
Advances in gene therapy for cartilage repair
Articular cartilage defects: pathophysiology and current clinical options
The clinical treatment of articular cartilage defects remains problematic and their association with osteoarthritis (OA) is long recognized (1). Articular cartilage has a limited intrinsic capability for self-healing and none of the current therapeutic options can securely and durably regenerate the wounded tissue in its original structure and function in sites of injury thus representing a burden that affects millions of individuals worldwide.
Articular cartilage defects disturb the cartilage surface, either as chondral defects restricted to the articular cartilage or as osteochondral defects extending into the subchondral bone (2). They are mainly caused by acute or repetitive trauma, OA, osteochondritis dissecans, and osteonecrosis. As chondral defects do not have a connection to the subchondral bone, access to pluripotent stem cells is reduced, originating mainly from the synovial lining and the Hoffa fat pad, as the chondrocytes adjacent to the defects do not significantly contribute to the repopulation of the defect (3). Osteochondral defects disrupt the integrity of the osteochondral unit (4). They are, in contrast to chondral defects, spontaneously more efficiently repaired because mesenchymal stem cells (MSCs) from the bone marrow compartment (BM-MSCs) initiate a localized chondro-/osteogenic differentiation within the defect.
The unsolved clinical question of cartilage repair is reflected in a multitude of surgical interventions either replacing the damaged (osteo)chondral tissue or aiming to induce repair. Replacement strategies include the transplantation of autologous or allogeneic osteochondral grafts or focal metallic resurfacing implants. Cartilage repair can chiefly be induced by marrow stimulation techniques such as microfracture (indicated for small symptomatic defects) and autologous chondrocyte implantation (ACI) (indicated for larger defects). Marrow stimulation techniques rely on the chondrogenesis within the chondral defect originating from MSCs mobilized by surgically penetrating the subchondral bone plate. ACI is a two-part surgical procedure (5). First, during arthroscopy and following an evaluation of the defect, (osteo)chondral biopsies are removed from which the chondrocytes are subsequently isolated and cultivated under laboratory conditions. After some weeks in vitro, the chondrocytes are implanted in a second intervention into the defect. While historically the cells were injected in the defect that was sealed with a periosteal flap, current techniques often attach the chondrocytes to a biodegradable solid matrix, a procedure termed matrix-assisted ACI (MACI). Long-term data from a randomized multicenter trial comparing periosteal flap-based ACI with microfracture showed no superiority of either technique for mid-size defects at ~15 years (6). Overall, such findings show the clear need for novel, more effective approaches for improved cartilage repair.
Principles of gene therapy
Gene therapy may provide powerful tools to enhance cartilage repair. Gene therapy is the concept of delivering genetic material in target cells using a gene transfer vector as a means to support the expression of therapeutic factors over prolonged periods of time relative to the application of recombinant molecules that have a short half-life (minutes to hours) (7,8). To date, about 2,600 gene therapy trials have been initiated worldwide to treat a variety of human disorders (9). Applications include gene addition, gene correction, gene silencing, and gene editing (10) via direct gene delivery in a recipient (in vivo approach) or upon indirect administration of genetically modified cells (ex vivo approach) (8,11). Gene transfer vectors include non-viral and viral-based constructs, both showing advantages and limitations for gene therapy (12,13) (Table 1).
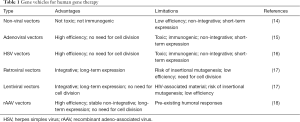
Full table
Non-viral vectors (14) have no size limitations and they are easy to generate, being safe and non-immunogenic and avoiding the risk of replication competence. Nevertheless, such vectors promote low gene transfer efficiencies (20–40%) only for short periods of time (some days to week), making them better suited for ex vivo approaches. Various types of viral vectors are available for gene therapy, including systems based on adenoviruses, herpes simplex virus (HSV), retro-/lentiviruses, and adeno-associated virus (AAV). Adenoviral and HSV vectors (15,16) can target dividing and non-dividing cells at high efficiencies (~100%), allowing for in vivo approaches, but mediating short-term transgene expression (some days to 1–2 weeks) as a result of their maintenance as unstable episomes, and can raise deleterious immune responses. Retroviral vectors (17) stably integrate in the host genome, thus promoting persistent transgene expression (months to years). Still, they are not adapted for direct approaches as they are much less efficient than other vectors (<20% efficiencies) while requiring cell division for integration and potentially initiating insertional mutagenesis and oncogene activation. Lentiviral vectors (17) can integrate in the genome of both dividing and non-dividing cells but they have the potential to carry material derived from the human immunodeficiency virus (HIV). Replication-defective recombinant adeno-associated virus (rAAV) vectors (18) can target dividing and non-dividing cells at high efficiencies (~100%) over prolonged periods of time (months to years) due to their maintenance as mostly stable episomes, thus enabling in vivo approaches. While a low risk for insertional mutagenesis has been evoked with rAAV (19), no causative effects could be reported regarding the occurrence of pathological events in the human population (20). However, the presence of neutralizing antibodies against the AAV capsid has been evidenced in individuals, including in the joints (21,22), a potential issue for effective translational human gene therapy (23).
