
Behind-remnant approach for anatomic anterior cruciate ligament reconstruction
Introduction
In anterior cruciate ligament (ACL) reconstruction, femoral tunnel position is especially important to determine knee kinematics and consequently clinical outcome. In double-bundle (DB) ACL reconstruction, each kinematics of anteromedial bundle (AMB) and posterolateral bundle (PLB), as well as their complementarity, is considered more critical. The majority of anatomic studies regarding native ACL revealed that the ACL insertion was positioned posteriorly to the lateral intercondylar ridge (1-4). However, how to create femoral tunnel for ACL graft is still controversial among surgeons; some described a relatively narrow insertion area (5-7), while other studies indicated a greater femoral insertion that extended widely to the articular cartilage margin (1,3,8,9).
Recent morphologic studies of native ACL using human cadaveric knees have shown that femoral insertion can be histologically and macroscopically divided into direct and indirect insertions (10,11). Surgeons should also consider large variations among knees, especially in terms of bony morphology and ACL itself. Usually, bony landmarks such as intercondylar ridge indicate where to place drill holes, however, even such bony landmarks have large variety among knees (12). On the other hand, with consideration of graft function, some surgeons recommend femoral tunnel placement near posterior articular cartilage margin (2). These differences among ACL surgeons might make them confuse the ideal or targeting femoral tunnel position in the anatomic ACL reconstructions. Moreover, what kind of anatomic landmarks could be available when the bony ridges were found to exist very anteriorly or not to exist during surgery (12).
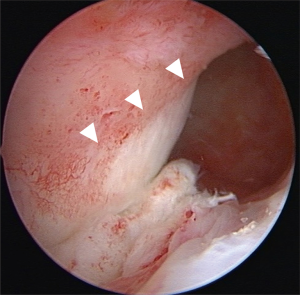
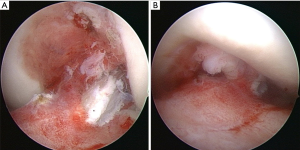
A recent trend in ACL reconstruction has been toward remnant-preserving technique based on the advantages such as better proprioceptive function (13-16), stability preservation (17,18), and better graft healing (19-22). Among them, we have developed a new approach for femoral tunnel creation, called a behind-remnant (BR) approach, in remnant-preserving DB ACL reconstruction (23). This technique has been developed based on a cadaveric study, reporting that the fan-like extension fibers, which attach to the indirect insertion, become tense only at extension, whereas the midsubstance fibers, which attach to the direct insertion, are tense at all flexion angle during knee extension-flexion (10). Consequently, a fold is observed at the margin between the direct and indirect insertions at knee-flexed position, and the fold can be confirmed arthroscopically behind the ACL. We have also reported that, with arthroscopic observation from the anteromedial portal, the direct insertion of the proximal portion of the injured ACL were especially well preserved in the majority of the cases, whereas the ruptured part of the midsubstance was covered with synovial tissue and the fan-like extension was differently preserved among the cases (Figure 1) (24). The midsubstance covered with synovial tissue would indicate the direct insertion of the ruptured ACL (23,24). Based on these findings, we found that the BR approach enabled us to create femoral tunnels at the posterior border of the midsubstance, the direct insertion, without any removal of the ACL remnant.
In this article, detailed surgical technique of the BR approach in remnant-preserving DB ACL reconstruction were described. Biomechanics of the ACL grafts especially focusing on graft tensions and femoral tunnel positions of the grafts were also evaluated. In addition, short-term results using this technique were reported.
