
Multiligament knee injuries in athletes, is it possible to return to play? —a rehabilitation perspective
Introduction
Multiligament knee injury (MLKI) is a catchall phrase used to describe a spectrum of ligamentous knee injuries ranging from injury of two ligaments to disruption of all four ligaments. While rare relative to other injuries in the knee, MLKI can be devastating and impact other structures of the knee including the neurovascular, menisci, chondral, tendon, and capsular structures that may further complicate a care plan (1).
While controversy still exists regarding optimal surgical management, the evolution of anatomical ligament reconstruction of these injuries has resulted in improved restoration of joint stability and patient satisfaction (2). These results have naturally led to greater expectations for what level of activity can be achieved after MLKI, and they are both reasonable and possible. For the rehabilitation professional, this increased expectation surrounding outcomes has forced a critical evaluation of postoperative restrictions that are used to protect the surgical reconstructions as well as the methods employed to restore preinjury levels of strength and function. For the above mentioned reasons, the purpose of this paper is twofold: to understand the factors that limit return to play (RTP) in MLKI patients, and to outline a rehabilitation program and functional assessment that maximizes a patient’s chance for RTP while guiding the decision making process for clinicians.
RTP rates
MLKI are complex with many variables involved in outcome statistics. As a result, RTP rates vary. Everhart et al. performed a systematic review investigating return to work or sport rates after MLKI. RTP rate at any level of sport was 59.1% for surgically managed MLKI (3). Return to a similar level of sport was much lower, with reported rates of 22% for competitive athletes and 33% for elite athletes (3). One study looking exclusively at 50 NFL athletes after MLKI reported a 64% RTP rate (4). Return to preinjury level of sport rates were lower, with only 30% of the NFL athletes achieving preinjury status (4). While possible to RTP, these statistics demonstrate the difficulty of returning to a high level of activity.
Factors limiting RTP
The complexity of MLKI makes it difficult to understand the extent to which individual factors affect a patient’s ability to RTP. Concrete cause and effect conclusions of how one factor amongst the large number of variables may impact RTP cannot be drawn. Therefore, the goal is to review what other studies have identified as barriers and provide clinical commentary to assist medical providers in returning patients to athletic performance.
In general, more severe injuries tend to have worse outcomes, especially when both cruciates are involved (3,5). Mechanisms of injury that create MLKI may involve high velocities that inflict extensive injuries beyond the knee. These are classified as poly-traumatic MLKI. Woodmass et al. compared outcomes between MLKI and poly-traumatic MLKI. Patients with poly-traumatic injuries reported lower functional scores, and it was concluded that the knee is not the limiting factor to returning to prior level of function in poly-traumatic MLKI (5). In non-poly-traumatic cases, results are more mixed and necessitate consideration of ligaments involved, neurovascular injury, joint fracture, cartilage injury, and meniscal injury. After injury is considered, the impact of arthrofibrosis, ligament laxity, and muscle weakness must be evaluated as factors inhibiting RTP.
Specific ligaments involved
Everhart et al. performed a systematic review investigating return to work or sport after MLKI (3) They found that studies including patients with Schenck types IV and V injuries (6) reported lower return to work rates compared to studies without grade IV and V (3). Bakshi et al. studied RTP rates of NFL players after MLKI. Overall, they found that ligament injury pattern significantly affects RTP (P=0.047) (4). There was a 68.2% next-season RTP rate for athletes with ACL/MCL injury compared to 37% for athletes with combined ACL/PCL/FCL injury (4). Further, a higher percentage of players with ACL/MCL injury (43.5%) returned to preinjury level of function compared to players with ACL/PCL/FCL injury (18.5%) (P<0.001) (4). Explanations provided by the authors for these differences include different mechanisms of injury, different forces contributing to other concomitant injuries, and the non-operative healing potential of MCL injuries.