Classical gene therapy for cartilage repair
Gene therapy to treat focal cartilage defects has been reported in experimental animal models in vivo using direct and indirect gene transfer approaches (Table 2). Enhanced repair has been reported by direct administration of adenoviral (24,25) and rAAV vectors (26-30) using the basic fibroblast growth factor (FGF-2) (26,27), insulin-like growth factor I (IGF-I) alone (28) or combined with an interleukin-1 receptor antagonist (IL-1Ra) (24), transforming growth factor beta (TGF-β) (29), bone morphogenetic proteins (BMP-2, -6) (25), and the sex-determining region Y-type high mobility group box 9 (SOX9) transcription factor (30) in chondral (24,27) and osteochondral defects (25,26,28-30) in rabbits (26-28,30), minipigs (29), and horses/ponies (24,25) for periods ranging from 3 to 52 weeks. Improved repair was also evidenced upon implantation of genetically modified BM-MSCs (34), bone marrow aspirates (31,32), and fat or muscle tissue grafts (33) using adenoviral (31-33) and lentiviral vectors (34) coding for TGF-β (32), BMP-2 (31,33), the zinc-finger protein 145 (ZNF145) transcription factor (34), and the Indian hedgehog (Ihh) signalling molecule (31) in chondral (32) and osteochondral defects (31,33,34) in rats (34), rabbits (31,33), and sheep (32) for up to 24 weeks.
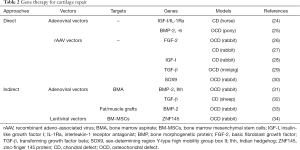
Full table
Despite promising results, many obstacles remain for the effective gene-based therapy of cartilage defects, especially in the view of clinical translation in patients. They include a number of barriers that may impair gene transfer and expression in vivo like the joint environment (synovial fluid, dense extracellular matrices, dissemination) and the presence of agents that may interfere with gene vector adsorption on a cell targets (clinically used heparin, neutralizing antibodies against viral capsid or envelope proteins, helper CD4+ and cytotoxic CD8+ T cell responses against transgene-expressing modified cells) (11,21-23,35-37). While diverse approaches have been developed to tackle these issues (alternative routes of vector administration, use of permissive clinical compounds such as hirudin, administration of transient immunosuppressors, plasmapheresis, use of vector decoys, vector engineering) (38-42), none have been satisfactorily capable of addressing the problem of adapted gene therapy for cartilage repair, showing the crucial need for novel, more effective systems.
