Pitfalls of revision reverse replacement part I: dealing with instability and glenoid bone loss
Reverse shoulder arthroplasty (RSA) was designed by Grammont in the 1980s to treat painful pseudoparalytic rotator cuff arthropathy (1,2). The design is based on medializing the center of rotation of the joint and lowering the humerus to tension the deltoid muscle, thus improving function in cuff-deficient shoulders. In the mid-1990s the first short-term follow-up series confirmed excellent functional outcomes which led to a dramatic increase in use of RSA worldwide (3). Over 35,000 procedures performed in the US in 2015, almost exceeding the number of conventional total shoulder arthroplasties (4). European national arthroplasty registries also reported high incidence of RSA varying from 6 to 34 RSAs per 100,000 inhabitants per year in 2012 (5).
Reliable and reproducible functional outcomes contributed to expanding indications for RSA beyond its original scope to more complex cases. Recent midterm clinical outcomes support the use of RSA to treat massive cuff tears without arthritis, proximal humerus fractures, fracture sequelae, rheumatoid arthritis, osteoarthritis with abnormal glenoid morphology, glenoid bone loss, cuff tears in elderly patients, tumors, and failed anatomic or reverse shoulder replacement (2-4).
Consequently, complications have also increased dramatically (6). Most recent systematic review reported global complication rate of 24% and revision RSAs had twice as frequent complications as primary RSA (33.3% vs. 13.4%) (3). Small case series with heterogeneous indications for RSA have reported complication and revision rates as high as 40% (7-9). A recent study based on the US Manufacturer and User Facility Device Experience (MAUDE) reported 2,390 RSA complications between 2012 and 2016, with the most common failure modes being instability (32%), infection (13.8%) and failed baseplate (10.4%) (10). These are devastating complications that result in disability, and revision RSA is highly problematic because of bone loss, poor soft tissue quality, dysfunctional deltoid, absent subscapularis and teres minor, heterotopic ossification, and extreme fibrosis with high risk of iatrogenic nerve injuries (11).
Dealing with failed RSA requires a comprehensive and individualized analysis of each patient with an effort to identify the mechanism of failure and potential risk factors. A good physician-patient relationship and active patient participation in decisions are crucial because outcomes of revision RSA may be poorer than primary procedures.
Instability after RSA
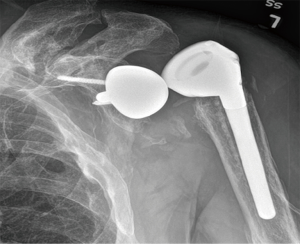
Instability is the most common postoperative complication of RSA (Figure 1), with an overall prevalence of 4.7%, and reoperation is required for the majority of cases (87.5%) (3). The prevalence of instability doubles in revisions (9.4%) compared to primary RSA (4.1%) (2). The largest case series on instability after RSA demonstrated a rate of 14.7% (6), while earlier studies reported rates up to 50% (7,12-19).
Although outcomes of RSA are generally excellent, the complication of instability is still the most difficult to treat, as seen from the high rate of recurrence (3,14,20). The increasing number of RSA procedures performed in the last 10 years and expanded indications to more complex cases raise concern about the current limited clinical understanding of instability after RSA. The available literature consists of level IV retrospective small case series (2,3,6), with only few prospective cohorts (levels II and III) (13,21-24). No randomized controlled trials have been reported.
Some risk factors have been associated with instability after RSA, but these reports do not identify etiology or help to guide treatment, and some risk factors cannot be modified, like male sex, obesity, previous operations with early dislocations, and revision surgery (25,26). Other predisposing factors include inadequate soft tissue tensioning, deltoid weakness, acromial fractures, os acromiale, subscapularis deficiency, undersized implants, prosthetic mal-positioning, polyethylene wear, glenoid medialization, mechanical failure, and heterotopic ossification (6,24). Most of these factors occur in either inability to maintain compressive forces between the glenosphere and the humerosocket or impingement of range of motion (6). Due to limited comparative studies regarding risk factors, it remains unclear which of these factors are actually associated with instability (24).