Neurovascular injury
Both neuro and vascular injuries can occur with MLKI. In these injuries, the common fibular nerve (CFN) and the popliteal artery are often the structures affected. Although the CFN may be damaged with variations of MLKI, Moatshe et al. found that CFN injury is 42 times more likely to occur when the PLC is injured compared to no PLC involvement (6). Also, the popliteal artery is nine times more likely to be injured when the PLC is involved in the MLKI (6). It is worth noting that MLKI involving severe intra-articular fractures were not included in this study (6).
The severity of fibular nerve palsy impacts recovery of muscle function (7). Full recovery (Medical Research Council score =5/5) was identified in 87.3% of patients with partial CFN palsy versus only 38.4% of patients with complete CFN palsy achieving functional recovery (MRC ≥3/5) (7). Krych et al. investigated whether or not fibular nerve injury leads to worse function after MLKI. When confounding variables were controlled for, no difference in Lysholm or IKDC scores were identified for those with and without fibular nerve injury (8). Unfortunately, functional testing (i.e., hop testing) and rates of RTP were not specified. Therefore, it cannot be concluded how fibular nerve injury affects what level of sport patients reach.
Even with these statistics, it is challenging to predict to what extent and on what timeline nerve function will be restored. Peskun et al. retrospectively reviewed 91 patients with MLKI, 26 of whom had fibular nerve injury (defined by lack of dorsal foot sensation and 0/5 MRC score) (8). The only variable associated with fibular nerve recovery was younger age (9). Incomplete nerve palsy may resolve without surgical intervention (7-9). For complete nerve palsy, surgical intervention is often necessary (10). Unfortunately, nerve grafting generally has poor results due to the fact that up to 15 cm of the nerve may be affected (11). Larger, more severe injuries requiring longer lengths of nerve grafting tend to have worse results (12). When nerve grafting is not a suitable option, or the grafting fails, posterior tibialis tendon transfer may be used to restore dorsiflexion function (10). However, studies measuring dorsiflexion strength after posterior tibial tendon transfer have found an average dorsiflexion strength index of 30% (13) and 42% (14) compared to the uninvolved side. Although this is a considerable strength deficit, Molund et al. reported that four of the twelve patients available for evaluation after posterior tibial tendon transfer were able to return to high levels of activity in marathon running and downhill mountain biking (14). Others were able to return to recreational levels of activity in biking, cross country skiing, dancing, squash, and football (14).
Sanders et al. performed a retrospective matched-cohort analysis comparing function after MLKI in those with and without vascular injury (15). Patients with vascular injury reported significantly lower IKDC (59.7% vs. 83.8%, P=0.002) and Lysholm scores (62.5% vs. 86.4%, P=0.001) than those without vascular injury (15). Although the authors stated they did not completely understand why results were worse in the vascular injury group, explanations included: prolonged tissue ischemia, external fixation, fasciotomy procedures, increased surgical morbidity, vascular graft-related complications, and prolonged immobilization (15). Many of these factors could lead to thinking that range of motion (ROM) would be negatively affected, but no differences in postoperative ROM were identified (15).
Articular cartilage and meniscal injury
Articular cartilage lesions may complicate recovery in MLKI, but to the authors’ knowledge, no study has specifically investigated how the combination of MLKI and cartilage lesions impacts RTP. The best information available is a study by Schmitt et al. who performed a systematic literature review of functional outcomes after surgical management of articular cartilage knee injuries (16). To summarize the findings, deficits in functional performance may persist for five to seven years following articular cartilage surgical procedures (16). Although the population studied wasn’t a MLKI population, it provides a clear view of how cartilage injury impacts recovery, and it may provide insight as to how cartilage injury impacts recovery after MLKI.
One reason cartilage injury and surgical intervention could complicate MLKI recovery is that rehabilitation timelines for cartilage procedures are often slow in order to allow protection of healing tissue. Delayed WB postpones all subsequent phases of rehab and ultimately leads to a longer rehabilitation. Strength training must be delayed, and the potential effects of this are observed with some strength impairments persisting up to two to seven years after articular cartilage repair (16). More progressive WB protocols that allow full weight bearing (FWB) at six weeks versus eight weeks after surgery may restore strength sooner after autologous chondrocyte implantation (17). How rapidly WB can be progressed without negatively impacting healing integrity is unclear. Further, important gait impairments persist regardless of progressive versus conservative rehabilitation (16). Sagittal plane knee kinematics and kinetics during gait are identified up to 12 months after surgery (16).
