
Stemless reverse shoulder arthroplasty: indications, technique and European experience
The evolution of shoulder replacement
Early attempts of shoulder replacement in Europe involved constrained stemmed prostheses for cases of infection (Emil Pean, 1893) (1) and tumor to deal with the considerable loss of bone and soft tissue (Jackson-Borrows) (2). In the USA, Neer et al. (3) developed a stemmed unconstrained humeral prosthesis specifically for the treatment of four-part fractures. In both cases, the stem served as a scaffold around which the proximal humerus could be rebuilt. As this was a successful design, it was later used for shoulder arthritis, and glenoid components were developed.
All these prostheses had a diaphyseal stem and neither of them was specifically designed for use in arthritis of the shoulder.
A major conceptual change was introduced by Stephen Copeland in the mid-1980s (over 30 years ago) with the use of cementless surface replacement arthroplasty in the degenerative shoulder (4-8).
Copeland and Levy (4-6,9-12) have promoted the concept that there is no need for a diaphyseal stem for anatomic TSA.
It took about 15 years to convince the shoulder world that a long stem is not needed for shoulder replacement for arthritis and a change in perception occurred.
It was not until the late 90s to the mid-2000s, after long term results with the Copeland shoulder were published that interest started with resurfacing prostheses (4-8).
Following this change in perception, other resurfacing implants appeared (Global Cap, Aequalis resurfacing, EPOCA, and others). These were followed by various stemless anatomic TSA designs without violation of the humeral diaphysis like the TESS (Biomet) in 2004, the Eclipse (Arthrex) in 2005 and followed recently by many other manufacturers.
It seems that the evolution of reverse TSA follows in the footsteps of the Anatomic TSA evolution.
Reverse shoulder prostheses are increasingly used in recent years for treatment of glenohumeral arthropathy with deficient rotator cuff such as: rotator cuff arthropathy, rheumatoid arthritis, proximal humeral fractures sequela, irreparable rotator cuff tears, and failed shoulder replacement. Good mid-term and long-term results with restoration of active elevation have been reported. However, early studies showed relatively high rates of complications (24–50%) (13-15) and many of them require further surgery (13-15).
Most of the current designs of rTSA are with a humeral diaphyseal stem. A significant part of the reported intra-operative and postoperative complications, as well as difficulties arising during revision surgery, are related to the diaphyseal humeral component (16-18).
Therefore, preservation of bone stock has become a major goal. Metaphyseal cementless implants without a diaphyseal stem have been developed to preserve bone and resect only minimal amount of bone (19-26).
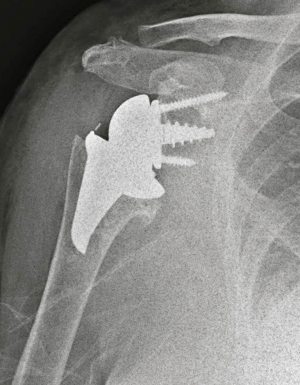
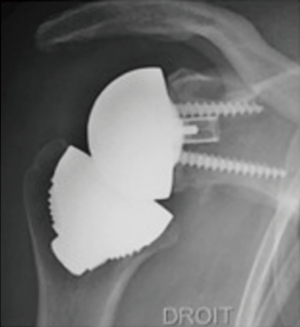
In 2005 the first stemless reverse TSAs were introduced for clinical use in Europe, with the Verso shoulder [Innovative Design Orthopaedics, London, UK (formerly Biomet, UK)] in UK (Figures 1,2) and the TESS reverse shoulder (Biomet, France) in France (Figures 3,4).


This review examines the European experience with stemless metaphyseal reverse TSAs, the history, the design rationale, the indications and the clinical and radiological outcome.
The European experience with stemless metaphyseal reverse TSAs now spans over 13 years, with results that are at least equal with the stemmed implants (26). Specific design biomechanical considerations will be discussed.
The design rationale of metaphyseal stemless rTSA is to achieve metaphyseal cementless fixation with preservation of bone utilizing minimal bone resection. The short metaphyseal humeral implant is canal sparing, preserves native bone and avoids complications relating to the humeral shaft. This will allow better bone stock for any future surgery should the need arise. Avoiding the need to address the humeral medullary canal during preparation makes the surgical procedure shorter. These implants are not limited by the alignment relation to the diaphyseal shaft.
The indications for the metaphyseal stemless rTSA are the same as for stemmed reverse arthroplasty:
All patients with glenohumeral arthropathy with deficient rotator cuff such as: rotator cuff arthropathy, rheumatoid arthritis, proximal humeral fractures sequela, irreparable rotator cuff tears, and failed shoulder replacement.