To arrange predisposing factors in a concise and intuitive way, Abdelfattah et al. (6) proposed a treatment-guiding classification for instability after RSA based on 143 revisions. They divided instability cases into three major groups: loss of compression (undersized implants, humeral height loss, subscapularis deficiency, acromial/scapular fractures, loss of deltoid contour, and deltoid dysfunction), loss of containment (mechanical failure and shallow humerosocket depth), and impingement (obesity, prosthetic malalignment, and soft tissue or bone notching). Although this first attempt to create a management algorithm to treat RSA instability showed high interobserver and intraobserver agreement, its applicability is limited because most cases present more than one factor causing instability and it is not possible to identify which factor mainly contributes to instability. Also, this study was limited by a small sample size, and only lateralized RSA prostheses were used.
Deltopectoral versus superolateral approach
A systematic review of RSA complications including studies before 2008 suggested that the deltopectoral approach was associated with a higher complication rate and instability when compared to the superolateral approach (3). However, the deltopectoral cases consisted of 79% of cases analyzed by the authors of the review, which represents possible bias. Also, the prosthetic components positioning and the status of the subscapularis were not controlled in those studies. More recent studies have shown no association between deltopectoral approach and instability or complications (6,15,23).
The superolateral approach has been shown to increase the likelihood of superior tilt of the glenosphere and valgus position of the stem (27). This approach has also been related to a more difficult release of the long head of the triceps and increased risk of inferior scapular spurs, which may predispose to notching and impingement related instability (2). In one of the few level III studies available, Tashjian et al. (24) reported that instability after RSA is associated with greater superior baseplate inclination and less inferior correction of the beta angle of the glenoid. Issues like body habitus, risk of deltoid dehiscence, and axillary nerve injury may also have limited the popularity of the superolateral approach (2).
Subscapularis tendon
Subscapularis management in RSA continues to be debated. Some authors recommend fixing the subscapularis (8,14,25,28,29) to improve stability, decrease dead space, improve blood supply to the proximal humerus, and improve internal rotation, whereas others suggest that the subscapularis does not have a significant impact on stability, can limit functional outcomes causing an iatrogenic Hornblower sign, and increases deltoid work to elevate the arm (30,31). Onstot et al. (31) showed that RSA with intact subscapularis requires 460% more posterior cuff force to external rotation, and 132% more force on the deltoid to elevate the arm. Also the immobilization period necessary to healing the repaired tendon may be a disadvantage (30).
Insufficiency of the subscapularis increased dislocation rates in a study by Edwards et al. (28). However, that study was uncontrolled and the majority of instability cases were revisions with proximal humeral bone loss. Cheung et al. (23) reported that patients with a successful subscapularis repair are significantly less likely to experience dislocation than if the tendons were not repaired. Abdelfattah et al. (6) did not find subscapularis deficiency causing instability among 43 revision cases using a lateralized RSA design. Clark et al. (32) and Friedman et al. (33) showed no difference between RSA with and without subscapularis repair. In a level III study that investigated subscapularis repair and instability after 202 medial glenosphere-lateral humerus RSAs, Vourazeris et al. (30) concluded that primary RSA with or without subscapularis repair resulted in similar clinical outcomes and dislocation rate.
Differences in RSA designs may partly explain these contradictory findings. Chalmers et al. (25) found an instability rate of 5% among studies using a medialized design RSA compared with 2.4% in studies using lateralized prosthesis designs. Traditional medial humerus-medial glenosphere designs (Grammont-style) seems to benefit from subscapularis repair in terms of increased stability (4,28,29,32). Newer generations of RSA with lateralized humerus, glenoid, or both allow for greater deltoid wrapping effect and improved tension of the remaining posterior cuff, increasing the compression force between the humerosocket and the glenosphere (4,6). Thus, for these lateralized RSA designs the available literature suggests that the subscapularis may be left unrepaired without compromising stability (6).
Although it is still unclear whether the subscapularis has an impact on RSA stability, we recommend repairing the subscapularis when possible and using lateralized designs for revision cases in which repair of subscapularis is not possible due to scarred, immobile, or poor quality tissue.
Loss of humerosocket-glenosphere compression
The mechanical goal of RSA is glenohumeral joint stability by balancing soft tissues, thus providing a stable fulcrum for deltoid function (6,15). Loss of compression has been described as the most common mode of instability after RSA (6,15,23) resulting in laxity or gapping between glenosphere and humerosocket. This can be caused by deltoid dysfunction, undersized implants, loss of humeral height, loss of deltoid contour, and acromial/scapular fractures (6). Revision surgery can reliably stabilize many of these cases except for those with deltoid dysfunction and postoperative acromial/scapular fractures (6,26).