Surgical technique
A routine procedure for intra-articular abnormalities was performed through anteromedial and anterolateral portals using 30-degree arthroscope before starting ACL reconstruction. A detailed observation was performed to define the portion, type and extent of the injured ACL. For the concomitant meniscus and articular cartilage injuries, appropriate procedures were determined based on the injury status. An oblique 3 cm incision was made on the anteromedial tibial surface at the level of the pes anserinus. Both gracilis and semitendinosus (ST) tendons were identified, and only ST tendon was harvested with an open-loop tendon stripper (Smith & Nephew Endoscopy, Andover, MA, USA). The harvested ST tendon was cut into halves and folded, creating 2 double-stranded grafts of at least 5.5 cm in length, then grafts were looped with the EndoButton CL-BTB (Smith & Nephew Endoscopy). The open end of each graft was closed with two Krackow sutures and a Bunnel suture using No. 2 braided polyester sutures. The 2 grafts were kept with tension for more than 10 minutes on the Graftmaster (Smith & Nephew Endoscopy) by Suture Vise with Tensiometer (Smith & Nephew Endoscopy).
Femoral tunnel creation was performed with the BR approach. None of the remnant tissue was removed for creating femoral tunnels. Arthroscopic observation via the anteromedial portal with a 30-degree arthroscope was practiced (Figures 2,3). Here we describe outside-in technique, but transportal technique as well as transtibial technique can also be utilized in the BR approach. For the PL tunnel creation, a guide wire was aimed at the deepest corner of the femoral articular cartilage with a 5-mm margin from the articular surface at 90-degree knee flexion. For the AM tunnel creation, a guide wire was aimed at the posterior border of the direct insertion of the native ACL with reference to the articular surface and its upper end. For femoral guide wire insertion with outside-in technique, an Antero-Lateral Entry Femoral Aimer (Smith and Nephew Endoscopy) was used and was set at an insertion angle of 10° for the AMB and −10° for the PLB. The angle of the femoral tunnel in relation to the joint line was aimed at 40° for the AMB and 30° for the PLB in the axial plane. For both AM and PL tunnels, with the knee at 90° flexion, a guide wire was introduced from the lateral femoral cortex, over-drilled using an EndoDrill (Smith and Nephew Endoscopy), and replaced by a FlipCutter (Arthrex, Naples, FL), creating a femoral socket with a diameter matched with the graft diameter. The remnant tissue of the ruptured ACL at the tibial side was not removed at all for tibial tunnel creation
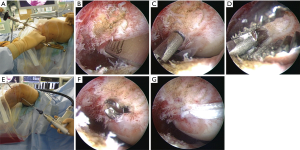
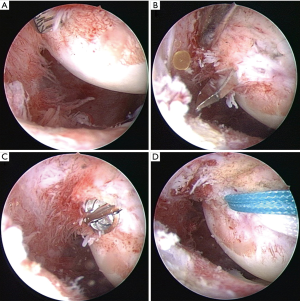
For tibial tunnel creation, the remnant of the injured ACL was also not removed at all at the tibial insertion (Figure 4). Tibial guide wires for AM and PL tunnels were introduced to the original tibial footprint from the anteromedial surface of the tibia at the level of the tibial tubercle with the anatomic landmarks of the ACL tibial footprint and medial intercondylar eminence using ACUFEX Director Tip Aimer (Smith & Nephew Endoscopy). For the AM tunnel, the tibial aimer was set at an angle of 60°, and the guide wire was aimed 3 mm posterior to the anterior margin of the ACL remnant and just lateral to the medial intercondylar eminence at an angle of 65° from the joint line in the coronal plane. For the PL tunnel, the tibial aimer was set at an angle of 55°, and the guide wire for the PLB was aimed just lateral to the spine of the medial intercondylar eminence at an angle of 45° from the joint line in the coronal plane. Then, tibial tunnels of the AMB and PLB were created by a cannulated reamer with the same diameter as each graft.