Scaffold-mediated gene therapy for cartilage repair
The manipulation of biomaterials employed in tissue engineering strategies, especially those already in clinical use (43-45), may provide powerful tools to improve the current gene therapy procedures for cartilage repair. Such scaffold-mediated gene transfer systems would be the basis to generate off-the-shelf products as cartilage supportive matrices and cargos for the effective, spatiotemporal delivery and expression of candidate genes in sites of cartilage injury (44,46). Optimally, the scaffolds would be biodegradable, support cell homeostasis, survival, and/or differentiation, display adapted biomechanical properties, and be capable of integration with the adjacent tissues. Biomaterials used to treat cartilage lesions include solid scaffolds and hydrogels based on natural and synthetic compounds. Solid scaffolds [polylactic acid (PLA), polyglycolic acid (PGA), natural type-I/III collagen membrane] (47,48) may be provided by arthrotomy while hydrogels (alginate, collagen, hyaluronic acid) (49,50) can be prepared as injectable formulations compatible with arthroscopic procedures. Nevertheless, none of these systems provide sufficient biologically active cues to promote adapted cartilage repair, an issue that may be addressed by supplementing their use with gene therapy approaches. On the other side, combining the current gene transfer procedures with the use of a scaffold may overcome the remaining barriers to effective gene therapy (51). Thus far, such a concept has been largely reported based on the delivery of genetically modified cells seeded on biomaterials in sites of cartilage lesions (Table 3). Improved cartilage repair has been achieved by administration of cells (chondrocytes, progenitor cells) genetically modified with nonviral (52-56), adenoviral (57-60), retroviral (61), and rAAV vectors (62) and next seeded on scaffolds made of PLA (56), PGA (57,60,61), poly(lactic-co-glycolic) acid (PLGA) (59), chitosan (58), collagen (54,55,62), and alginate (52,53). Gene transfer was performed to overexpress FGF-2 (53,62), IGF-I (52), TGF-β (56,58), BMPs (54,60,61), the cartilage-derived morphogenetic protein 1 (CDMP-1) (55), the connective tissue growth factor (CTGF) (59), SOX9 (57), and the sonic hedgehog (Shh) signalling molecule (61) in osteochondral defects in rabbits (52-59,61,62) and pigs (60), with enhanced repair noted for up to 26 weeks.
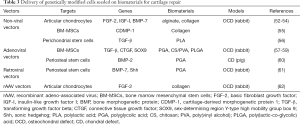
Full table
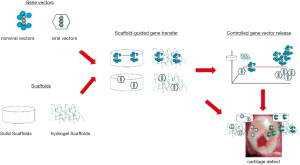
Nevertheless, such approaches require the complex and invasive preparation, genetic modification, and subsequently seeding of cells on a biomaterial, while direct coating or encapsulation of gene transfer vectors in a scaffold may provide less demanding procedures that may be well suited to promote cartilage repair (11,63-66) (Figure 1). Controlled release of gene transfer vectors from biomaterials in strategies that aim at enhancing the processes of cartilage repair have been reported using solid, hydrogel, and hybrid compounds (Table 4). The Guilak’s group for instance has pioneered the use of scaffold-guided delivery of lentiviral vectors to target MSCs via three-dimensional (3D) woven poly(ε-caprolactone) (PCL) scaffolds as a means to enhance the commitment of the cells towards chondrogenesis and support the formation of mechanically functional cartilage (TGF-β gene transfer) (70) and to protect them from inflammation and degradation in conditions of joint resurfacing (IL-1Ra gene transfer) (71,72). Hydrogels and micellar systems based on fibrin (73), alginate (74), self-assembling peptides (75), and poloxamers or poloxamines (74,76-80) have been also manipulated to deliver rAAV vectors. These systems were developed to target MSCs (79,80) and enhance their chondrogenic potential (TGF-β gene transfer) (73) and to modify (77,79,80) and remodel (TGF-β and SOX9 gene transfer) (79,80) experimental human osteochondral defects in their natural 3D environment. Hybrid scaffolds were also created to transfer non-viral vectors using systems based on fibrin, PLGA, and poly(ethylene oxide)-b-poly (L-lysine) (PEO-b-PLL) to deliver TGF-β in rabbit osteochondral defects (67-69) as a means to increase cartilage repair and integration with the surrounding knee joint cartilage.

Full table
Conclusions
Controlled release of gene therapy vectors from biomaterials is a relatively novel field of research that may provide powerful, non-invasive tools to enhance the processes of cartilage repair in sites of injury. Such scaffold-guided gene transfer systems may also allow to overcome the existing physiological barriers to effective cell and tissue modification in vivo (joint environment such as the synovial fluid and extracellular matrices, immune responses, etc.). Even though relatively few data are available in clinically relevant models of cartilage defects in vivo, the growing body of evidence showing the benefits of such approaches in vitro and in situ suggests that controlled scaffold-mediated gene transfer will open new avenues of research to treat articular cartilage lesions in patients in the future, especially when combining this concept with classical clinical procedures like marrow stimulation techniques.
Acknowledgments
Funding: Work supported by a grant from the Deutsche Forschungsgemeinschaft (DFG VE 1099/1-1 to M Cucchiarini).