Meniscus tears are another intra-articular factor that impact rehabilitation and RTP. That being said, Jenkins et al. found no correlation between the presence of or lack of a meniscus tear and IKDC or SMFA scores (18). While some meniscus tears may have very little impact on WB timeframes and ROM progressions, repairs stressed by hoop tension, such as meniscal root tears, require protection timeframes for both ROM and WB. Meniscal root tears have a reported occurrence of 20.2% in MLKI cases (19).
Arthrofibrosis
There are varying grades of arthrofibrosis (20), but all impact patient function. With a loss of knee extension alone, basic gait pattern is disrupted and cannot be normalized due to physical block in terminal extension. Reported incidence of arthrofibrosis after MLKI varies (7,21,22). As mentioned earlier, it is not clear whether acute versus delayed management provides superior results (21,22). However, a retrospective case control study identified combined ACL/PCL/PLC injury (OR =17.08), knee dislocation (OR =12.84), and use of an external fixator (OR =12.81) as significant risk factors for stiffness (23).
Laxity
While stiffness after MLKI is a concern, laxity is also a concern. Great care is taken with anatomical reconstruction to restore as normal mechanics as possible. If laxity develops postoperatively, the overall integrity and function of the knee may be affected. Due to this concern, rehabilitation protocols err on the side of caution when determining initial WB status and appropriate progression. Precautions to protect ligament healing often extend up to four months and beyond, depending on the ligament reconstructed (Table 1). Most of these precautions have not been systematically compared to more progressive alternatives. Instead, they are based on anticipated physiological healing and tissue maturation timelines. However, one study did compare NWB for six weeks to partial weight bearing (PWB) at 40% of body weight with crutches for six weeks after isolated FCL reconstruction or ACL/FCL reconstruction (24). At the six months follow up, no differences in laxity between the two groups were found (24) Further investigation is necessary to determine the effects of progressive WB on laxity and function for other ligamentous repairs.
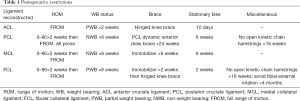
Full table
Strength deficits
Many studies report IKDC and Lysholm scores after MLKI, but physical testing of strength and function (i.e., quadriceps index, hop test) are rarely discussed. To the authors’ knowledge, there is only one study that reports strength indices (summarized in Table 2 (18). The main takeaway is that strength deficits in the quadriceps persist at two years after surgery. This is comparable to several ACL studies that show it takes up to two years to resolve impairments in quadriceps and hamstrings strength after ACL reconstruction (25-28). This is of concern because patients are typically cleared prior to two years after surgery, and strength deficits are often observed after patients are cleared to RTP (29). Normalizing strength prior to RTP is an important issue to address due to the negative impact of residual weakness on function (30).
Rehabilitation recommendations
The rehabilitation plan and process following surgery for a MLKI is a key component of achieving a successful outcome and providing the opportunity for patients to return to their previous level of sporting activity. The variation of reconstructed structures makes for some differences in the specific rehabilitation protocols; however, the following principles are common to the acute management phase of post-surgical:
- Protect the surgical reconstruction and restore joint range of motion (ROM);
- Manage the scarring process;
- Minimize muscular atrophy and restore preinjury levels of muscular strength;
- Utilize return to sport testing to guide decision making.
Protect the surgical reconstruction & restore joint ROM
Following early surgery, immediate ROM has been reported to reduce the incidence of flexion loss of >10º and extension loss >5º (22). This is significant since residual stiffness is the most common complication after a multiligament knee reconstruction (31). While there are numerous benefits of early mobility, there remains a large variation in the literature for timing of post-surgical ROM and the amplitude allowed (1). As a guideline for clinicians, PROM of 0–90º for two weeks and then full ROM thereafter has been reported as safe ROM restriction for all ligamentous injury patterns (24,32,33).