There are some limitations for the use of stemless rTSA: the stemless reverse implants are not suitable for acute proximal humerus comminuted fractures, for nonunions and for some revisions of stemmed implants.
Specific design rationale and surgical technique of the Verso and the TESS stemless rTSA
The Verso rTSA (Figures 1,2) (19,21,25,27)
The Verso was designed to be a simple prosthesis and with a simple surgical technique.
The procedure can be performed through the anterosuperior (Neviaser-MacKenzie) (5,28) or the deltopectoral approaches to the shoulder.
The Verso implant has a short triple-tapered metaphyseal humeral component with three thin fins for cementless cancellous bone fixation. This structure provides a small volume implant with large surface area, hence, provides immediate three dimensional press fit of the prosthesis to the cancellous humeral metaphysis with good load share to the bone, and further biological fixation to the titanium porous and hydroxyapatite coating. There are four sizes of humeral components, differing only by the size of the fins.
The Verso surgical technique includes cancellous bone graft impaction. Beyond the preservation of bone with minimal bone resection, the resected humeral bone is further ‘recycled’ and used for bone graft impaction in the humerus. This improves the quality and density of the bone, and improves the immediate press-fit fixation of the prosthesis. This design provides direct load transfer to the humeral cancellous bone in the metaphysis, which minimizes stress shielding and leads to improved bone quality and density underneath the prosthesis (25). Therefore, a bony defect under the humeral cut, any inadequate or soft bone or osteoporotic bone are not a contraindication for the use of the Verso prosthesis (19,21,22,24,25).
The Verso humeral cut is performed at a 155° neck-shaft angle and with the 10° inclined dial-able liner the final implant angle of 145°. The humeral liners can be dialed in a way that the correct version and offset of the liner can be determined and adapted to each patient even after the definitive metal implants have been implanted. This 10° angled liners are providing larger impingement-free arc of rotation around the glenoid.
The Verso glenoid baseplate has a central tapered screw (hydroxyapatite-coated titanium) with the screw largest core diameter of 9 mm. The baseplate fixation to the glenoid bone relies on this central tapered screw with the two additional screws serving as anti-rotational screws, superiorly and inferiorly. The glenosphere is fixed with a Morse taper to the baseplate. The glenosphere is lateralized 3 mm from the glenoid face, this is built in the thickness of the baseplate and the glenosphere.
The baseplate has six screw holes that enable use of two anti-rotation screws superiorly and inferiorly. In case of fracture or deficient glenoid bone these can be used for osteosynthesis as well.
This glenoid baseplate structure proved to provide the best fixation in a study comparing six different reverse shoulder designs by Hopkins et al. from Imperial College London (29).
The humeral polyethylene liner is designed as 10° angled rim dialable liner in order to reduce glenoid notching and improve rotational movements. This 10° angled rim inclined shape, achieved by removing the redundant polyethylene walls inferiorly-medially and respectively on both sides. This provides a very low profile medially, which reduces the impingement between the polyethylene liners to the glenoid neck and provides larger arc of impingement-free rotation movements.
Humerus preparation
A guide is used to resect the top 20-mm slice of proximal humeral bone in 155° neck-shaft angle in 30° of retroversion. The resected bone is used for bone graft impaction into the humerus.
The proximal humerus is now prepared to receive the humeral shell implant. The size of the humeral shell can be determined using pre-operative templates or intra-operatively. The punch size should be limited within the cancellous metaphysis without encroaching the cortical bone.
The humeral punch is impacted using the humeral inserter. This completes the preparation of the humerus.
The humeral punch with cover/protector is than depressed and slowly impacted further by the assistant using the forked retractor whilst the glenoid is being prepared. The constant pressure of the forked retractor on the humeral shell while preparing the glenoid further impacts the cancellous bone of the proximal humerus under the shell and creates good bone impaction with gentle continuous pressure.
Glenoid preparation
A thorough release of the capsule and labrum around the glenoid is performed.
Central drill hole is drilled with a 5-mm stop drill bit aimed at the desired point at centre of the lower circle of the glenoid with slight inclination of 10°. Both cortices should be penetrated.
Preparation of the glenoid continues with sequential use of the glenoid face reamer and the step removal reamer. Any peripheral osteophyte or uncut bone is trimmed.
The cortex around the central glenoid face hole is now enlarged using the glenoid peg reamer/burr.