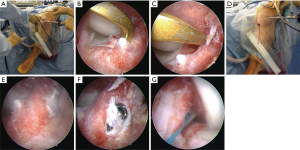
The PL graft was first introduced with arthroscopic observation, then the AM graft in the same manner. Femoral side fixation was performed using the EndoButton CL-BTB. Each graft was fixed to an anchor staple with sutures at the tibial site at 20° of flexion. Each graft was fixed with the tension adjusted to the graft diameter on the basis of 25N for the graft diameter of 6 mm, so that the initial tensions of both the AM and PL grafts were equally stressed (25,26). Graft impingement was checked at knee full extension after final fixation (Figure 5).
Postoperative management
The postoperative rehabilitation was undergone for every patient with the same protocol. The protocol was not modified even when meniscus surgery was performed along with ACL reconstruction. The postoperative rehabilitation protocol was as follows: knee range of motion exercise started at 2 days postoperatively from 0° of extension to gradual flexion up to 120° of flexion by 4 weeks postoperatively. Twenty kg partial weight-bearing was allowed at 2 days, and was gradually increased. Crutches were removed at 4 weeks. The closed kinetic exercises start 6 weeks postoperatively as a quarter squatting, then a half squatting. Jogging was allowed after accomplishing stable one-leg half squatting. The running speed was encouraged to increase gradually. After a patient could perform 80% of full speed run, athletic exercises related to the previous sports or hopeful activities started with definite instructions. Athletic exercises were specified to each patient step by step to desired sports along with the patient’s athletic level. Full athletic activities, then return to sports, were allowed 6 months after surgery if the patient did not show any problematic symptoms in the joint with sufficient muscle recovery after specified athletic training had been accomplished.
Graft tension changes and femoral tunnel position
We compared graft tension change of each the AMB and PLB and their femoral tunnel positions of the BR approach with the control. The study included 23 patients who underwent remnant-preserving DB ACL reconstruction using the BR approach (BR group) and 25 patients who underwent DB ACL reconstruction before we introduced the BR approach (control group). In the control group, the intercondylar ridge was detected and used as a landmark for femoral tunnel creation, and femoral tunnels were created based on the bony landmarks. The demographic data and laxity data between the 2 groups were not significantly different.
This study was approved by our institutional review board, and all the patients provided informed written consent.
Graft tension changes
During surgery, each AM and PL graft was fixed to the SE Graft Tensioning System (Linvatec, Largo, FL, USA) provisionally using sutures at the tibial site after the two grafts had been fixed on the femoral bony surface with the EndoButton CL-BTB (27). A graft-tensioning device, named the SE Graft Tensioning System, can help quantifying and applying consistent amounts of tension to each graft, with an accuracy of 0.83±0.03 N (mean ± standard deviation) according to the manufacturer’s guidelines.
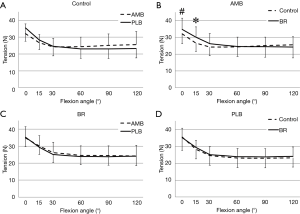
Each AM graft and PL graft was fixed at 20° of knee flexion. The tension was applied to each graft adjusted to be equal-stress tensioned per cross sectional area on a basis of 25 N per 6 mm in diameter, so that both bundles were equal-stress tensioned at the time of the measurements (25). The graft tension changes of each AM and PL bundles were evaluated during knee range of motion. Knee flexion-extension movement was manually applied from 0° to 120° with avoiding any translational or rotational forces to the leg. The knee flexion angle-graft tension curves were described with each graft tension measured at 0°, 15°, 30°, 60°, 90° and 120°. The same one surgeon performed all measurements. He was very careful to apply a same motion or load to the knee joint in order to make the inter-rater variability small. He performed all measurements three times with the results shown as an average.
The reciprocal tension curves during flexion-extension was observed between the AM and PL grafts. That is, the PL graft had greater tension than the AM graft between 0° and 30°, whereas the AM graft showed greater tension beyond 60° of knee flexion with no statistical difference (Figure 6A). On the other hand, AM and PL grafts showed equivalent tension curves in the BR group (Figure 6B). The results of the comparison of the average flexion angle of the knee to graft tension curves of the AMB indicated that the graft tension of the BR group tended higher at 0 degree, and that was significantly higher at 15 degrees than that of the control group (P=0.14 and 0.038, respectively) (Figure 6C). The tension of the 2 groups showed no significant difference over 30° of knee flexion. The tension curves of the PL graft were not significantly different between the 2 groups (Figure 6D).