For the majority of injury patterns, patients are NWB for a period of six weeks (32,33). This is to limit WB load on the reconstructed ligaments and the resultant risk of graft elongation and joint laxity. An exception to this is patients with combined ACL/FCL reconstructions. In a randomized controlled trial, LaPrade and colleagues demonstrated there were no significant differences in pain, ROM, subjective outcome, stability, or swelling between the control NWB group and the experimental PWB group (24).
For PCL-based MLKI’s it is recommended to utilize a dynamic brace for six months. This brace provides a dynamic anterior draw force to the proximal tibia, limiting posterior tibial sag and PCL stress with increased degrees of flexion. In a three year follow up cohort study of 100 PCL patients, the use of an anterior draw brace for six months combined with a double bundle anatomic reconstruction and early ROM that began day one post-surgery improved preoperative posterior tibial translation from 11.0±3.5 to 1.6±2.0 mm postoperatively (33). For ACL-based MLKI, patients are advised to utilize a knee immobilizer for a 6-week duration, with the patient needing to demonstrate a straight leg raise with less than a 5º quadriceps lag to open up the brace during WB 91). A list of postoperative restrictions is found in Table 1.
Manage the scarring process
The most frequent MLKI complication of joint stiffness is largely related to the development of scar tissue and subsequent arthrofibrosis. Scarring in the anterior knee is shown to result in the negative consequences of increased patellofemoral and tibiofemoral joint contact pressures, reduced moment arm of the extensor mechanisms, and a cause of anterior knee pain (34,35). While there is no single rehabilitation intervention to reduce the incidence of arthrofibrosis, a treatment plan that includes early ROM (day one postoperative), patellofemoral mobilization, and appropriate loading decisions that help resolve of joint effusion and inflammation is prudent. Mueller et al., advocate manual postoperative patellofemoral mobilization as a means to mitigate the restriction of normal joint mechanics by scar tissue (36). This manual patellofemoral mobilization influences the entire anterior knee, with a specific focus on maintaining the integrity of the anterior interval and suprapatellar pouch.
Minimize muscular atrophy
Given that significant strength deficits have been reported following surgery for MLKI, special attention and effort should be given reducing muscular atrophy. The use of blood flow restriction training (BFR) shows promise in this area. BFR involves the occlusion of venous return and the restriction of arterial inflow through the application of an extremity tourniquet. Muscle hypertrophy is promoted when completing exercise with the tourniquet inflated through a combination of processes including cell signaling, hormonal changes that stimulate protein synthesis, and proliferation of myogenic cells (37-39). While lacking the clinical trials to help elicit the optimal dosing, it has been suggested that the following protocol may be beneficial in resisting atrophy: initiate BFR treatment once proximal thigh sensation is normal, perform at a frequency of three to six days per week, utilize 80% limb occlusion pressure, and complete a set and repetition structure of 1×30, 3×15 with 30 seconds rest between sets for each muscle group of interest (40).
Restoration of muscular strength
With strength deficits persisting until 2 years post-surgery (18), the restoration of strength takes on particular significance in MLKI patients. One method of organizing the development of strength is through periodization. Periodization is the division of a training or rehabilitation program into smaller phases (periods) as a means of creating more manageable segments (41). Further, periodized programs increase specificity of training, are effective in creating gains in strength, and help avoid training plateaus (42). Tables 3, 4, and 5 describe the parameters and sample treatments.

Full table

Full table

Full table
Return to sport testing
The importance of return to sport testing and its role in reducing reinjury risk is well described in the ACL literature (43,44). Despite this, little work exists in the MLKI rehabilitation literature surrounding the understanding of residual muscular strength and movement pattern deficits following MLKI surgery or the effectiveness of RTP testing. Since muscular strength deficiencies have been demonstrated as far out as two years post-surgery (18) and reduced muscular strength and movement pattern deficiencies are related to knee injuries (43,45-47) it seems appropriate to follow a return to sport testing protocol similar to that used after ACL reconstruction. In addition to the cornerstone strength and power tests of RTP criterion, it is important that movement quality also be observed (48,49).