The thread of the central glenoid drill hole is then gently prepared with the glenoid tap, very carefully performed by hand.
The definitive glenoid baseplate is then screwed gently into the tapped hole until the baseplate is in contact with the bone face and cannot screwed in further. The superior and inferior drill holes of the baseplate are drilled and two peripheral titanium screws are inserted.
After trial reduction with the trial glenosphere and trial liner, the trial components are removed.
Bone graft impaction with morselized bone from the resected humeral head is used as autograft to improve the bone quality of the proximal humerus. If there is not enough autograft, bone graft substitute in the form of Tri-Calcium Phosphate (TCP) granules or putty, mixed along with the patient’s blood, can be used as graft.
A matching size humeral shell (to the punch) is impacted into place with counter pressure provided under the elbow by the assistant.
The glenosphere is inserted into the Morse taper of the glenoid baseplate and impacted firmly.
A trial liner can be inserted at this stage to assess the best size, position (version), stability and range of motion after trial reduction. The selected definitive humeral liner size is dialed into the best selected position (version), impacted and locked into the humeral shell.
The joint is reduced, any remnants of the teres minor and subscapularis are attempted to be approximated to the proximal humerus.
The TESS reverse TSA (Figures 3,4)
The TESS prosthesis is designed by 12 French and Belgian surgeons based on Grammont’s concept (30). The TESS implant consists of a humeral reverse stemless cup (reverse corolla) a central pegged glenoid baseplate with four surround screws and corresponding glenosphere, and a polyethylene liner.
The TESS intended humeral cut is at a 155°–150° neck-shaft angle. The humeral reverse stemless cup (reverse corolla) made of cobalt-chrome with titanium plasma spay and hydroxyapatite coating. The outer surface of the corolla has six anti-rotational shallow wings for better press fit of the prosthesis to the bone and further biological fixation to the titanium porous and hydroxyapatite coating. There are four sizes of reverse corolla. There is a stem option, with an angulation of 150°.
The TESS glenoid baseplate has a central peg. The baseplate fixation to the glenoid bone relies on four surrounding screws: superior, inferior, anterior and posterior locking screws.
The glenosphere is fixed with a taper and secured with a central screw to the baseplate. The glenosphere is lateralized 3 mm from the glenoid face, this is built in the thickness of the baseplate and the glenosphere.
The TESS surgical procedure can also be performed through the anterosuperior (5,27) or the deltopectoral approaches to the shoulder.
Humerus preparation
A guide is used to resect the upper part of the proximal humeral bone in 155°–150° neck-shaft angle in 20° of retroversion. The humeral metaphysis is reamed to remove bone to the size of the reverse corolla from the metaphysis. The reverse corolla is then impacted into the centre of the excavated humeral metaphysis.
The TESS shoulder prosthesis (Stemless or stemmed) should only be implanted when there is sufficient proximal humerus around the corolla-stem junction that encase at least 2/3 of the reverse corolla.
Glenoid preparation
After exposure and release of the glenoid, a central drill hole is drilled and the glenoid face reamed.
The glenoid baseplate central peg is inserted and secured by four locking screws. The glenosphere taper is inserted and the glenosphere secured with a central screw to the baseplate.
The corresponding sized polyethylene insert (to the reverse corolla and the glenosphere sizes) is inserted. The joint is reduced.
Clinical and radiographic results
Clinical experience with the Verso rTSA and the TESS span over 13 years. The results with these stemless prostheses are at least at level with the results with the stemmed implants (21,24,25,27,30-36).
Atoun et al. (21) presented promising preliminary short-term clinical and radiographic results of the Verso stemless metaphyseal prosthesis in thirty-one patients, with a mean follow-up of 36 months (range, 24–52 months). The average Constant score improved from 12.7 to 56.2 with age/sex adjusted CS improved from 17.8 to 80.2. The improvement was statistically significant (P<0.0001) for all subgroups of the Constant score: pain, activity of daily living, range of motion and power. In addition, they found a marked improvement in patient satisfaction of their shoulders from 2.4 to 8.5/10.
Levy et al. (25) found similar improvements that have been maintained in a longer follow-up of 2–7 years in 102 consecutive patients who underwent rTSA with this implant. Ninety eight (20 men, 78 women) were available for follow-up. Mean age was 74.4 years (range, 38-93 years). The average follow-up was 50 months (4 years and 2 months) (range, 24–82 months). Indications for rTSA were: 65 with cuff arthropathy, 12 fracture sequelae, 13 rheumatoid arthritis, 3 failed RC repairs, 3 for loosening of anatomical prosthesis and 2 for acute trauma (dislocation with massive rotator cuff tear and preceding arthritis). Seventeen of these patients were operated as revision arthroplasty. Sixteen were revisions of resurfacing prostheses and one revision of a stemmed prosthesis.