Undersized implants must be suspected when early recurrent RSA dislocation occurs (6,20). Successful management includes revision RSA using a thicker polyethylene, bigger humeral insert, large glenosphere, or a combination of these to achieve better intraoperative conjoint tendon and deltoid tensions and appropriate prosthetic stability (6,23). Loss of compression can also occur late with concentric polyethylene wear (6).
Proximal humerus fractures, malunions, tuberosity bone loss, and loss of humeral height may incur in deficient deltoid wrapping and a loose deltoid, predisposing to instability (6,23). Full-length scaled radiographs of both arms can be valuable in preoperative planning to assess humeral shortening and medialization (2,20). These are complicated cases, and there is no consensus for appropriate treatment.
Boileau (20) proposed an algorithm to treat humeral shortening and medialization. If shortening is less than 15 mm and there is no humeral loosening, malpositioning or excessive medialization of the glenosphere the humeral height can be restored by adding a metal and/or thicker polyethylene heightener. Also, the humerus can be lengthened using a larger glenosphere, an inferiorly eccentric glenosphere, and/or placing the baseplate in a lower position with a slight inferior tilt. When shortening exceeds 15 mm, the humeral component has to be replaced and repositioned at a higher length relative to the contralateral humerus. One should consider structural proximal humerus bone allograft to improve stem fixation and deltoid wrapping for bone defects with loss of proximal humerus contour. Another recent option to manage bone defects is a proximal humerus endoprosthesis.
Medialization should be considered when instability persists despite adequate humeral length. A combination of larger prosthesis sizes and lateralizing the glenosphere increases deltoid wrapping, thus improving stability (20). Further lateralizing of the glenosphere can be achieved either with bone grafting under a regular metal plate (bone-increased offset RSA) or metallic increased offset baseplates (34).
The choice of revision RSA design due to instability must be judicious. Although lateralized design RSAs are good options for improving stability, offsetting the glenosphere also increases shear forces on the glenoid component (2,3,13,17), which may result in early loosening of the glenoid component (4). In a systematic review, Wright el al found that prostheses with a lateralized center of rotation had a significantly higher glenoid loosening rate (4.8%) and need for revision (10.5%) compared with medialized designs (1.8% and 5.6%, respectively) (2). Offsetting the humeral component by changing from inlay to onlay is another way the most modern RSA designs can compensate for the relative joint medialization without increasing stress over the glenosphere fixation (4,20,35). Reducing the inclination of the humeral tray (145º rather than 155º) also increases the humeral lateralization (20), but Cheung et al. (23) found that more vertical neck-shaft angle may be associated with improved RSA instability.
Deltoid dysfunction is considered to be the cause of instability after RSA when none of these other factors can be identified. Possible causes are axillary nerve palsy, muscle weakness, atrophy or rupture, or cervical radiculopathy. Deltoid dysfunction is underdiagnosed although it has been shown to be the most common cause of instability after RSA in some series, and outcomes have been poor with high recurrent instability rates (6,15).
Excessive tension of the deltoid may overload the acromion with risk of fracture after RSA. Some authors suggest that preoperative acromion fracture or os acromiale did not influence functional outcomes of RSA (20,36), but postoperative scapular fractures were associated with poor function. Crosby et al. (36) proposed a classification based on a review of 400 RSA: avulsion fractures of the acromion (type I), fractures posterior to the acromioclavicular joint (type II), and fractures of the scapular spine (type III). Non-operative treatment was suggested for types I and II. The authors recommended to avoid the superior screw in the baseplate because it could act as a stress riser for acromion fractures. There is no consensus to date for treatment of instability caused by acromial and scapular fractures after RSA. Most series report treating these fractures non-operatively with 4 to 6 weeks of immobilization in a sling with arm abducted 30º to promote deltoid shortening, but recurrent instability and reoperation rates are high (6,20,37,38).