RTP assessment has been incorporated into the progression criteria for each periodized phase in the sample rehabilitation program outlined in this paper. Rehabilitation professionals can use these progression criteria as a means to assess patient progress, with the cumulative passing of each phase’s assessment resulting in clearance to RTP. Alternatively, clinicians may use the criteria outlined with the power progression (Table 5) as the final RTP assessment.
Conclusions
While return to a competitive level of sports is very difficult after MLKI, it is possible for the athlete to overcome significant injury through surgery and rehabilitation. We advocate for future MLKI studies to structure rehabilitation into periodized progressions to optimize recovery. Finally, it is encouraged that all athletes recovering from MLKI undergo rigorous RTP testing to help ensure safety and success in returning to sport.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Camilo Partezani Helito and Jorge Chahla) for the series “The Multiligament Injured Knee” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.06). The series “The Multiligament Injured Knee” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch AD, Chmielewski T, Bailey L, et al. Current concepts and controversies in rehabilitation after surgery for multiple ligament knee injury. Curr Rev Musculoskelet Med 2017;10:328-45. [Crossref] [PubMed]
- Geeslin AG, LaPrade RF. Outcomes of Treatment of Acute Grade-III Isolated and Combined Posterolateral Knee Injuries. J Bone Joint Surg Am 2011;93:1672-83. [Crossref] [PubMed]
- Everhart JS, Du A, Chalasani R, et al. Return to work or sport after multiligament knee injury: A systematic review of 21 Studies and 524 patients. Arthroscopy 2018;34:1708-16. [Crossref] [PubMed]
- Bakshi NK, Khan M, Lee S, et al. Return to play after multiligament knee injuries in National Football League athletes. Sports Health 2018;10:495-9. [Crossref] [PubMed]
- Woodmass JM, Johnson NR, Mohan R, et al. Poly-traumatic multi-ligament knee injuries: is the knee the limiting factor? Knee Surg Sports Traumatol Arthrosc 2018;26:2865-71. [Crossref] [PubMed]
- Moatshe G, Dornan GJ, Løken S, et al. Demographics and injuries associated with knee dislocation: A prospective review of 303 patients. Orthop J Sports Med 2017;5:2325967117706521 [Crossref] [PubMed]
- Woodmass JM, Romatowski NPJ, Esposito JG, et al. A systematic review of peroneal nerve palsy and recovery following traumatic knee dislocation. Knee Surg Sports Traumatol Arthrosc 2015;23:2992-3002. [Crossref] [PubMed]
- Krych AJ, Giuseffi SA, Kuzma SA, et al. Is peroneal nerve injury associated with worse function after knee dislocation? Clin Orthop Relat Res 2014;472:2630-6. [Crossref] [PubMed]
- Peskun CJ, Chahla J, Steinfeld ZY, et al. Risk Factors for Peroneal Nerve Injury and Recovery in Knee Dislocation. Clin Orthop Relat Res 2012;470:774-8. [Crossref] [PubMed]
- Giuseffi SA, Bishop AT, Shin AY, et al. Surgical treatment of peroneal nerve palsy after knee dislocation. Knee Surg Sports Traumatol Arthrosc 2010;18:1583-6. [Crossref] [PubMed]
- Sedel L, Nizard R. Nerve grafting for traction injuries of the common peroneal nerve. A report of 17 cases. J Bone Joint Surg Br 1993;75:772-4. [Crossref] [PubMed]
- Kim DH, Murovic JA, Tiel RL, et al. Management and outcomes in 318 operative common peroneal nerve lesions at the Louisiana State University Health Sciences Center. Neurosurgery 2004;54:1421-8; discussion 1428-9. [Crossref] [PubMed]
- Yeap J, Birch R, Singh D. Long-term results of tibialis posterior tendon transfer for drop-foot. Int Orthop 2001;25:114-8. [Crossref] [PubMed]
- Molund M, Engebretsen L, Hvaal K, et al. Posterior tibial tendon transfer improves function for foot drop after knee dislocation. Clin Orthop Relat Res 2014;472:2637-43. [Crossref] [PubMed]