Femoral tunnel position
The femoral and tibial tunnel positions were evaluated by three-dimensional computed tomography (3D-CT) taken 1week postoperatively (Figure 7). For assessing the femoral tunnel position, the sagittal view with neutral rotation of the lateral femoral condyle was used (28). The quadrant method reported by Bernard et al. was used to describe the center of the femoral bone tunnels of the AM and PL grafts (29). The measurement was independently performed by two observers, who were blinded to the intra-operative data, using ImageJ software (http://rsb.info.nih.gov/ij/). Analysis of inter-observer reliability yielded an intra-class correlation coefficient of 0.992 (95% confidence interval; 0.987–0.995) (30).
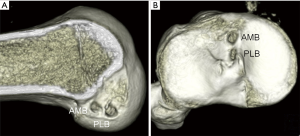
The AM tunnel position in the BR group was significantly more posterior than that in the control group (Table 1). Tunnel position of the PLB in the BR group was significantly lower than that in the control group. Smaller variances of the AM tunnel position in the BR group was indicated with the F test compared with the control group.

Full table
Clinical outcomes
The followed-up patients with the BR approach consisted of 29 male and 19 female (48 in total) with a mean age of 23 (range, 14–41) years at the time of surgery. The mean time from injury to surgery was 13 (range, 1–72) months. Eleven combined medial meniscus injuries were treated with 8 repaired and 3 partially removed; 11 lateral meniscus injuries were treated with 9 repaired and 2 partially removed; 2 had both medial and lateral meniscus injuries (both repaired). This study was approved by our institutional review board, and all the patients provided informed written consent.
During the follow-up period, one patient sustained ACL graft tear with obvious re-injury episode. The other patient had contralateral ACL injury in a noncontact situation after returning to her pre-injury activity level. With regard to new meniscus injury, 1 patient had retear of the repaired medial meniscus injury (partially removed) and 3 had new medial meniscus injury (repaired). Two patients required additional arthroscopic synovectomy due to the prolonged extension loss of the operated knee. In both cases, a Cyclops lesion was observed in front of the reconstructed ACL, and the loss of extension was improved after resection of the Cyclops lesion. No patient left more than 5-degree extension loss at the final follow-up.
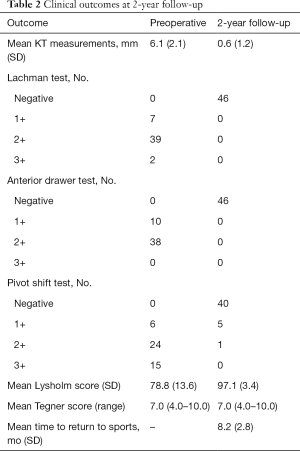
Clinical outcomes are shown in Table 2. Mean KT measurements improved from 6.1 mm preoperatively to 0.6 mm at final follow-up, and the Lachman test and the anterior drawer test became negative in all cases. Pivot shift result was also improved, although 6 cases had residual pivot shift (grade 1: 5 cases, grade2: 1 case) at final follow-up. Preoperative 79 points Lysholm score improved to 98 points averagely at final follow-up. Sports activity level represented by the Tegner score was maintained from preinjury to final follow-up, with mean time required to return to athletic activities of 8 months.
Full table
Discussion
The current article showed that the BR approach for a remnant-preserving DB ACL reconstruction validated consistency of femoral tunnel creation and good clinical outcomes. Femoral tunnel creation by the BR approach showed higher reproducibility than the conventional approach in our institute.
