
Anatomic reconstruction of anterior cruciate ligament: concept, indication and its efficacy
Introduction
Among the most commonly performed procedures by sports medicine surgeons, the technique for anterior cruciate ligament (ACL) reconstruction has endured many alterations. From low anterior medial drilling to double-bundle reconstruction, the majority of such changes have been predicated on the goal of performing the most anatomic reconstruction possible. This trend continues today as a renewed focus has been placed not only on graft positioning, but on appropriate graft sizing based on the dimension of the original, injured ACL.
Of course, one need nothing more than basic intuition to understand that a ‘one size fits all’ approach to ACL reconstruction likely leads to suboptimal results. Just as orthopedists aim for anatomic reduction of a fracture, it reasons that the size of the ACL graft used should mimic the individual patient’s anatomy, rather than simply using a graft of an average reported size. As previously defined, “anatomic” reconstruction is the functional restoration of the ACL to its native dimension, collagen orientation, and insertion sites (1). In this article we review the current literature in terms of indications, rationale, and outcomes for the ever-evolving technique of anatomic ACL reconstruction.
ACL anatomy and function
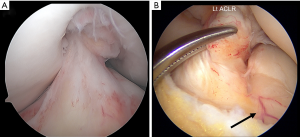
The ACL is a dynamic structure, rich in neurovascular supply and comprised of distinct bundles, which function synergistically to facilitate normal knee kinematics in concert with bony morphology. Characterized by individual uniqueness, the ACL is inherently subject to both anatomic and morphological variations as well as physiologic aging (2). The ACL is anatomically composed of two bundles, the anteromedial (AM) and posterolateral (PL) bundles, which are named based on their respective insertion site on the tibia. The native ACL is covered by synovial tissue, which is highly vascularized and serves to isolate the native ACL from the synovial fluid of the knee joint (Figure 1A). The vascular supply of the femoral insertion site originates from the posterior soft tissue. The tibial insertion site is vascularized in part by vessels that enter from the anterior horn of lateral meniscus (Figure 1B) (3). The ACL is also rich in mechanoreceptors, providing proprioceptive information and initiating protective and stabilizing muscular reflexes (4-6).
The femoral insertion site of the ACL possesses a complex geometry but has been characterized as oval- or ellipse-shaped (7,8). The tibial insertion site is equally resistant to simple geometric description, but fans into a broad insertion as it extends distally from the midsubstance. Both the femoral and tibial insertion sites contain regions of direct insertion architecture, with its characteristic fibrocartilaginous transition zone, as well as indirect insertion architecture in which the ligament transitions abruptly into bone (8,9). The isthmus of ACL exists at its midsubstance, approximately equidistant from the tibial and femoral insertion sites. The cross-sectional area of the isthmus is smallest in knee extension and increases with knee flexion. Furthermore, the isthmus is less than half the area of tibial and femoral insertion sites. The ACL length is shortest at 90° knee flexion and increases with knee extension (9). The morphology of the ACL often changes with age and fatty degeneration, especially in the PL bundle, is often seen in elderly patients.
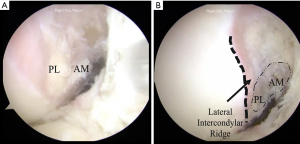
The ACL insertion sites are associated with particular bony landmarks. For the femoral insertion site (Figure 2A), the lateral wall of the intercondylar notch often possesses two visible ridges. The lateral intercondylar (i.e., resident’s) ridge demarcates the anterior border of the ACL, and the lateral bifurcate ridge runs perpendicular to the lateral intercondylar ridge, separating the femoral insertions of the AM and PL bundles. Absent femoral remnants following ACL rupture, these bony landmarks may be used to identify the borders of the native femoral ACL insertion site (Figure 2B) (10). Compared with the femoral attachment, there are fewer studies on the bony landmarks for the tibial insertion site. However, the medial intercondylar eminence, the anterior horn of the lateral meniscus, and the intermeniscal ligament (when visualized) may be used as reference points for the ACL tibial insertion site (10,11).