- Sanders TL, Johnson NR, Levy NM, et al. Effect of vascular injury on functional outcome in knees with multi-ligament injury. J Bone Joint Surg Am 2017;99:1565-71. [Crossref] [PubMed]
- Schmitt LC, Quatman CE, Paterno MV, et al. Functional outcomes after surgical management of articular cartilage lesions in the knee: A systematic literature review to guide postoperative rehabilitation. J Orthop Sports Phys Ther 2014;44:565-A10. [Crossref] [PubMed]
- Edwards PK, Ackland TR, Ebert JR. Accelerated weightbearing rehabilitation after matrix-induced Autologous Chondrocyte Implantation in the tibiofemoral joint. Am J Sports Med 2013;41:2314-24. [Crossref] [PubMed]
- Jenkins PJ, Clifton R, Gillespie EM, et al. Strength and function recovery after multiple-ligament reconstruction of the knee. Injury 2011;42:1426-9. [Crossref] [PubMed]
- Kosy JD, Matteliano L, Rastogi A, et al. Meniscal root tears occur frequently in multi-ligament knee injury and can be predicted by associated MRI injury patterns. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Shelbourne KD, Patel DV, Martini DJ. Classification and management of arthrofibrosis of the knee after anterior cruciate ligament reconstruction. Am J Sports Med 1996;24:857-62. [Crossref] [PubMed]
- Hohmann E, Glatt V, Tetsworth K. Early or delayed reconstruction in multi-ligament knee injuries: A systematic review and meta-analysis. Knee 2017;24:909-16. [Crossref] [PubMed]
- Mook WR, Miller MD, Diduch DR, et al. Multiple-Ligament Knee Injuries: A systematic review of the timing of operative intervention and postoperative rehabilitation. J Bone Joint Surg Am 2009;91:2946-57. [Crossref] [PubMed]
- Bodendorfer BM, Keeling L, Michaelson E, et al. Predictors of knee arthrofibrosis and outcomes after arthroscopic lysis of adhesions following ligamentous reconstruction: A retrospective case–control study with over two years' average follow-up. J Knee Surg 2018; [Epub ahead of print]. [PubMed]
- LaPrade RF, DePhillipo NN, Cram TR, et al. Partial controlled early postoperative weightbearing versus nonweightbearing after reconstruction of the fibular (lateral) Collateral Ligament: A randomized controlled trial and equivalence analysis. Am J Sports Med 2018;46:2355-65. [Crossref] [PubMed]
- Aglietti P, Giron F, Buzzi R, et al. Anterior Cruciate Ligament Reconstruction: one-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial. J Bone Joint Surg Am. 2004;86-A:2143-55. [Crossref] [PubMed]
- Aune AK, Holm I, Risberg MA, et al. Four-strand hamstring tendon autograft compared with patellar tendon-bone autograft for Anterior Cruciate Ligament reconstruction. Am J Sports Med 2001;29:722-8. [Crossref] [PubMed]
- Inagaki Y, Kondo E, Kitamura N, et al. Prospective clinical comparisons of semitendinosus versus semitendinosus and gracilis tendon autografts for anatomic double-bundle anterior cruciate ligament reconstruction. J Orthop Sci 2013;18:754-61. [Crossref] [PubMed]
- Maletis GB, Cameron SL, Tengan JJ, et al. A Prospective Randomized Study of Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2007;35:384-94. [Crossref] [PubMed]
- Toole AR, Ithurburn MP, Rauh MJ, et al. Young athletes after anterior cruciate ligament reconstruction cleared for sports participation: How many actually meet recommended return-to-sport criteria cutoffs? J Orthop Sports Phys Ther 2017;47:825-33. [PubMed]
- Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2012;42:750-9. [Crossref] [PubMed]
- Sisto DJ, Warren RF. Complete knee dislocation. A follow-up study of operative treatment. Clin Orthop Relat Res 1985;94-101. [PubMed]
- LaPrade RF, Wijdicks CA. The management of injuries to the medial side of the knee. J Orthop Sports Phys Ther 2012;42:221-33. [Crossref] [PubMed]