Loss of containment and mechanical failure
Mechanical failure destabilizes the fulcrum of the glenosphere-humerosocket articulation, including glenosphere-base plate or humerosocket-stem dissociation, humeral stem fracture, and eccentric polyethylene wear (6,15). Somerson et al. (10) reported component dissociation rates of 12.2% of the glenosphere-base plate and 5.5% of the humerosochet-stem among 2,390 RSA complications reported to the US Food and Drug Administration from 2012 to 2016. Further, 88% of orthopedic devices in US were cleared through a 510(k) premarket notification in 2012, of which 17.8% were subsequently recalled (10). Surgeons should closely monitor device complication rates and recall notices, as well as dedicate more time intra-operatively to ensure RSA components are securely assembled.
Most mechanical failure complications require revision surgery to change the prosthetic components, with good outcomes (6). Eccentric polyethylene wear results in late-stage instability due to loss of the rim buttress (15). This may be related to prosthesis design (Grammont style), notching, and/or component malpositioning (6). Changing to a semi-constrained polyethylene may improve stability by increasing the humerosocket depth.
Component malpositioning
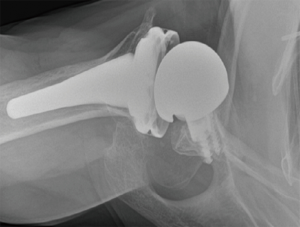
Prosthetic malalignment (Figure 2) can cause dislocation of RSA if excessive anterior version of the glenosphere and/or excessive retroversion of the humeral component, resulting in abutment of the humerosocket against the glenoid neck during internal rotation (3,6,12,15,25,34). However, it is still unclear how much misalignment of the component can be considered a risk to RSA stability.
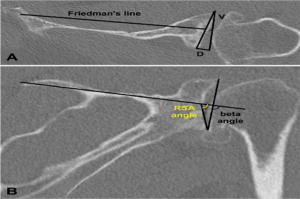
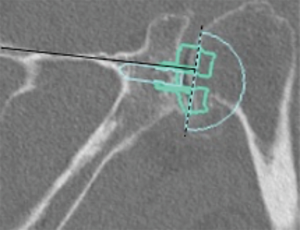
In a level III study, Tashjian et al. analyzed 168 RSAs with 13% of instability found the only risk factor significantly associated with instability was the superior inclination of the baseplate after controlling for age, sex, body mass index, and primary versus revision procedure (24). This fact emphasizes the importance of preoperative planning and meticulous placement of the baseplate. Inclination of the glenoid can be reliably measured on true anteroposterior radiographs of the shoulder joint using the beta-angle described by Maurer et al. (39). Given that the correct position of the baseplate occupies the inferior two thirds of the glenoid, recently Daggett et al. proposed a modification to this measurement called the “reverse shoulder angle” to avoid underestimation of glenoid inclination (40) (Figure 3). Although 15º of inferior tilt of the baseplate was demonstrated to be associated with decreased rates of glenoid loosening and failure (2,16), further studies are necessary to correlate baseplate inclination with instability risk.
Impingement
Impingement-related instability has been reported in several previous studies (3,6,12,15,25,34). It is more common in patients with history of proximal humerus fractures with inadequate debridement of scar tissue, callus, or heterotopic bone when the RSA was performed. A CT scan can be helpful in differentiating bone and soft tissue impingement (6).
Glenoid bone loss on revision RSA
Glenoid bone loss is found frequently in RSA, especially among revisions. It has been estimated that around 40% of primary RSAs present glenoid bone defects at the time of surgery (34,42). Glenoid bone deficiency presents a challenge when surgeons have limited access to autograft (43). Failure to restore glenoid bone stock during revision RSA may lead to insufficient baseplate fixation and malpositioning, predisposing to early loosening, notching, instability, and poor functional outcomes (41,43,44).
Although RSA has become a successful treatment option for salvage revisions (13,42,43) there is still little information regarding the need for and use of bone graft in revision RSAs. Most reports available are small cases series from high-volume referral centers that demonstrate reasonable short- and mid-term outcomes for pain relief and function (43), but graft subsidence remains a problem (41,44).