The ACL is the primary structure that contributes to anteroposterior and rotatory stability of the knee, especially at lower flexion angles (12). The AM and PL bundles work together as a unit to provide both anterior stability and rotatory stability of the knee in response to complex loads throughout the entire range of motion. That said, the bundles exhibited different tensioning patterns throughout a full range of motion. The AM bundle exhibits relatively consistent tension from full extension to 90° of flexion, but diminishing tension with further flexion. The tension of PL bundle diminishes dramatically when flexion exceeds 30° (13). Functionally the AM bundle plays a role in controlling the anterior tibial translation at more than 60° knee flexion, and the PL bundle shows more significant role in controlling anteroposterior and rotatory stability at lower knee flexion angles (14). The principal purpose of ACL reconstruction surgery is to restore these functions so as to provide for dynamic stability of the knee joint.
Current understanding of anatomic reconstruction
One cannot begin a discussion of anatomic ACL reconstruction without considering tunnel placement. Non-anatomic tunnel positioning has been well demonstrated to act as a key contributor to both poor outcomes and failure following ACL reconstruction (15,16). Further, while the complications of non-anatomic tunnel placement such as impingement and loss of range of motion are well documented, recent studies have also suggested that anatomic reconstruction also allows for an improved kinematic relationship of the implanted graft with adjacent native stabilizers, such as the PCL (17). Given such findings, a renewed focus on achieving anatomic placement has also called into question the long-practiced technique of transtibial tunnel drilling.
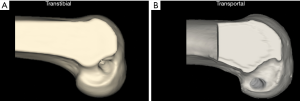
In this regard, the adoption of femoral drilling through a low AM portal has facilitated more anatomic tunnel creation (18), and improved outcomes in knee kinematic testing (19,20). In a comparison of tunnel positioning following transtibial and transportal femoral tunnel drilling, creation of a transtibial femoral tunnel was found to consistently fail to achieve the anatomic position of the ACL attachment (Figure 3) (21). Furthermore, a recent systematic review of studies comparing transtibial vs. AM drilling found that the latter yielded better objective (i.e., Lachman and Pivot Shift tests) and subjective (i.e., IKDC scores) outcomes compared to the former (22). Further, in a cadaveric study of tunnel placement, an anatomic location of femoral and tibial ACL tunnels resulted in graft force and anterior laxity that more closely resembled a native uninjured ACL than in specimens where the femoral tunnel was placed in a more vertical position (23). However, it may be possible to modify the transtibial approach to achieve anatomical placement; regardless of drilling technique, a thorough understanding of ACL anatomy is needed.
As the native ACL is divided into AM and PL bundles, a double bundle reconstruction technique was championed as it more closely restores joint kinematics in cadaveric testing models. Indeed, double bundle reconstruction has been shown to result in less anterior to posterior laxity versus a non-anatomic technique, although the clinical effect of such findings remains unclear (24-26). In a recent review, van Eck et al. created a step-by-step algorithm for anatomic ACL reconstruction, based upon a synthesis of a comprehensive literature review (27). The senior author favors a single bundle technique in the case of multi-ligamentous trauma or advanced arthritic changes, as well as in patients with open physes or a narrow femoral notch (28). Additionally, it has been suggested that a double bundle technique can be especially challenging in patients with a measured tibial insertion site of less than 14 mm, although there is a paucity of evidence to definitively support strict thresholds (28). Of course, it must be emphasized that the true predictor of outcomes is appropriate tunnel placement, rather than the use of a single or double-bundle technique. In the senior author’s experience, an anatomic single bundle reconstruction can achieve the same clinical outcomes as a double bundle technique (29).