- Laprade RF, Cinque ME, Dornan GJ, et al. Double-bundle Posterior Cruciate Ligament Reconstruction in 100 patients at a mean 3 years’ follow-up: Outcomes were comparable to Anterior Cruciate Ligament Reconstructions. Am J Sports Med 2018;46:1809-18. [Crossref] [PubMed]
- Ahmad CS, Kwak D, Ateshian GA, et al. Effects of patellar tendon adhesion to the anterior tibia on knee mechanics. Am J Sports Med 1998;26:715-24. [Crossref] [PubMed]
- Mikula JD, Slette EL, Dahl KD, et al. Intraarticular arthrofibrosis of the knee alters patellofemoral contact biomechanics. J EXP ORTOP. J Exper Orthop 2017;4:1-7. [Crossref]
- Mueller BT, Moulton SG, O'Brien L, et al. Rehabilitation following meniscal root repair: A clinical commentary. J Orthop Sports Phys Ther 2016;46:104-13. [Crossref] [PubMed]
- Gundermann DM, Walker DK, Reidy PT, et al. Activation of mTORC1 signaling and protein synthesis in human muscle following blood flow restriction exercise is inhibited by rapamycin. Am J Physiol Endocrinol Metab 2014;306:E1198-204. [Crossref] [PubMed]
- Nielsen JL, Aagaard P, Bech RD, et al. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J Physiol 2012;590:4351-61. [Crossref] [PubMed]
- Takarada Y, Takazawa H, Ishii N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med Sci Sports Exerc 2000;32:2035-9. [Crossref] [PubMed]
- DePhillipo NN, Kennedy MI, Aman ZS, et al. The role of blood flow restriction therapy following knee surgery: Expert opinion. Arthroscopy 2018;34:2506-10. [Crossref] [PubMed]
- Chahla J, O'Brien L, Godin JA, LaPrade RF. Return to Play After Multiple Knee Ligament Injuries. In: Return to Play in Football. Berlin, Heidelberg: Springer Berlin Heidelberg; 2018. pp. 637-47.
- Lorenz DS, Reiman MP, Walker JC. Periodization: Current review and suggested implementation for athletic rehabilitation. Sports Health. 2010;2:509-18. [Crossref] [PubMed]
- Grindem H, Snyder-Mackler L, Moksnes H, et al. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: The Delaware-Oslo ACL cohort study. Br J Sports Med 2016;50:804-8. [Crossref] [PubMed]
- Kyritsis P, Bahr R, Landreau P, et al. Likelihood of ACL graft rupture: Not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med 2016;50:946-51. [Crossref] [PubMed]
- Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict Anterior Cruciate Ligament Injury risk in female athletes: A prospective study. Am J Sports Med 2005;33:492-501. [Crossref] [PubMed]
- Ithurburn MP, Paterno MV, Ford KR, et al. Young athletes with quadriceps femoris strength asymmetry at return to sport after Anterior Cruciate Ligament Reconstruction demonstrate asymmetric single-leg drop-landing mechanics. Am J Sports Med 2015;43:2727-37. [Crossref] [PubMed]
- Nawasreh Z, Logerstedt D, Cummer K, et al. Do patients failing return-to-activity criteria at 6 months after anterior cruciate ligament reconstruction continue demonstrating deficits at 2 years? Am J Sports Med 2017;45:1037-48. [Crossref] [PubMed]
- O’Brien LT, Mueller BT. Rehabilitation principles for non-operative and surgical approaches for medial collateral and posteromedial corner injuries. In: Chahla J, LaPrade RF. The Medial Collateral Ligament and the Posteromedial Corner. A Comprehensive Analysis. New York: Nova Medicine & Health Publishers Inc. 2018:139-60.
- Wren TAL, Mueske NM, Prophy CH, et al. Hop distance symmetry does not indicate normal landing biomechanics in adolescent athletes with recent anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 2018;48:622-9. [Crossref] [PubMed]
Cite this article as: Mueller BT, O’Brien LT. Multiligament knee injuries in athletes, is it possible to return to play? —a rehabilitation perspective. Ann Joint 2018;3:92.