A recent level III study by Wagner et al. (43) concluded that revision RSAs requiring bone graft had higher rates of glenoid loosening and implant failure than procedures in which bone graft were not necessary. Survival free-of-revision rates at 2 and 5 years for grafted revision RSAs were 88% and 76%, respectively, compared to 94% and 93% for those without bone-grafting (43). Also, radiographic glenoid loosening was worse among shoulders that required grafting than in those without it (43).
Preoperative glenoid morphology and bone loss evaluation
Compared with primary RSA, in which the pattern of glenoid bone loss generally follows the underlying etiology with posterior erosion and subluxation of the humeral head in degenerative joint osteoarthritis, superior erosion in cuff tear arthropathy, or medializing central wear in inflammatory arthritis, revision RSA often presents with a multi-plane glenoid bone loss dependent on the previous implant used (peg, keel, cage, metal-back), mode of failure, cement, and techniques used to remove the implant (42). Thus, Walch (45), Lévigne (46) and Lévigne (47) classifications of glenoid wear that are all mono-planar are not suitable for guiding treatment in revision RSAs with cavitary complex glenoid bone defects.
Williams and Iannotti proposed a simple classification based on the location and extent of the glenoid bone loss (48). Cavitary defects (grade A) can be filled with impacted autograft or cancellous wedges substitutes, whereas uncontained glenoid wall defects (grade B) and complex defects (grade C) require tricortical iliac crest autograft or structural allografts (20).
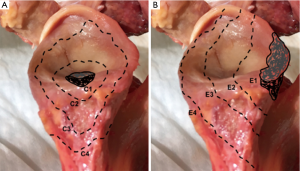
Gupta et al. (44) also presented a classification system to guide intra-operative decision-making for the management of glenoid bone defects (Figure 4). Defects are classified as centric (C), or eccentric (E), and then subclassified according to size (1 to 4) and location (anterior, posterior, inferior or superior). They reported 83% of eccentric (E), 63% of moderate to severe (grades 3 and 4), and 51% of anterior defects in a cohort of 54 revision RSAs (44).
Detailed imaging of the shoulder including true anterior-posterior in the scapular plane (Grashey), axillary and scapular Y view X-rays must be complemented by computed tomography (CT). CT scan allows the best accuracy to assess glenoid morphology (42,49). Precise measurements of version, inclination, and depth of the glenoid are important to central peg and fixation screw placement in areas with the best remaining native bone stock. The goal is to implant the baseplate as low as possible, placing the central peg parallel within 10º to 15º of inferior tilt in relation to the line of the supraspinatus fossa (34). Three-dimensional preoperative planning software and patient-specific instrumentation are useful tools that have been validated (42), but outcome and cost-effectiveness data are not yet available.
Glenoid version in the axial plane can be measured by different methods: Friedman, vault and scapular body line (50) (Figure 3A). In a level III study, Rouleau et al. (50) concluded that the Friedman method is the most reliable measurement of glenoid version. However, the reference points necessary for the Friedman method may not be available when dealing with severe bone loss revision RSA, and the only method of version measurement available may be the scapula spine axis (44). In these cases the three-dimensional computed tomography may be helpful.
Hill and Norris (41) described a useful measurement of eccentric bone defects using axillary X-rays. It was adapted for use with CT scans, helping to determine the size of structural bone grafts necessary to fill in the eccentric uncontained glenoid defects (Figure 3A).
Glenoid inclination in the vertical plane is another important measurement when planning glenoid baseplate positioning. Inferior tilt greater than 15º was shown to correlate with early glenoid loosening and failure (2,16). Tashjian et al. reported increased rates of instability after RSA with increased beta-angle (24). Although glenoid inclination can be reliably measured using the beta angle (39,42), it can be underestimated when planning a RSA. Recently Daggett et al. proposed the “reverse shoulder angle” which seems to be more specific for RSA baseplate fixation (40) (Figure 3B).
Graft selection
Moderate to severe uncontained glenoid bone loss (Seebauer C3/4 and E3/4) requires structured bone grafting (44). Bone graft can be attained from humeral head and iliac crest (autograft) or femoral head (allograft). Most revision surgeries no longer have enough good quality bone remaining in the humeral head, which limits structural grafts to tricortical iliac crest or allograft.