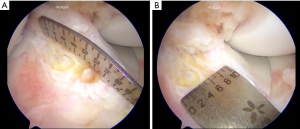
Another often overlooked aspect of ACL reconstruction is the use of individualized, anatomic graft sizes. While some author had initially recommended the use of a graft of at least 7 mm in diameter (30,31), more recent reviews have demonstrated a graft diameter of less than 8mm may be associated with higher revision rates (32,33). However, it is not simply the size of the graft, but rather the size of the graft in relationship to an individual’s anatomy that must be considered intra-operatively with the stated goal of restoring the native femoral and tibial attachment sites (34). Further, no one autograft has been shown to reliably recreate the ACL’s anatomic attachment sites (35), while individual characteristics such as height and weight have been demonstrated to be poor predictors of actual ACL size (36). For this reason, it is the senior author’s practice to take intra-operative measurements of the ACL tibial footprint (Figure 4), as cadaveric studies have shown the ACL midsubstance is 50.2%±15% of the tibial insertion area (37). Once these measurements are obtained, a successful anatomic reconstruction will implement a graft with an area constituting 50% to 80% of the measured insertion size.
Interestingly, many studies have shown that smaller grafts will eventually enlarge following implantation. One MRI study demonstrated large increases in hamstring autografts 12 months after implantation (38), with similar increases demonstrated in animal studies (39,40). Further, smaller graft sizes have also been shown to undergo increased long-term hypertrophy (41). Given these findings, it should be emphasized that the importance of anatomic graft sizing is to prevent failure in the first 12–14 months, before such changes have time to occur (33).
Surgical approach to anatomic ACL reconstruction
The first key to anatomic reconstruction is visualization. Visualization is optimized with a three-portal approach: (I) anterolateral (AL) portal, (II) AM portal, and (III) accessory anteromedial (AAM) portal. The AL portal is placed at the level of the inferior pole of the patella and just lateral to the lateral border of the patellar tendon. This should avoid Hoffa’s fat pad by being above it. Use of a spinal needle under direct arthroscopic visualization localizes the AM portal and the AAM portal. The AM portal is transtendinous, or immediately medial to the patellar tendon, aiming toward the central (medial-lateral) and inferior third of the intercondylar notch. The AAM portal is placed just above the medial joint line and 2 cm medial to the medial border of the patellar tendon. It is important to ensure there is at least 2 mm between the localizing spinal needle and the medial femoral condyle to avoid chondral injury during instrumentation. In this approach, the AAM portal provides the best visualization of the femoral footprint, but all portals are used to comprehensively evaluate the ACL insertion sites. For the majority of the surgery, the AM portal is used for visualization and the AAM portal is used for instrumentation.
The graft options for anatomic ACL reconstruction include quadriceps tendon autograft with or without bone plug, bone-patellar tendon-bone autograft, hamstring autograft, and allograft. Allografts have been shown to have higher failure rates in young, active patients, and should be avoided in young patients when possible (41,42). Graft choice is individualized for each patient based on numerous factors such as age, activity level, and preoperative imaging. Preoperative evaluation should include measurements of the thickness of the quadriceps and patellar tendon on sagittal MRI, as well as an assessment for tendinopathy (43), while hamstring tendon size can also be measured on preoperative ultrasound (44).
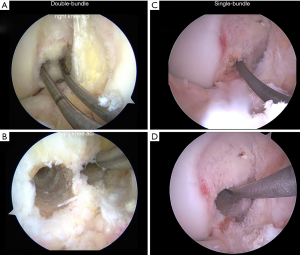
Non-anatomic tunnel placement is the most common technical reason for graft failure (15,45). The correct positions for anatomic tunnel placement are identified during reconstruction surgery by noting the native ACL remnant tissue and the lateral intercondylar and lateral bifurcate ridges of the femur (11,46). Viewing from both the AM portal and AAM portal can facilitate accurate localization. First, the femoral tunnel is drilled through the AAM portal with the arthroscope in the AM portal. For double bundle reconstruction, the PL bundle femoral tunnel is drilled first followed by the AM bundle tunnel in the respective insertion sites (Figure 5A,B). For single bundle reconstruction, the femoral tunnel is drilled at the midpoint of the two bundles (Figure 5C,D). The tibial tunnel drilling is performed viewing from the AL portal. The AM bundle tunnel is drilled with the tibial guide at 55 degrees and the PL bundle at 45 degrees for double bundle reconstruction. The tunnels should be at least 2 cm apart on the anterior tibial cortex to maintain a bony bridge. In single bundle reconstruction, the tibial tunnel is drilled at the midpoint of the two bundles with the tibial guide set at 55 degrees.