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.07). MC serves as an unpaid editorial board member of Annals of Joint from Jun 2018 to May 2020. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Madry H, Kon E, Condello V, et al. Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24:1753-62. [Crossref] [PubMed]
- Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc 2010;18:419-33. [Crossref] [PubMed]
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage 2002;10:432-63. [Crossref] [PubMed]
- Madry H, Orth P, Cucchiarini M. Role of the subchondral bone in articular cartilage degeneration and repair. J Am Acad Orthop Surg 2016;24:e45-6. [Crossref] [PubMed]
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889-95. [Crossref] [PubMed]
- Knutsen G, Drogset JO, Engebretsen L, et al. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Joint Surg Am 2016;98:1332-9. [Crossref] [PubMed]
- Wirth T, Parker N, Yla-Herttuala S. History of gene therapy. Gene 2013;525:162-9. [Crossref] [PubMed]
- Dunbar CE, High KA, Joung J, et al. Gene therapy comes of age. Science 2018;359:eaan4672.
- Ginn SL, Amaya AK, Alexander IE, et al. Gene therapy clinical trials worldwide to 2017: An update. J Gene Med 2018;20:e3015 [Crossref] [PubMed]
- Blighe K, DeDionisio L, Christie KA, et al. Gene editing in the context of an increasingly complex genome. BMC Genomics 2018;19:595-614. [Crossref] [PubMed]
- Cucchiarini M. Human gene therapy: novel approaches to improve the current gene delivery systems. Discov Med 2016;21:495-506. [PubMed]
- Madry H, Orth P, Cucchiarini M. Gene therapy for cartilage repair. Cartilage 2011;2:201-25. [Crossref] [PubMed]
- Evans CH, Ghivizzani SC, Robbins PD. Gene delivery to joints by intra-articular injection. Hum Gene Ther 2018;29:2-14. [Crossref] [PubMed]
- Goodwin T, Huang L. Nonviral vectors: we have come a long way. Adv Genet 2014;88:1-12. [Crossref] [PubMed]
- Athanasopoulos T, Munye MM, Yáñez-Muñoz RJ. Nonintegrating gene therapy vectors. Hematol Oncol Clin North Am 2017;31:753-70. [Crossref] [PubMed]
- Artusi S, Miyagawa Y, Goins WF, et al. Herpes simplex virus vectors for gene transfer to the central nervous system. Diseases 2018;6:E74 [Crossref] [PubMed]
- Elsner C, Bohne J. The retroviral vector family: something for everyone. Virus Genes 2017;53:714-22. [Crossref] [PubMed]
- Grimm D, Büning H. Small but increasingly mighty: latest advances in AAV vector research, design, and evolution. Hum Gene Ther 2017;28:1075-86. [Crossref] [PubMed]
- Nault JC, Datta S, Imbeaud S, et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet 2015;47:1187-93. [Crossref] [PubMed]
- Gil-Farina I, Fronza R, Kaeppel C, et al. Recombinant AAV integration is not associated with hepatic genotoxicity in nonhuman primates and patients. Mol Ther 2016;24:1100-5. [Crossref] [PubMed]
- Chirmule N, Propert K, Magosin S, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther 1999;6:1574-83. [Crossref] [PubMed]
- Cottard V, Valvason C, Falgarone G, et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol 2004;24:162-9. [Crossref] [PubMed]
- Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev 2017;8:87-104. [Crossref] [PubMed]
- Morisset S, Frisbie DD, Robbins PD, et al. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res 2007;221-8. [Crossref] [PubMed]
- Menendez MI, Clark DJ, Carlton M, et al. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthritis Cartilage 2011;19:1066-75. [Crossref] [PubMed]
- Cucchiarini M, Madry H, Ma C, et al. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol Ther 2005;12:229-38. [Crossref] [PubMed]
- Hiraide A, Yokoo N, Xin KQ, et al. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum. Gene Ther 2005;16:1413-21. [Crossref] [PubMed]
- Cucchiarini M, Madry H. Overexpression of human IGF-I via direct rAAV-mediated gene transfer improves the early repair of articular cartilage defects in vivo. Gene Ther 2014;21:811-9. [Crossref] [PubMed]
- Cucchiarini M, Asen AK, Goebel L, et al. Effects of TGF-β overexpression via rAAV gene transfer on the early repair processes in an osteochondral defect model in minipigs. Am J Sports Med 2018;46:1987-96. [Crossref] [PubMed]