It would be technically difficult to place the tunnels in the appropriate anatomic position if the arthroscopic observation is performed only from the frontside of the ACL remnant when the surgeon attempts to perform the remnant preservation ACL reconstruction. The bony landmarks as the intercondylar ridge is hard to detect without any removal of the remnant insertion. Therefore, it is reported to confirm the tunnel position by X-ray (19) or C-arm during surgery. Otherwise, some extents of the remnant tissue have to be detached from the femoral insertion to identify the bony landmarks. Furthermore, there are various patterns of intercondylar ridge, and there are some cases which do not present the intercondylar ridge obviously (12). In such cases, it will be difficult to create the femoral tunnels appropriately and accurately only dependent on the bony landmarks. With consideration of the amount of tissue removal, the BR approach could preserve the remnant tissue as much as possible without any removal of the remnant tissue at the femoral side. Important findings are that the majority of the ACL injured knees indicates the original direct insertion of proximal portion of the injured ACL with synovial proliferation, which can be used as a landmark for the AM tunnel creation. On the other hand, the original insertion of the PLB was less preserved with less synovial proliferation; therefore, aiming position for center of the PL tunnel at 5 mm from the deepest articular cartilage surface at 90° of knee flexion, with use of other remnant tissue as a landmark, was useful for surgeons to create highly reproducible femoral PL tunnel. Our important experience of injured ACL with the observation from the behind-remnant indicated that the remnant of the direct insertion did not move from the original position although the freed remnant scar from the femoral attachment hanged down anteriorly in some cases. On the other hand, the original direct insertion of the injured ACL varied among patients in height from the articular surface; some had higher insertion site with wider fan-like extension, while others had vice versa.
By use of the BR approach, the AM tunnel position was more posterior than those by the conventional approach in our experience. Regarding the better and more appropriate femoral tunnel creation, further discussion will be necessary to achieve better clinical outcome. AM tunnel position was created lower and deeper than those by conventional approach. According to our results of graft tension pattern measurements during surgery, the control group indicated reciprocal tension pattern by average, which was more similar to the tension patterns of native ACL. These results will be rational because the centers of the AM and PL tunnels in the control group seem to be placed at the centers of the direct insertion site of the AMB and PLB. On the other hand, deeply placed AM tunnels in the BR group indicated higher tension in extension; consequently, the BR group showed an equivalent pattern during knee flexion-extension. When the femoral tunnel is more posteriorly placed, the graft tension becomes tighter with extension and looser with flexion (30-32). This might over-constrain the reconstructed ACL in the knee than normal ACL, causing loss of extension or graft retear (31). However, in our case series at 2-year follow-up, only 1 patient (2%) had graft retear and there was no patient with extension deficit, although 2 patients required resection of the Cyclops lesion. In addition, clinical results were satisfactory in both subjective and objective findings.
Nevertheless, we believe that it is more appropriate to create the femoral tunnel a little more posterior to the native direct insertion during an anatomic ACL reconstruction, aiming at the posterior margin of the direct insertion of the normal ACL for the tunnel center. It will be especially the case when soft -tissue grafts are selected, considering tunnel expansion and graft deviation in the tunnel during the early period after surgery (32,33). Still, further studies by randomized controlled trial comparing with conventional approach would be needed to verify the validity of the posteriorly placed femoral tunnel created by BR approach.