The ideal graft fixation technique has not been proven to date. Typically, soft tissue grafts are fixed with suspensory devices on the femoral side. Metal or bioabsorbable interference screws are also an option, and no difference has been found in clinical outcomes between suspensory and interference fixation (47). Grafts with bone blocks are fixed with interference screws on the femoral side. Tibial side fixation of both soft tissue and bone plug grafts is performed with interference screws because of the ease of insertion and minimal graft slippage. There is no definitive consensus on preconditioning, tensioning, or knee flexion angle during fixation of the graft (48). In general, for single bundle reconstruction, the graft is fixed at 0–20 degrees of flexion with near maximal manual tension. Fixation in greater flexion may increase the risk of loss of extension (49).
Future directions
Unfortunately, to date there is a dearth of long-term studies comparing anatomic and non-anatomic ACL reconstruction, with most studies demonstrating only short-term differences in graft rupture rates (50,51). Similarly, while biomechanical studies have shown better restoration of native joint kinematics with an anatomic approach, there is a paucity of literature with regard to differences in the long-term development of degenerative changes for the two techniques. Interestingly, one study comparing 10-year outcomes between single and double bundle reconstructions did find a significantly lower rate of graft failure with a double bundle technique (26). However, the reasons for such findings remain unclear with further investigation of long-term outcomes needed to make a concrete assessment of functional outcomes.
Additionally, another recent practice that has gained favor is the technique of remnant preservation during reconstruction. This technique offers several theoretical advantages. Firstly, the preserved tissue may aid in graft placement, acting as a marker for anatomic tunnel creation during reconstruction. Secondly, incorporation of native tissue into the implanted graft may protect the graft, allowing for earlier progression of rehabilitation (52). Finally, remnant preservation may increase the adjacent vascularity by preserving the ACL’s vascularized synovial envelope (53), possibly improving graft healing and incorporation. However, recent studies have failed to demonstrate a clear clinical benefit with remnant preservation techniques (54,55), with any potential benefit to date remaining merely theoretical.
Of course, no discussion of the future of anatomic ACL reconstruction would be complete without mentioning graft augmentation. In one recent study, standard hamstring autograft reconstruction was compared to cruciate repair augmented with a synthetic scaffolding material. While the augmented repairs did perform slightly worse in terms of Lachman exam, initial results were promising, with no evidence of adverse reaction or failure in any augmented-repair patients (56). Additionally, while primary allograft reconstruction has been demonstrated to result in higher re-rupture rates (42), the idea of augmenting an individual’s native tissue with allograft offers a potential solution for situations when the desired graft size is unachievable with autograft alone. However, while several smaller studies have suggested poor results following allograft augmentation, a recent meta-analysis was unable to conclusively demonstrate either a positive or negative effect on outcomes (57).