Patients’ satisfaction (Subjective Shoulder Value) improved from 0.8/10 to 8.5/10. The Constant score improved from 14 to 59 [age-and-sex adjusted CS 86 (P<0.0001)]. Range of motion improved from 47° to 129° in elevation, 10° to 51° in external rotation, and 21° to 65° in internal rotation. All patients but one, resumed normal or near normal daily and leisure activities. Video recordings of the ROM of all the patients were taken preoperative and on every follow-up (Figure 5A).
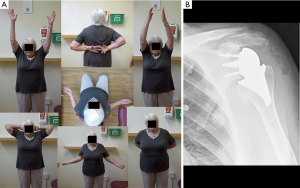
Radiographic analysis showed no radiolucencies around either the humeral or glenoid components at latest follow-up. There were no prosthetic humeral or glenoid migration, change in position or loosening of the stemless reverse humeral and the glenoid components. There was no subsidence of the prostheses and no evidence of proximal resorption of bone around the humeral implant to suggest stress shielding (Figure 5B).
Humeral component related complications: Two cases had a crack of the humeral metaphysis due to excessive bone impaction, these healed completely around the implants at three months with conservative treatment with no effect on the outcome. They did not show any lucencies or loosening at the follow-up.
The good outcome has been maintained over time in a long follow up study of more than 5 years (5–11 years follow-up) (26). One hundred and seventy two consecutive shoulders underwent rTSA between 2005 to December 2011, 149 with short metaphyseal stemless implant and 23 with stemmed implant. The average follow up was 89 months (6.25 years) (range 60–138 months). There were 41 males and 131 females; the mean age at surgery was 74.3 y (range, 38–93 y). One hundred and eleven patients for severe rotator cuff deficiency: 86 cuff tear arthropathy, 19 for fracture sequelae, 24 for rheumatoid arthritis, 16 patients were after failed RC repair or massive irreparable cuff tear, 4 for osteoarthritis with cuff deficiency or eroded glenoid, 18 for failed anatomical prosthesis with cuff deficiency, and 5 for acute trauma. 13 patients (26 shoulders) underwent bilateral (staged) rTSA at that period. Fifty patients were operated as revision arthroplasty (21 from stemmed implants to stemmed rTSA, 29 to short metaphyseal stemless rTSA (3 of them from stemmed implant to stemless implant).
Patients’ satisfaction (SSV) improved from 1.1/10 to 9.3/10. Mean Constant Score (for all diagnoses) improved from 15.9±8.6 preop to 59.7±20.4 at last follow-up. Age/sex adjusted Constant score improved from 22.6±12.3 preoperatively to 89.2±30.3 at the last follow-up (P<0.0001). The mean active range of movement improved from 53.8° to 131.9° active elevation, 20.7° to 34.6° active external rotation and 32.3° to 68.8° active internal rotation. Radiographic analysis showed no lucencies, subsidence or stress shielding around the humeral or glenoid components. Glenoid notching was found in 40 shoulders (23.2%) [34 grade 1–2 (19.8%) and 6 cases grade 3 (3.5%)].
In 2013, Ballas and Beguin (23) published the results of a prospective, single-surgeon series of 56 TESS stemless implants implanted between 2004 and 2009, reviewed at a mean of 58 months (range, 38–95 months). The Constant-Murley score improved from 29 to 62 points. Active elevation in forward flexion improved from 79º to 140º. There was one intraoperative complication which consisted of a metaphyseal-diaphyseal humeral bone crack without consequence. At the latest follow-up, there were no peri-prosthetic humeral radiolucencies, migration, or loosening of the reverse humeral cup in the metaphysis. In 1 case, significant lysis of the greater tuberosity was noted, without secondary displacement of the humeral corolla. Five grade 1 scapular notches appeared (9% of cases). Revision surgery was necessary in 4 cases (7%). Dissociation of the glenoid components occurred in 3 cases, whereas there was one case of early instability.
Kadum et al. (34) reported the results of 40 shoulders in 37 patients (23 women and 14 men), mean age at surgery 72.0 years (range, 60–88 years). Operated with either Stemmed or stemless TESS rTSA between 2007–2011. The mean follow-up was 39 months (15–66 months). Indications were CTA (n=14), primary OA with rotator cuff dysfunction (n=10), RA (n=7) and proximal humeral fracture sequelae (n=9).
