revision in cases with low suspicion for infection. The clinical importance of positive cultures in this scenario is yet to be determined, however it does not appear to affect the overall outcome in cases of one-stage revisions (34).
Resection arthroplasty
Resection arthroplasty is an option for low demand patients when revision arthroplasty is not possible. Rates of recurrence of infection range from 0–30%; however, this is typically at the cost of a poor functional outcome (28,29,35). Commercially produced articulating spacers are another option and have been used as definitive treatment in low demand patients (36).
Arthrodesis
Arthrodesis is uncommonly employed as a definitive treatment after shoulder PJI; however, it remains an option for patients with sufficient bone stock. Typically, a 4.5 mm pelvic reconstruction plate is used for fixation along the lateral humeral cortex and placed along the scapular spine with several screws. In cases of severe bone loss (>6 cm proximal humerus), a vascularized fibula graft may be used to aid in healing (37). The fibular graft should be 4–5 cm longer than the defect in order for docking in the distal humerus. Wick et al. reported significantly improved pain relief in a series of patients with septic arthritis of the shoulder treated with arthrodesis. Although 90% of patients had significantly improved pain, no patient was pain-free (38).
Summary
The heterogeneity of the patient samples within the available literature, combined with relatively low patient numbers makes drawing firm conclusions regarding treatment quite difficult. The gold standard for revision RTSA remains removal of all components (and cement) and placement of revision components via a single or dual-stage reconstruction. While two-stage reconstruction is preferred for patients with PJI caused by virulent organisms, one-stage revision is likely sufficient for most cases of PJI with less virulent organisms.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.02). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. MAS reports personal fees from DJO Surgical, personal fees from KCI Medical, outside the submitted work. SN reports research funding from DJO Surgical, Flexion Therapeutics, Integra Life Sciences, Wright Medical Technologies, Zimmer Biomet, Arthrex, DePuy, Roche, OREF, Exactech and Biedermann Motech. In addition, Dr Namdari is a consultant for DJO Surgical, Depuy, Biedermann Motech, Vivo Capital, and InGeneron and he receives royalties from DJO Surgical, Elsevier, SLACK, Wolter Kluwer-Lippincott, Aevumed and Biedermann Motech, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics 1993;16:65-8. [PubMed]
- Kim SH, Wise BL, Zhang Y, et al. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am 2011;93:2249-54. [Crossref] [PubMed]
- Morris BJ, O'Connor DP, Torres D, et al. Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:161-6. [Crossref] [PubMed]
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg 2010;19:1076-84. [Crossref] [PubMed]
- Portillo ME, Salvado M, Alier A, et al. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res 2013;471:3672-8. [Crossref] [PubMed]
- Hsu JE, Somerson JS, Vo KV, et al. What is a "periprosthetic shoulder infection"? A systematic review of two decades of publications. Int Orthop 2017;41:813-22. [Crossref] [PubMed]
- Hsu JE, Bumgarner RE, Matsen FA 3rd. Propionibacterium in Shoulder Arthroplasty: What We Think We Know Today. J Bone Joint Surg Am 2016;98:597-606. [Crossref] [PubMed]
- Parvizi J, Gehrke T. International Consensus Group on Periprosthetic Joint I. Definition of periprosthetic joint infection. J Arthroplasty 2014;29:1331. [Crossref] [PubMed]
- . Workgroup Convened by the Musculoskeletal Infection S. New definition for periprosthetic joint infection. J Arthroplasty 2011;26:1136-8. [Crossref]
- Shields MV, Abdullah L, Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elbow Surg 2016;25:1034-40. [Crossref] [PubMed]
- Grosso MJ, Frangiamore SJ, Saleh A, et al. Poor utility of serum interleukin-6 levels to predict indolent periprosthetic shoulder infections. J Shoulder Elbow Surg 2014;23:1277-81. [Crossref] [PubMed]
- Nelson GN, Paxton ES, Narzikul A, et al. Leukocyte esterase in the diagnosis of shoulder periprosthetic joint infection. J Shoulder Elbow Surg 2015;24:1421-6. [Crossref] [PubMed]