Conclusions
In remnant-preserving DB ACL reconstruction, femoral tunnels could be created without any remnant tissue removed by using the behind remnant (BR) approach. The femoral tunnel creation could be possible with high reproducibility with the BR approach. More posteriorly placed tunnel by the BR approach technique indicated equivalent tension pattern both in the AMB and PLB. Short-term clinical results after remnant-preserving DB ACL reconstruction using the BR approach were satisfactory.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.12.01). The series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” was commissioned by the editorial office without any funding or sponsorship. TM served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iwahashi T, Shino K, Nakata K, et al. Direct anterior cruciate ligament insertion to the femur assessed by histology and 3-dimensional volume-rendered computed tomography. Arthroscopy 2010;26:S13-20. [Crossref] [PubMed]
- Shino K, Suzuki T, Iwahashi T, et al. The resident's ridge as an arthroscopic landmark for anatomical femoral tunnel drilling in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2010;18:1164-8. [Crossref] [PubMed]
- Ferretti M, Ekdahl M, Shen W, et al. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy 2007;23:1218-25. [Crossref] [PubMed]
- Hutchinson MR, Ash SA. Resident's ridge: assessing the cortical thickness of the lateral wall and roof of the intercondylar notch. Arthroscopy 2003;19:931-5. [Crossref] [PubMed]
- Hara K, Mochizuki T, Sekiya I, et al. Anatomy of normal human anterior cruciate ligament attachments evaluated by divided small bundles. Am J Sports Med 2009;37:2386-91. [Crossref] [PubMed]
- Mochizuki T, Muneta T, Nagase T, et al. Cadaveric knee observation study for describing anatomic femoral tunnel placement for two-bundle anterior cruciate ligament reconstruction. Arthroscopy 2006;22:356-61. [Crossref] [PubMed]
- Yasuda K, Kondo E, Ichiyama H, et al. Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy 2004;20:1015-25. [Crossref] [PubMed]
- Colombet P, Robinson J, Christel P, et al. Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy 2006;22:984-92. [Crossref] [PubMed]
- Harner CD, Baek GH, Vogrin TM, et al. Quantitative analysis of human cruciate ligament insertions. Arthroscopy 1999;15:741-9. [Crossref] [PubMed]
- Mochizuki T, Fujishiro H, Nimura A, et al. Anatomic and histologic analysis of the mid-substance and fan-like extension fibres of the anterior cruciate ligament during knee motion, with special reference to the femoral attachment. Knee Surg Sports Traumatol Arthrosc 2014;22:336-44. [Crossref] [PubMed]
- Sasaki N, Ishibashi Y, Tsuda E, et al. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy 2012;28:1135-46. [Crossref] [PubMed]
- Tsukada S, Fujishiro H, Watanabe K, et al. Anatomic variations of the lateral intercondylar ridge: relationship to the anterior margin of the anterior cruciate ligament. Am J Sports Med 2014;42:1110-7. [Crossref] [PubMed]
- Denti M, Monteleone M, Berardi A, et al. Anterior cruciate ligament mechanoreceptors. Histologic studies on lesions and reconstruction. Clin Orthop Relat Res 1994;29-32. [PubMed]
- Adachi N, Ochi M, Uchio Y, et al. Mechanoreceptors in the anterior cruciate ligament contribute to the joint position sense. Acta Orthop Scand 2002;73:330-4. [Crossref] [PubMed]
- Lee BI, Min KD, Choi HS, et al. Immunohistochemical study of mechanoreceptors in the tibial remnant of the ruptured anterior cruciate ligament in human knees. Knee Surg Sports Traumatol Arthrosc 2009;17:1095-101. [Crossref] [PubMed]
- Lee BI, Kwon SW, Kim JB, et al. Comparison of clinical results according to amount of preserved remnant in arthroscopic anterior cruciate ligament reconstruction using quadrupled hamstring graft. Arthroscopy 2008;24:560-8. [Crossref] [PubMed]
- Nakamae A, Ochi M, Deie M, et al. Biomechanical function of anterior cruciate ligament remnants: how long do they contribute to knee stability after injury in patients with complete tears? Arthroscopy 2010;26:1577-85. [Crossref] [PubMed]