The final decision as to whether a stemmed or stemless humeral implant would be used was made intraoperatively depending on bone quality and stability of the humeral component. They chose the stemmed version if primary stability of the humeral implants could not be achieved.
In their series, over 60% of the cases they have used stemmed TESS (9 fracture sequela + 15 cases) and only 16 cases had stemless TESS. Of these 16 stemless implants, two (12.5%) had to be revised at three and four days post-operatively due to corolla displacement.
These authors (34) recommend that in fracture sequela cases to use only stemmed TESS implants since implant-bone stability is questionable in osteoporotic bone.
However, marked improvement in shoulder function, quality of life and reduction of pain for both stemmed and stemless TESS versions and for all diagnoses. When analyzing the stemmed and stemless TESS in arthritis patients (without fracture sequela patients), the two groups were comparable except that more women received stemmed implants (<0.05).
In 2015, Teissier et al. (30) described 101 patients with 105 stemless TESS implants, with a minimum follow-up period of 24 months. Ninety one implants in 87 patients (61 men and 26 women), with a mean age of 73 years, at a mean follow-up of 41 months (range, 24–69 months) were analyzed, as 6 patients had died and 8 had moved overseas. The mean Constant score improved from 40 points preoperatively to 68 points at last follow-up (P<0.001). Mean flexion was 143° (range, 90°–170°), and mean external rotation was 39° (range, 20°–70°). Ninety-six percent of patients rated their satisfaction as good or excellent.
Radiographically, inferior scapular notching occurred in 17 cases (19%). The notching rate was higher when the glenometaphyseal angle increased (P<0.001), when the inferior tilt decreased (P=0.003), and when the neck-shaft angle increased. There was no evidence of component loosening.
Von Engelhardt et al. (36) published in 2015 a study evaluating 67 patients (56 non-stemmed, 11 stemmed TESS) after a mean follow-up of 17.5 months. A significant increase of the relative Constant (11.3% vs. 78.8%) and DASH scores (73.7 vs. 31.8). Loosening of the non-stemmed humeral component was observed in one case of the revision arthroplasty group. Scapular notching was observed in 9 cases (13.4%).
Discussion
The European experience with stemless metaphyseal reverse TSAs now spans over 13 years, with results that are at least equal with the stemmed implants. These stemless metaphyseal prostheses without a diaphyseal stem resect only minimal amount of bone, preserve bone and does not violate the humeral shaft, avoiding most of the intra-operative complications relating to the preparation of the humeral shaft. As there is no need for preparation of humeral shaft, the procedures are much shorter than with stemmed implants.
The rTSA is mainly performed in the older population. This patient age group has tendency to suffer from trips and falls. Therefore, have an increased risk to suffer late traumatic periprosthetic fractures. If a stemmed prosthesis is used, the periprosthetic humeral fracture tends to happen at mid-shaft of the humerus (at the metal-bone interface stress riser). Using short metaphyseal without a diaphyseal stem prosthesis reduces the risk of diaphyseal periprosthetic fracture. Using the stemless implants, does not reduce the risk of falls in this patients age group, however, if fracture is to happen, it will involve the metaphysis rather than the humeral shaft. Metaphyseal fractures may heal better than diaphyseal ones with conservative treatment.
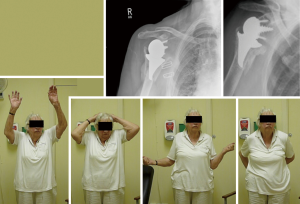
Levy et al. (26) in their series had eight patients (of 149) that sustained periprosthetic fractures of the proximal humerus (metaphyseal fractures) following a fall. Six of them were treated conservatively and all healed with good to reasonable functional outcome (Figure 6). Only Two patients with displaced meta-diaphyseal periprosthetic fracture had to be operated and were revised to a stemmed reverse prosthesis. Performing stemless reverse TSA allows for another “revision stage”, if needed, to a stemmed implant, and therefore, better suitable for use in younger patients that may need to be revised during their life time.
Natera et al. (37) assessed the clinical results of the Verso metaphyseal rTSA in young patients aged 65 or younger operated between 2006 and 2015 with 2 to 11 years follow up. They had 44 patients (29 F, 15 M) with a mean age of 59±6 years (range, 39–65 years).