Conclusions
Though fraught with complexities, the goal of performing a truly anatomic reconstruction of the injured ACL has advanced significantly over the years. It is our belief that as our understanding of knee anatomy and its resulting effects on kinematics continues to improve, it is incumbent upon us to continue incorporating such knowledge into our reconstructive techniques. Ultimately the goal of any surgery, ACL reconstruction or otherwise, should be to restore an individual’s anatomy as best as possible, as we continue to strive for the best possible outcomes in treating our patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takeshi Muneta) for the series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.12.11). The series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” was commissioned by the editorial office without any funding or sponsorship. FHF serves as an Editor-in-Chief of Annals of Joint from Mar 2016 to Aug 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Eck C, Working Z, Fu F. Current Concepts in Anatomic Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction. Phys Sportsmed 2011;39:140-8. [Crossref] [PubMed]
- Fu FH, Nagai K. Editorial Commentary: The Anterior Cruciate Ligament Is a Dynamic Structure. Arthroscopy 2018;34:2476-7. [Crossref] [PubMed]
- Ferretti M, Levicoff EA, Macpherson TA, et al. The Fetal Anterior Cruciate Ligament: An Anatomic and Histologic Study. Arthroscopy 2007;23:278-83. [Crossref] [PubMed]
- Freeman MA, Wyke B. The innervation of the knee joint. An anatomical and histological study in the cat. J Anat 1967;101:505-32. [PubMed]
- MacDonald PB, Hedden D, Pacin O, et al. Proprioception in Anterior Cruciate Ligament-Deficient and Reconstructed Knees. Am J Sports Med 1996;24:774-8. [Crossref] [PubMed]
- Johansson H, Sjölander P, Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res 1991;161-78. [PubMed]
- Norman D, Metcalfe AJ, Barlow T, et al. Cortical Bony Thickening of the Lateral Intercondylar Wall: The Functional Attachment of the Anterior Cruciate Ligament. Am J Sports Med 2017;45:394-402. [Crossref] [PubMed]
- Sasaki N, Ishibashi Y, Tsuda E, et al. The Femoral Insertion of the Anterior Cruciate Ligament: Discrepancy Between Macroscopic and Histological Observations. Arthroscopy 2012;28:1135-46. [Crossref] [PubMed]
- Moulton SG, Steineman BD, Haut Donahue TL, et al. Direct versus indirect ACL femoral attachment fibres and their implications on ACL graft placement. Knee Surg Sports Traumatol Arthrosc 2017;25:165-71. [Crossref] [PubMed]
- Fu FH, Jordan SS. The lateral intercondylar ridge--a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am 2007;89:2103-4. [PubMed]
- Ferretti M, Doca D, Ingham SM, et al. Bony and soft tissue landmarks of the ACL tibial insertion site: an anatomical study. Knee Surg Sports Traumatol Arthrosc 2012;20:62-8. [Crossref] [PubMed]
- Lipke JM, Janecki CJ, Nelson CL, et al. The role of incompetence of the anterior cruciate and lateral ligaments in anterolateral and anteromedial instability. A biomechanical study of cadaver knees. J Bone Joint Surg Am 1981;63:954-60. [Crossref] [PubMed]
- Amis AA, Dawkins GP. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br 1991;73:260-7. [Crossref] [PubMed]
- Zantop T, Herbort M, Raschke MJ, et al. The Role of the Anteromedial and Posterolateral Bundles of the Anterior Cruciate Ligament in Anterior Tibial Translation and Internal Rotation. Am J Sports Med 2007;35:223-7. [Crossref] [PubMed]
- Morgan JA, Dahm D, Levy B, et al. Femoral Tunnel Malposition in ACL Revision Reconstruction. J Knee Surg 2012;25:361-8. [Crossref] [PubMed]
- Chen JL, Allen CR, Stephens TE, et al. Differences in mechanisms of failure, intraoperative findings, and surgical characteristics between single- and multiple-revision ACL reconstructions: a MARS cohort study. Am J Sports Med 2013;41:1571-8. [Crossref] [PubMed]
- Iriuchishima T, Tajima G, Ingham SJM, et al. PCL to graft impingement pressure after anatomical or non-anatomical single-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20:964-9. [Crossref] [PubMed]