Iliac crest has the advantage of better graft incorporation, no disease transmission when compared to allograft, and no additional cost when compared with allograft or augmented patient-specific baseplates (34). The disadvantage is possible donor site morbidity (34). Jones et al. recently showed higher rates of graft incorporation for autograft RSA compared with allografts (86% versus 66%, respectively) (51). Norris et al. (52) described implanting the long peg baseplate directly on the iliac crest before harvesting the bone graft, providing solid fixation of the baseplate to the tricortical iliac crest autograft.
Allografts may be chosen to avoid morbidity associated with harvesting iliac crest, obesity, or previous iliac crest bone harvest (44). Although several types of allograft have been described, the femoral neck has been the preference because its dimensions mimic the native glenoid size (42) (Figure 5).
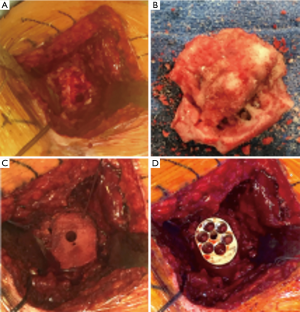
Immediate graft fixation and compression obtained by a combination of a long central peg baseplate (>25 mm) and screws have shown to provide compression forces in abduction that may be favorable to graft healing and incorporation (35).
Glenoid component selection
The glenoid baseplate seems to have an important role for management of a glenoid with severe bone loss. Werner et al. (53) suggested that a minimum of 50% contact between the baseplate and the native glenoid is essential to baseplate stability, and a minimum of 10 mm of the central peg should also anchor into native glenoid bone. A biomechanical study from Formaini et al. demonstrated that less than 50% of contact between the baseplate and the native glenoid significantly increased micromotion to unaccepted levels that might result in early baseplate failure (54).
Every attempt to preserve as much native glenoid bone stock as possible must be considered when dealing with severe uncontained glenoid defects. Frich et al. demonstrated that 2-mm reaming led to a 70% decrease in the compressive strength of the remaining native glenoid bone stock, indicating that excessive reaming should be avoided to preserve glenoid native bone stock (55).
Convex-back baseplates (Figure 6) recently introduced require less bone reaming and allow greater contact area between the baseplate and the native glenoid than flat-back baseplates (44). Convex-back baseplates has also demonstrated less tilt and displacement when subjected to eccentric loads when compared to flat-back ones, suggesting that convex designs may have better fixation and lower loosening rates (44). Anglin et al. confirmed this theory in a biomechanical study demonstrating that convex-backed baseplates lead to stress transmission in compression of the underlying bone rather than shear forces when compared with a standard flat-back design (56).
One-step versus two-step reconstruction
In most cases, glenoid reimplantation is feasible in a one-step procedure if the baseplate provides adequate compression to the graft and stable fixation to the remaining native scapular bone. Gupta et al. proposed the “50% rule” as a prerequisite for single-stage RSA revisions. It consists in having a minimum of 50% of the baseplate resting on native glenoid bone, a minimum of 50% of the central peg in native bone, and 50% (minimum of 2) of the screws fixed into native glenoid (44).
However, if the glenoid defect is so severe that graft fixation may not be reliable, excessive tension is needed to reduce the joint, or infection is suspected, a two-step procedure with 3 to 6 months between each stage may be a better option to allow for adequate graft incorporation.
Acknowledgments
We thank Lyn Camire, MA, ELS, of our department for editorial assistance.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.11). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. AMM reports other from AAOS board, other from American Journal of Orthopedics, other from American Shoulder and Elbow Surgeons, other from Arthrex, other from Current Orthopaedic Practice, other from Globus Medical, other from Ignite Orthopaedics, other from Wolters Kluwer Health-Lippincott Williams & Wilkins, other from Journal of Shoulder and Elbow Arthroplasty, other from Journal of Shoulder and Elbow Surgery, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grammont PM, Trouilloud P, Laffay J, et al. Study of the performance of a new shoulder prosthesis. Rhumatologie 1987;39:407-18.