Indications were cuff tear arthropathy, rheumatoid arthritis, primary osteoarthritis and fracture sequelae. Seventeen of them were revision arthroplasty. At 2 years’ follow-up, the mean CS improved from 18.1±11.9 to 60.1±18.6 (P<0.001) and the mean subjective shoulder value increased from 0.79/10 to 8.5/10 (P<0.001). The mean active elevation was 141.1°±41.9° (P<0.001) with an improvement of 82.7°±39.9°, the active abduction 136.8°±44.7° (P<0.001) with an improvement of 87°±39.9°, the active abduction 136.8°±44.7° (P<0.001) with an improvement of 87°±42°, the active external rotation 36.9°±21.4° (P<0.001) with an improvement of 16°±22° and the active internal rotation 66.2°±23.1° (P<0.001) with an improvement of 40.1°±35°. All the patients rated their shoulder as much better or better than before the operation, with 68% excellent. There were no statistically significant differences between outcome at 12 months and last follow up.
No differences were observed between primary and revision cases. No lucencies, subsidence, stress-shielding, glenoid notching or implant loosening were evident radiographically at last follow up. The good clinical and radiological results were maintained over time.
Reverse total shoulder arthroplasty is providing good shoulder elevation, yet less predictable rotations. Good rotations are crucial for performance of activities of daily living (ADLs), including personal hygiene. Concerns remain regarding bilateral rTSA over lack of rotations bilaterally and resultant difficulties with ADLs. Levy et al. (27) examined the outcome of 19 patients (15 women, 4 men; 38 shoulders) with bilateral rTSA in restoration of function and ADLs.
The mean follow-up was 48.4 months (range, 24–75 months). Mean duration between staged operations was 18.2 months (range, 3–46 months). The Constant score improved from 18.7 to 65.1 points (age- and sex-adjusted, 100.2). Elevation improved from 57.5° to 143°, internal rotation (IR) from 9° to 81° (30 shoulders could reach above the sacroiliac joint), and external rotation (ER) from 20° to 32° (35 shoulders had >20° ER in adduction, 31 shoulders had full ER in elevation). The Subjective Shoulder Value improved from 2.1 of 10 to 9.2 of 10. Mean ADLEIR (Activities of Daily Living External and Internal Rotations) score was 33 of 36 (P<0.001 for all). Most patients resumed their leisure and sport activities (gardening, golf, swimming, bowling).
Bilateral rTSA results in marked and predictable improvement in all movements, pain relief, and functional outcomes, with high patient satisfaction and high ADLEIR score. All patients were able to perform perineal hygiene after their rTSA. Most patients had no limitation in ADLs and their leisure activities (Figure 7).

Another difficult population with theoretical higher risk of dislocation and loosening are patients with ‘weight-bearing’ shoulders, using wheelchair or crutches. Arealis et al. (31) assessed the long-term results of stemless Verso rTSA in 24 patients (30 shoulders) with ‘weight-bearing’ shoulders. The results in this group mirrored the results in the non ‘weight-bearing’ shoulders group. Constant score improved from 9.4 (range, 2–26) points preoperatively to 59.8 (range, 29–80) points at final follow up (P=0.001). Range of motion improved from 46° to 130° of elevation, 13° to 35° of external rotation and 29° to 78° internal rotation (P=0.001). The ADLEIR score was 32.4/36.
No dislocations or instability occurred. Radiographically, no lucencies, subsidence, stress shielding, lucencies around the implants or implant loosening were evident on the X-rays both on the humeral and the glenoid side. There were 3 Sirveaux-Nerot grade 1 (10%) and 3 grade 2 (10%) glenoid notching. No intraoperative, early or long term complications were noted. However, they had to be treated carefully in the first 6 weeks after surgery, having to be hoisted to the wheelchair and avoiding any weight through their shoulders for the first 6 weeks after surgery.
Therefore, it can be concluded that both stemless reverse implants show reliable excellent outcome, with less invasive systems and a low rate of complications (25,26,30).
Despite the good results obtained, the use of TESS stemless reverse prostheses was limited for fear of failure of bone fixation, due to greater forces on the implant. Moroder et al. (38) investigated the short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stemmed implants, using the stemless TESS reverse prosthesis between 2009 and 2013. The authors were able to use the stemless TESS reverse only in 18.4% of cases due to the bone quality. Clinical and radiological outcomes of both groups were comparable. At 35 months follow-up (range, 24–75 months), no significant difference was noted regarding Constant score, ASES score, subjective shoulder value, pain score, patient satisfaction, strength, and range of motion between the groups. One case of traumatic dislocation was observed in the stemless RSA group. Scapular notching grade 1 was detected in two cases of the stemless group, while in the stemmed group five cases with grade 1 and four cases with grade 2 notching were observed. No loosening of the humeral component was observed in neither group. The authors concluded that at short to mid-term follow-up, stemless rTSA does not feature inferior clinical or radiological outcomes.