- Cucchiarini M, Orth P, Madry H. Direct rAAV SOX9 administration for durable articular cartilage repair with delayed terminal differentiation and hypertrophy in vivo. J Mol Med (Berl) 2013;91:625-36. [Crossref] [PubMed]
- Sieker JT, Kunz M, Weißenberger M, et al. Direct bone morphogenetic protein 2 and Indian hedgehog gene transfer for articular cartilage repair using bone marrow coagulates. Osteoarthritis Cartilage 2015;23:433-42. [Crossref] [PubMed]
- Ivkovic A, Pascher A, Hudetz D, et al. Articular cartilage repair by genetically modified bone marrow aspirate in sheep. Gene Ther 2010;17:779-89. [Crossref] [PubMed]
- Evans CH, Liu FJ, Glatt V, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater 2009;18:96-111. [Crossref] [PubMed]
- Liu TM, Guo XM, Tan HS, et al. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum 2011;63:2711-20. [Crossref] [PubMed]
- Mi Z, Ghivizzani SC, Lechman E, et al. Adverse effects of adenovirus-mediated gene transfer of human transforming growth factor beta 1 into rabbit knees. Arthritis Res Ther 2003;5:R132-9. [Crossref] [PubMed]
- Schuettrumpf J, Zou J, Zhang Y, et al. The inhibitory effects of anticoagulation on in vivo gene transfer by adeno-associated viral or adenoviral vectors. Mol Ther 2006;13:88-97. [Crossref] [PubMed]
- Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295-304. [Crossref] [PubMed]
- Halbert CL, Standaert TA, Wilson CB, et al. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998;72:9795-805. [PubMed]
- Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther 2012;19:694-700. [Crossref] [PubMed]
- Mingozzi F, Anguela XM, Pavani G, et al. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med 2013;5:194ra92 [Crossref] [PubMed]
- Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 2013;122:23-36. [Crossref] [PubMed]
- Rey-Rico A, Frisch J, Venkatesan JK, et al. Determination of effective rAAV-mediated gene transfer conditions to support chondrogenic differentiation processes in human primary bone marrow aspirates. Gene Ther 2015;22:50-7. [Crossref] [PubMed]
- Filardo G, Kon E, Roffi A, et al. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy 2013;29:174-86. [Crossref] [PubMed]
- Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater 2013;25:248-67. [Crossref] [PubMed]
- Kon E, Roffi A, Filardo G, et al. Scaffold-based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015;31:767-75. [Crossref] [PubMed]
- Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 2015;11:213-22. [Crossref] [PubMed]
- Patrascu JM, Krüger JP, Böss HG, et al. Polyglycolic acid-hyaluronan scaffolds loaded with bone marrow-derived mesenchymal stem cells show chondrogenic differentiation in vitro and cartilage repair in the rabbit model. J Biomed Mater Res B Appl Biomater 2013;101:1310-20. [Crossref] [PubMed]
- Gao L, Orth P, Cucchiarini M, et al. Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Thier S, Baumann F, Weiss C, et al. Feasibility of arthroscopic autologous chondrocyte implantation in the hip using an injectable hydrogel. Hip Int 2018;28:442-9. [Crossref] [PubMed]
- Vega SL, Kwon M, Burdick JA. Recent advances in hydrogels for cartilage tissue engineering. Eur Cell Mater 2017;33:59-75. [Crossref] [PubMed]
- Cucchiarini M, Madry H, Guilak F, et al. A vision on the future of articular cartilage repair. Eur Cell Mater 2014;27:12-6. [Crossref] [PubMed]
- Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 2005;12:1171-9. [Crossref] [PubMed]
- Kaul G, Cucchiarini M, Arntzen D, et al. Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo. J Gene Med 2006;8:100-11. [Crossref] [PubMed]
- Che JH, Zhang ZR, Li GZ, et al. Application of tissue-engineered cartilage with BMP-7 gene to repair knee joint cartilage injury in rabbits. Knee Surg Sports Traumatol Arthrosc 2010;18:496-503. [Crossref] [PubMed]
- Katayama R, Wakitani S, Tsumaki N, et al. Repair of articular cartilage defects in rabbits using CDMP1 gene-transfected autologous mesenchymal cells derived from bone marrow. Rheumatology (Oxford) 2004;43:980-5. [Crossref] [PubMed]
- Guo X, Zheng Q, Yang S, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene. Biomed Mater 2006;1:206-15. [Crossref] [PubMed]