- Muneta T, Koga H, Ju YJ, et al. Remnant volume of anterior cruciate ligament correlates preoperative patients' status and postoperative outcome. Knee Surg Sports Traumatol Arthrosc 2013;21:906-13. [Crossref] [PubMed]
- Yasuda K, Kondo E, Kitamura N, et al. A pilot study of anatomic double-bundle anterior cruciate ligament reconstruction with ligament remnant tissue preservation. Arthroscopy 2012;28:343-53. [Crossref] [PubMed]
- Ochi M, Iwasa J, Uchio Y, et al. The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br 1999;81:902-6. [Crossref] [PubMed]
- Gohil S, Annear PO, Breidahl W. Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularisation. A randomised prospective study with a one-year follow-up. J Bone Joint Surg Br 2007;89:1165-71. [Crossref] [PubMed]
- Ahn JH, Lee SH, Choi SH, et al. Magnetic resonance imaging evaluation of anterior cruciate ligament reconstruction using quadrupled hamstring tendon autografts: comparison of remnant bundle preservation and standard technique. Am J Sports Med 2010;38:1768-77. [Crossref] [PubMed]
- Muneta T, Koga H, Nakamura T, et al. A new behind-remnant approach for remnant-preserving double-bundle anterior cruciate ligament reconstruction compared with a standard approach. Knee Surg Sports Traumatol Arthrosc 2015;23:3743-9. [Crossref] [PubMed]
- Muneta T, Koga H, Nakamura T, et al. Behind-remnant arthroscopic observation and scoring of femoral attachment of injured anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 2016;24:2906-14. [Crossref] [PubMed]
- Conner CS, Morris RP, Vallurupalli S, et al. Tensioning of anterior cruciate ligament hamstring grafts: comparing equal tension versus equal stress. Arthroscopy 2008;24:1323-9. [Crossref] [PubMed]
- Koga H, Muneta T, Yagishita K, et al. Effect of posterolateral bundle graft fixation angles on clinical outcomes in double-bundle anterior cruciate ligament reconstruction: a randomized controlled trial. Am J Sports Med 2015;43:1157-64. [Crossref] [PubMed]
- Koga H, Muneta T, Yagishita K, et al. The effect of graft fixation angles on anteroposterior and rotational knee laxity in double-bundle anterior cruciate ligament reconstruction: evaluation using computerized navigation. Am J Sports Med 2012;40:615-23. [Crossref] [PubMed]
- Inoue M, Tokuyasu S, Kuwahara S, et al. Tunnel location in transparent 3-dimensional CT in anatomic double-bundle anterior cruciate ligament reconstruction with the trans-tibial tunnel technique. Knee Surg Sports Traumatol Arthrosc 2010;18:1176-83. [Crossref] [PubMed]
- Bernard M, Hertel P, Hornung H, et al. Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 1997;10:14-21; discussion 21-2. [PubMed]
- Koga H, Muneta T, Yagishita K, et al. Effect of femoral tunnel position on graft tension curves and knee stability in anatomic double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2014;22:2811-20. [Crossref] [PubMed]
- Rahr-Wagner L, Thillemann TM, Pedersen AB, et al. Increased risk of revision after anteromedial compared with transtibial drilling of the femoral tunnel during primary anterior cruciate ligament reconstruction: results from the Danish Knee Ligament Reconstruction Register. Arthroscopy 2013;29:98-105. [Crossref] [PubMed]
- Kobayashi M, Nakagawa Y, Suzuki T, et al. A retrospective review of bone tunnel enlargement after anterior cruciate ligament reconstruction with hamstring tendons fixed with a metal round cannulated interference screw in the femur. Arthroscopy 2006;22:1093-9. [Crossref] [PubMed]
- Tsuda E, Fukuda Y, Loh JC, et al. The effect of soft-tissue graft fixation in anterior cruciate ligament reconstruction on graft-tunnel motion under anterior tibial loading. Arthroscopy 2002;18:960-7. [Crossref] [PubMed]
Cite this article as: Koga H, Muneta T. Behind-remnant approach for anatomic anterior cruciate ligament reconstruction. Ann Joint 2018;3:109.