Conclusions
Based on the good results with the stemless metaphyseal implants described above, other stemless reverse implants are emerging and being introduced recently by different manufacturers. The percentage of usage of stemless reverse prostheses in Europe is constantly growing. This is expected to grow much further with the introduction of stemless implants to the US.
With the long term (>13 years) excellent clinical and radiologic results of certain stemless reverse TSA designs (25-27,30), that show long survivorship, together with being bone preserving and provide better revise-ability, should the need occur, the future use of stemless reverse TSAs will increase. However, as always, surgeons must be diligent in selecting and using proven implants that have shown good track record and long term good results.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. O Levy receives equity and royalties from Innovative Design Orthopaedics (IDO) as designing surgeon. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bankes MJ, Emery RJ. Pioneers of shoulder replacement: Themistocles Gluck and Jules Emile Pean. J Shoulder Elbow Surg 1995;4:259-62. [Crossref] [PubMed]
- Ross AC, Wilson JN, Scales JT. Endoprosthetic replacement of the proximal humerus. J Bone Joint Surg Br 1987;69:656-61. [Crossref] [PubMed]
- Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am 1982;64:319-37. [Crossref] [PubMed]
- Levy O, Funk L, Sforza G, et al. Copeland surface replacement arthroplasty of the shoulder in rheumatoid arthritis. J Bone Joint Surg Am 2004;86-A:512-8. [Crossref] [PubMed]
- Levy O, Copeland SA. Cementless surface replacement arthroplasty of the shoulder. 5- to 10-year results with the Copeland mark-2 prosthesis. J Bone Joint Surg Br 2001;83:213-21. [Crossref] [PubMed]
- Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg 2004;13:266-71. [Crossref] [PubMed]
- Copeland SA, Levy O, Brownlow HC. Resurfacing Arthroplasty of the Shoulder. Techniques in Shoulder & Elbow Surgery 2003;4:199-210. [Crossref]
- Copeland S. The continuing development of shoulder replacement: "reaching the surface J Bone Joint Surg Am 2006;88:900-5. [PubMed]
- Levy O, Tsvieli O, Merchant J, et al. Surface replacement arthroplasty for glenohumeral arthropathy in patients aged younger than fifty years: results after a minimum ten-year follow-up. J Shoulder Elbow Surg 2015;24:1049-60. [Crossref] [PubMed]
- Levy O. Shoulder resurfacing: is it really as good as total shoulder replacement? Current Orthopaedic Practice 2012;23:2-9. [Crossref]
- Mullett H, Levy O, Raj D, et al. Copeland surface replacement of the shoulder. Results of an hydroxyapatite-coated cementless implant in patients over 80 years of age. J Bone Joint Surg Br 2007;89:1466-9. [Crossref] [PubMed]
- Raj D, Mullett H, Even T, et al. Hydroxyapatite-coated Copeland surface replacement arthroplasty. Long term follow up. 19th Congress of the European Society for Surgery of the Shoulder and Elbow (ESSSE); September 21-24, 2005; Rome, Italy, 2005.
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Lévigne C, Boileau P, Favard L, et al. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2008;17:925-35. [Crossref] [PubMed]
- Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop 2010;34:1075-82. [Crossref] [PubMed]
- Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am 1996;78:603-16. [Crossref] [PubMed]
- Wall B, Nove-Josserand L, O'Connor DP, et al. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am 2007;89:1476-85. [PubMed]
- Andersen JR, Williams CD, Cain R, et al. Surgically treated humeral shaft fractures following shoulder arthroplasty. J Bone Joint Surg Am 2013;95:9-18. [Crossref] [PubMed]
- Levy O, Atoun E, Narvani A, et al. Does reverse shoulder need a stem? 2–7 years Follow-up with stemless reverse total shoulder Prosthesis. J Shoulder Elbow Surg 2013;22:e32-e3. [Crossref]
- Levy O, Young L, Tsvieli O, et al., editors. Stemless Reverse Total Shoulder Arthroplasty rTSA in Patients under 70 years Mid-term Results 25th SECEC-ESSSE Congress Istanbul 2014; Istanbul, Turkey, 2014.