- Cao L, Yang F, Liu G, et al. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011;32:3910-20. [Crossref] [PubMed]
- Qi BW, Yu AX, Zhu SB, et al. Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-beta1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood) 2013;238:23-30. [Crossref] [PubMed]
- Zhu S, Zhang B, Man C, et al. Combined effects of connective tissue growth factor-modified bone marrow-derived mesenchymal stem cells and NaOH-treated PLGA scaffolds on the repair of articular cartilage defect in rabbits. Cell Transplant 2014;23:715-27. [Crossref] [PubMed]
- Gelse K, Mühle C, Knaup K, et al. Chondrogenic differentiation of growth factor-stimulated precursor cells in cartilage repair tissue is associated with increased HIF-1alpha activity. Osteoarthritis Cartilage 2008;16:1457-65. [Crossref] [PubMed]
- Grande DA, Mason J, Light E, et al. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am 2003;85-A:111-6. [Crossref] [PubMed]
- Yokoo N, Saito T, Uesugi M, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum 2005;52:164-70. [Crossref] [PubMed]
- Lam J, Lu S, Kasper FK, et al. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev 2015;84:123-34. [Crossref] [PubMed]
- Rey-Rico A, Cucchiarini M. Controlled release strategies for rAAV-mediated gene delivery. Acta Biomater 2016;29:1-10. [Crossref] [PubMed]
- Cucchiarini M. New cell engineering approaches for cartilage regenerative medicine. Biomed Mater Eng 2017;28:S201-7. [Crossref] [PubMed]
- Venkatesan JK, Falentin-Daudré C, Leroux A, et al. Controlled release of gene therapy constructs from solid scaffolds for therapeutic applications in orthopedics. Discov Med 2018;25:195-203. [PubMed]
- Wang W, Li B, Li Y, et al. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 2010;31:5953-65. [Crossref] [PubMed]
- Li B, Yang J, Ma L, et al. Fabrication of poly(lactide-co-glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly(ethylene oxide)-b-poly(L-lysine)/TGF-beta1 plasmid DNA complexes for cartilage restoration in vivo. J Biomed Mater Res A 2013;101:3097-108. [PubMed]
- Li B, Li F, Ma L, et al. Poly(lactide-co-glycolide)/fibrin gel construct as a 3D model to evaluate gene therapy of cartilage in vivo. Mol Pharm 2014;11:2062-70. [Crossref] [PubMed]
- Brunger JM, Huynh NP, Guenther BM, et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc Natl Acad Sci USA 2014;111:E798-E806. [Crossref] [PubMed]
- Glass KA, Link JM, Brunger JM, et al. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014;35:5921-31. [Crossref] [PubMed]
- Moutos FT, Glass KA, Compton SA, et al. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc Natl Acad Sci U S A 2016;113:E4513-22. [Crossref] [PubMed]
- Lee HH, Haleem AM, Yao V, et al. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng Part A 2011;17:1969-78. [Crossref] [PubMed]
- Díaz-Rodríguez P, Rey-Rico A, Madry H, et al. Effective genetic modification and differentiation of hMSCs upon controlled release of rAAV vectors using alginate/poloxamer composite systems. Int J Pharm 2015;496:614-26. [Crossref] [PubMed]
- Rey-Rico A, Venkatesan JK, Frisch J, et al. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater 2015;18:118-27. [Crossref] [PubMed]
- Rey-Rico A, Venkatesan JK, Frisch J, et al. PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater 2015;27:42-52. [Crossref] [PubMed]
- Rey-Rico A, Frisch J, Venkatesan JK, et al. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl Mater Interfaces 2016;8:20600-13. [Crossref] [PubMed]
- Rey-Rico A, Babicz H, Madry H, et al. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int J Pharm 2017;531:492-503. [Crossref] [PubMed]
- Rey-Rico A, Venkatesan JK, Schmitt G, et al. rAAV-mediated overexpression of TGF-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int J Nanomedicine 2017;12:6985-96. [Crossref] [PubMed]
- Rey-Rico A, Venkatesan JK, Schmitt G, et al. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol Pharm 2018;15:2816-26. [Crossref] [PubMed]
Cite this article as: Cucchiarini M, Madry H. Advances in gene therapy for cartilage repair. Ann Joint 2018;3:97.