- Mook WR, Garrigues GE. Diagnosis and Management of Periprosthetic Shoulder Infections. J Bone Joint Surg Am 2014;96:956-65. [Crossref] [PubMed]
- Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res 2008;466:1363-7. [Crossref] [PubMed]
- Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg 2012;21:1304-9. [Crossref] [PubMed]
- Singh JA, Sperling JW, Schleck C, et al. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg 2012;21:1534-41. [Crossref] [PubMed]
- Sperling JW, Kozak TK, Hanssen AD, et al. Infection after shoulder arthroplasty. Clin Orthop Relat Res 2001;206-16. [Crossref] [PubMed]
- Piper KE, Fernandez-Sampedro M, Steckelberg KE, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLoS One 2010;5:e9358 [Crossref] [PubMed]
- Chalmers PN, Sumner S, Romeo AA, et al. Do Elevated Inflammatory Markers Associate With Infection in Revision Shoulder Arthroplasty? Journal of Shoulder and Elbow Arthroplasty 2018. doi:
10.1177/2471549217750465 . - Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg 2010;18:771-2. [Crossref] [PubMed]
- Dilisio MF, Miller LR, Warner JJ, et al. Arthroscopic tissue culture for the evaluation of periprosthetic shoulder infection. J Bone Joint Surg Am 2014;96:1952-8. [Crossref] [PubMed]
- Frangiamore SJ, Saleh A, Grosso MJ, et al. Early Versus Late Culture Growth of Propionibacterium acnes in Revision Shoulder Arthroplasty. J Bone Joint Surg Am 2015;97:1149-58. [Crossref] [PubMed]
- Coste JS, Reig S, Trojani C, et al. The management of infection in arthroplasty of the shoulder. J Bone Joint Surg Br 2004;86:65-9. [Crossref] [PubMed]
- Assenmacher AT, Alentorn-Geli E, Dennison T, et al. Two-stage reimplantation for the treatment of deep infection after shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1978-83. [Crossref] [PubMed]
- Padegimas EM, Narzikul A, Lawrence C, et al. Antibiotic Spacers in Shoulder Arthroplasty: Comparison of Stemmed and Stemless Implants. Clin Orthop Surg 2017;9:489-96. [Crossref] [PubMed]
- Levy JC, Triplet J, Everding N. Use of a Functional Antibiotic Spacer in Treating Infected Shoulder Arthroplasty. Orthopedics 2015;38:e512-9. [Crossref] [PubMed]
- Jacquot A, Sirveaux F, Roche O, et al. Surgical management of the infected reversed shoulder arthroplasty: a French multicenter study of reoperation in 32 patients. J Shoulder Elbow Surg 2015;24:1713-22. [Crossref] [PubMed]
- Ortmaier R, Resch H, Hitzl W, et al. Treatment strategies for infection after reverse shoulder arthroplasty. Eur J Orthop Surg Traumatol 2014;24:723-31. [Crossref] [PubMed]
- Romano CL, Borens O, Monti L, et al. What treatment for periprosthetic shoulder infection? Results from a multicentre retrospective series. Int Orthop 2012;36:1011-7. [Crossref] [PubMed]
- Nelson GN, Davis DE, Namdari S. Outcomes in the treatment of periprosthetic joint infection after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2016;25:1337-45. [Crossref] [PubMed]
- Hsu JE, Gorbaty JD, Whitney IJ, et al. Single-Stage Revision Is Effective for Failed Shoulder Arthroplasty with Positive Cultures for Propionibacterium. J Bone Joint Surg Am 2016;98:2047-51. [Crossref] [PubMed]
- Grosso MJ, Sabesan VJ, Ho JC, et al. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg 2012;21:754-8. [Crossref] [PubMed]
- Padegimas EM, Lawrence C, Narzikul AC, et al. Future surgery after revision shoulder arthroplasty: the impact of unexpected positive cultures. J Shoulder Elbow Surg 2017;26:975-81. [Crossref] [PubMed]
- Braman JP, Sprague M, Bishop J, et al. The outcome of resection shoulder arthroplasty for recalcitrant shoulder infections. J Shoulder Elbow Surg 2006;15:549-53. [Crossref] [PubMed]
- Coffey MJ, Ely EE, Crosby LA. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. J Shoulder Elbow Surg 2010;19:868-73. [Crossref] [PubMed]
- Bilgin SS. Reconstruction of proximal humeral defects with shoulder arthrodesis using free vascularized fibular graft. J Bone Joint Surg Am 2012;94:e94 [Crossref] [PubMed]
- Wick M, Muller EJ, Ambacher T, et al. Arthrodesis of the shoulder after septic arthritis. Long-term results. J Bone Joint Surg Br 2003;85:666-70. [Crossref] [PubMed]
Cite this article as: Stone MA, Namdari S. Reverse shoulder arthroplasty: diagnostic and treatment options for the infected reverse. Ann Joint 2018;3:91.