- Alentorn-Geli E, Samitier G, Torrens C, et al. Reverse Shoulder arthroplasty. Part 2: systematic review of reoperations, revisions, problems, and complications. Int J Shoulder Surg 2015;9:60-7. [Crossref] [PubMed]
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Routman HD, Flurin PH, Wright T, et al. Reverse shoulder arthroplasty prosthesis design classification system. Bull Hosp Jt Dis (2013) 2015;73:S5-14. [PubMed]
- Lubbeke A, Rees J, Barea C, et al. International variation in shoulder arthroplasty. Incidence, indication, type of procedure, and outcomes evaluation in 9 countries. Acta Orthop 2017;88:592-9. [Crossref] [PubMed]
- Abdelfattah A, Otto R, Simon P, et al. Classification of instability after reverse shoulder arthroplasty guides surgical management and outcomes. J Shoulder Elbow Surg 2018;27:e107-18. [Crossref] [PubMed]
- Werner CM, Steinmann PA, Gilbart M, et al. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am 2005;87:1476-86. [PubMed]
- Cheung E, Willis M, Walker M, et al. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011;19:439-49. [Crossref] [PubMed]
- Wall B, Nové-Josserand L, O’Connor DP, et al. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85. [PubMed]
- Somerson JS, Hsu J, Neradilek M, et al. Analysis of 4063 complications of shoulder arthroplasty reported to the US Food and Drug Administration from 2012 to 2016. J Shoulder Elbow Surg 2018;27:1978-86. [Crossref] [PubMed]
- Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop 2010;34:1075-82. [Crossref] [PubMed]
- Boileau P, Melis B, Duperron D, et al. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:1359-70. [Crossref] [PubMed]
- Boileau P, Watkinson DJ, Hatzidakis AM, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-161S. [Crossref] [PubMed]
- Gallo RA, Gamradt SC, Mattern CJ, et al. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg 2011;20:584-90. [Crossref] [PubMed]
- Kohan EM, Chalmers PN, Salazar D, et al. Dislocation following reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1238-45. [Crossref] [PubMed]
- Cuff D, Pupello D, Virani N, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am 2008;90:1244-51. [Crossref] [PubMed]
- Frankle M, Siegal S, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am 2005;87:1697-705. [PubMed]
- Seebauer L, Walter W, Keyl W. Reverse total shoulder arthroplasty for the treatment of defect arthropathy. Oper Orthop Traumatol 2005;17:1-24. [Crossref] [PubMed]
- Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br 2004;86:388-95. [Crossref] [PubMed]
- Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res 2016;102:S33-43. [Crossref] [PubMed]
- Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol 2007;19:185-208. [Crossref] [PubMed]
- Wall B, Nove-Josserand L, O’Connor D, et al. Reverse total shoulder arthroplasty: A review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85. [PubMed]
- Cheung EV, Sarkissian E, Sox-Harris A, et al. Instability after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2018;27:1946-52. [Crossref] [PubMed]
- Tashjian RZ, Martin BI, Ricketts CA, et al. Superior baseplate inclination is associated with instability after reverse total shoulder arthroplasty. Clin Orthop Relat Res 2018;476:1622-9. [Crossref] [PubMed]
- Chalmers PN, Rahman Z, Romeo AA, et al. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:737-44. [Crossref] [PubMed]
- Teusink MJ, Pappou IP, Schwartz DG, et al. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:621-7. [Crossref] [PubMed]
- Gillespie RJ, Garrigues GE, Chang ES, et al. Surgical exposure for reverse total shoulder arthroplasty: differences in approaches and outcomes. Orthop Clin North Am 2015;46:49-56. [Crossref] [PubMed]
- Edwards TB, Williams MD, Labriola JE, et al. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:892-6. [Crossref] [PubMed]
- Routman HD. The role of subscapularis repair in reverse total shoulder arthroplasty. Bull Hosp Jt Dis (2013) 2013;71:108-12. [PubMed]
- Vourazeris JD, Wright T, Struk A, et al. Primary reverse total shoulder arthroplasty outcomes in parients with subscapularis repair versus tenotomy. J Shoulder Elbow Surg 2017;26:450-7. [Crossref] [PubMed]
- Onstot B, Suslak AG, Colley R, et al. Consequences of concomitant subscapularis repair with reverse total shoulder arthroplasty. Proceedings of the 58th annual meeting of the Orthopaedic Research Society, 2012, Feb 4-7. San Francisco, California. Rosemont, IL: Orthopaedic Research Society, 2012.
- Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg 2012;21:36-41. [Crossref] [PubMed]
- Friedman RJ, Flurin PH, Wright TW, et al. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. J Shoulder Elbow Surg 2017;26:662-8. [Crossref] [PubMed]
- Boileau P, Morin-Salvo N, Gauci M, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty);a solution for the management glenoid bone loss and erosion. J Shoulder Elbow Surg 2017;26:2133-42. [Crossref] [PubMed]
- Lädermann A, Walch G, Lubbeke A, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:336-41. [Crossref] [PubMed]
- Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011;469:2544-9. [Crossref] [PubMed]
- Mayne IP, Bell SN, Wright W, et al. Acromial and scapular spine fractures after reverse total shoulder arthroplasty. Shoulder Elbow 2016;8:90-100. [Crossref] [PubMed]
- Otto RJ, Virani NA, Levy JC, et al. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg 2013;22:1514-21. [Crossref] [PubMed]
- Maurer A, Fucentese SF, Pfirrmann CW, et al. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg 2012;21:1096-103. [Crossref] [PubMed]
- Daggett M, Werner B, Gauci MO, et al. Comparison of glenoid inclination angle using different clinical imaging modalities. J Shoulder Elbow Surg 2016;25:180-5. [Crossref] [PubMed]
- Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am 2001;83-A:877-83. [Crossref] [PubMed]
- Seidl AJ, Williams G, Boileau P. Challenges in reverse shouldr arthroplasty: addressing glenoid bone loss. Orthopedics 2016;39:14-23. [Crossref] [PubMed]
- Wagner E, Houdek M, Griffith T, et al. Glenoid bone-grafting in revision to a reverse total shoulder arthroplasty. J Bone Joint Surg Am 2015;97:1653-60. [Crossref] [PubMed]
- Gupta A, Thussbas C, Kock M, et al. Management of glenoid bone defects with reverse shouldr arthroplasty – surgical technique and clinical outcomes. J Shoulder Elbow Surg 2018;27:853-62. [Crossref] [PubMed]
- Walch G, Badet R, Boulahia A, et al. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999;14:756-60. [Crossref] [PubMed]
- Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:925-35. [Crossref] [PubMed]
- Lévigne C, Franceschi J. Rheumatoid arthritis of the shoulder: radiological presentation and results of arthroplasty. In: Walch G, Boileau P. editors. Shoulder Arthroplasty. Berlin, Germany: Springer-Verlag, 1999:221-30.
- Williams GR Jr, Iannotti JP. Options for glenoid bone loss: composites of prosthetics and biologics. J Shoulder Elbow Surg 2007;16:S267-72. [Crossref] [PubMed]
- Nyffeler RW, Jost B, Pfirrmann CW, et al. Measurement of glenoid version: conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg 2003;12:493-6. [Crossref] [PubMed]
- Rouleau DM, Kidder JF, Pons-Villanueva J, et al. Glenoid version: how to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg 2010;19:1230-7. [Crossref] [PubMed]
- Jones RB, Wright TW, Zuckerman JD. Reverse total shoulder arthroplasty with structural bone grafting of large glenoid defects. J Shoulder Elbow Surg 2016;25:1425-32. [Crossref] [PubMed]
- Norris TR, Kelly JD, Humphrey SC. Management of glenoid bone defects in revision shoulder arthroplasty: a new application of the reverse total shoulder prosthesis. Tech Shoulder Elbow Surg 2007;8:37-46. [Crossref]
- Werner BS, Böhm D, Abdelkawi A, et al. Glenoid bone grafting in reverse shoulder arthroplasty for long-standing anterior shoulder dislocation. J Shoulder Elbow Surg 2014;23:1655-61. [Crossref] [PubMed]
- Formaini NT, Everding NG, Levy JC, et al. The effect of glenoid bone loss on reverse shoulder arthroplasty baseplate fixation. J Shoulder Elbow Surg 2015;24:e312-9. [Crossref] [PubMed]
- Frich LH, Jensen NC, Odgaard A, et al. Bone strength and material properties of the glenoid. J Shoulder Elbow Surg 1997;6:97-104. [Crossref] [PubMed]
- Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg 2000;9:323-31. [Crossref] [PubMed]
Cite this article as: Lobao MH, Murthi AM. Pitfalls of revision reverse replacement part I: dealing with instability and glenoid bone loss. Ann Joint 2018;3:99.