- Atoun E, Van Tongel A, Hous N, et al. Reverse shoulder arthroplasty with a short metaphyseal humeral stem. Int Orthop 2014;38:1213-8. [Crossref] [PubMed]
- Arealis G, Hope N, Levy O. Reverse total shoulder replacement (rTSA) for patients with ‘weight-bearing’ shoulders using wheelchair or crutches 26th SECEC-ESSSE Congress - European society for surgery of the shoulder and the elbow; Milano, Italy. 2015:OP-111.
- Ballas R, Beguin L. Results of a stemless reverse shoulder prosthesis at more than 58 months mean without loosening. J Shoulder Elbow Surg 2013;22:e1-e6. [Crossref] [PubMed]
- Levy O, Walecka J, Tsvieli O, et al. Bilateral Reverse Total Shoulder Arthroplasty RTSA - Functional Outcome and Activities of Daily Living ADLs 25th SECEC-ESSSE Congress Istanbul 2014. Istanbul, Turkey 2014:PO-255.
- Levy O, Narvani A, Hous N, et al. Reverse shoulder arthroplasty with a cementless short metaphyseal humeral implant without a stem: clinical and radiologic outcomes in prospective 2- to 7-year follow-up study. J Shoulder Elbow Surg 2016;25:1362-70. [Crossref] [PubMed]
- Levy O, Consigliere P, Witney-Lagen C, et al. editors. Metaphyseal Reverse Total Shoulder Arthroplasty without a stem - Long-Term Results With 5-11 Years Follow-Up. SECEC 2017; Berlin, Germany, 2017.
- Levy O, Walecka J, Arealis G, et al. Bilateral reverse total shoulder arthroplasty-functional outcome and activities of daily living. J Shoulder Elbow Surg 2017;26:e85-e96. [Crossref] [PubMed]
- Mackenzie DB. The antero-superior exposure for total shoulder replacement. Orthopedics and Traumatology 1993;2:71-7. [Crossref]
- Hopkins AR, Hansen UN. Primary stability in reversed-anatomy glenoid components. Proc Inst Mech Eng H 2009;223:805-12. [Crossref] [PubMed]
- Teissier P, Teissier J, Kouyoumdjian P, et al. The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: clinical and radiographic results. J Shoulder Elbow Surg 2015;24:45-51. [Crossref] [PubMed]
- Arealis G, Hope N, Atoun E, et al. Reverse Total Shoulder Replacement In Patients With ‘Weight-bearing’ Shoulders On Wheelchair Or Walking Aids 2016 AAOS Annual Meeting, March 1 - 5, 2016; Orlando, Florida, USA: AAOS; 2016. p. Poster #P309.
- Levy O, Young L, Tsvieli O, et al. Stemless Reverse Total Shoulder Arthroplasty rTSA in Patients under 70 years Mid-term Results 25th SECEC-ESSSE Congress Istanbul 2014 2014:PO-261.
- Huguet D, DeClercq G, Rio B, et al. Results of a new stemless shoulder prosthesis: radiologic proof of maintained fixation and stability after a minimum of three years' follow-up. J Shoulder Elbow Surg 2010;19:847-52. [Crossref] [PubMed]
- Kadum B, Mukka S, Englund E, et al. Clinical and radiological outcome of the Total Evolutive Shoulder System (TESS(R)) reverse shoulder arthroplasty: a prospective comparative non-randomised study. Int Orthop 2014;38:1001-6. [Crossref] [PubMed]
- Kadum B, Mafi N, Norberg S, et al. Results of the Total Evolutive Shoulder System (TESS): a single-centre study of 56 consecutive patients. Arch Orthop Trauma Surg 2011;131:1623-9. [Crossref] [PubMed]
- von Engelhardt LV, Manzke M, Filler TJ, et al. Short-term results of the reverse Total Evolutive Shoulder System (TESS) in cuff tear arthropathy and revision arthroplasty cases. Arch Orthop Trauma Surg 2015;135:897-904. [Crossref] [PubMed]
- Natera LG, Consigliere P, Witney-Lagen C, et al., editors. Clinical Results Of A Short Metaphyseal Reverse Total Shoulder Arthroplasty In Patients Aged 65 Or Younger: 2 To 11 Years Follow Up. EFORT 2018; Barcelona, Spain, 2018.
- Moroder P, Ernstbrunner L, Zweiger C, et al. Short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stem. Int Orthop 2016;40:2115-20. [Crossref] [PubMed]
Cite this article as: Levy O, Panagopoulos GN, Leonidou A, Atoun E. Stemless reverse shoulder arthroplasty: indications, technique and European experience. Ann Joint 2018;3:108.