- Sheean AJ, Musahl V. Editorial Commentary: Does “No Difference” Really Mean “No Difference”? Not All Anterior Cruciate Ligament Transtibial Drilling Techniques Are Created Equal. Arthroscopy 2018;34:2871-3. [Crossref] [PubMed]
- Kato Y, Ingham SJM, Kramer S, et al. Effect of tunnel position for anatomic single-bundle ACL reconstruction on knee biomechanics in a porcine model. Knee Surg Sports Traumatol Arthrosc 2010;18:2-10. [Crossref] [PubMed]
- Musahl V, Kopf S, Rabuck S, et al. Rotatory knee laxity tests and the pivot shift as tools for ACL treatment algorithm. Knee Surg Sports Traumatol Arthrosc 2012;20:793-800. [Crossref] [PubMed]
- Kopf S, Forsythe B, Wong AK, et al. Transtibial ACL reconstruction technique fails to position drill tunnels anatomically in vivo 3D CT study. Knee Surg Sports Traumatol Arthrosc 2012;20:2200-7. [Crossref] [PubMed]
- Chen H, Chen B, Tie K, et al. Single-bundle versus double-bundle autologous anterior cruciate ligament reconstruction: a meta-analysis of randomized controlled trials at 5-year minimum follow-up. J Orthop Surg Res 2018;13:50. [Crossref] [PubMed]
- Araujo PH, Asai S, Pinto M, et al. ACL Graft Position Affects in Situ Graft Force Following ACL Reconstruction. J Bone Joint Surg Am 2015;97:1767-73. [Crossref] [PubMed]
- Desai N, Alentorn-Geli E, van Eck CF, et al. A systematic review of single- versus double-bundle ACL reconstruction using the anatomic anterior cruciate ligament reconstruction scoring checklist. Knee Surg Sports Traumatol Arthrosc 2016;24:862-72. [Crossref] [PubMed]
- Desai N, Björnsson H, Musahl V, et al. Anatomic single- versus double-bundle ACL reconstruction: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 2014;22:1009-23. [Crossref] [PubMed]
- Järvelä S, Kiekara T, Suomalainen P, et al. Double-Bundle Versus Single-Bundle Anterior Cruciate Ligament Reconstruction: A Prospective Randomized Study With 10-Year Results. Am J Sports Med 2017;45:2578-85. [Crossref] [PubMed]
- van Eck CF, Lesniak BP, Schreiber VM, et al. Anatomic Single- and Double-Bundle Anterior Cruciate Ligament Reconstruction Flowchart. Arthroscopy 2010;26:258-68. [Crossref] [PubMed]
- Shen W, Forsythe B, Ingham SM, et al. Application of the anatomic double-bundle reconstruction concept to revision and augmentation anterior cruciate ligament surgeries. J Bone Joint Surg Am 2008;90:20-34. [Crossref] [PubMed]
- Hussein M, van Eck CF, Cretnik A, et al. Individualized Anterior Cruciate Ligament Surgery. Am J Sports Med 2012;40:1781-8. [Crossref] [PubMed]
- Maeda A, Shino K, Horibe S, et al. Anterior Cruciate Ligament Reconstruction with Multistranded Autogenous Semitendinosus Tendon. Am J Sports Med 1996;24:504-9. [Crossref] [PubMed]
- Bickel BA, Fowler TT, Mowbray JG, et al. Preoperative magnetic resonance imaging cross-sectional area for the measurement of hamstring autograft diameter for reconstruction of the adolescent anterior cruciate ligament. Arthroscopy 2008;24:1336-41. [Crossref] [PubMed]
- Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy 2013;29:1948-53. [Crossref] [PubMed]
- Magnussen RA, Lawrence JTR, West RL, et al. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy 2012;28:526-31. [Crossref] [PubMed]
- Rahnemai-Azar AA, Sabzevari S, Irarrázaval S, et al. Restoring Nature Through Individualized Anatomic Anterior Cruciate Ligament Reconstruction Surgery. Arch bone Jt Surg 2016;4:289-90. [PubMed]
- Offerhaus C, Albers M, Nagai K, et al. Individualized Anterior Cruciate Ligament Graft Matching: In Vivo Comparison of Cross-sectional Areas of Hamstring, Patellar, and Quadriceps Tendon Grafts and ACL Insertion Area. Am J Sports Med 2018;46:2646-52. [Crossref] [PubMed]
- Kopf S, Pombo MW, Shen W, et al. The ability of 3 different approaches to restore the anatomic anteromedial bundle femoral insertion site during anatomic anterior cruciate ligament reconstruction. Arthroscopy 2011;27:200-6. [Crossref] [PubMed]
- Fujimaki Y, Thorhauer E, Sasaki Y, et al. Quantitative In Situ Analysis of the Anterior Cruciate Ligament. Am J Sports Med 2016;44:118-25. [Crossref] [PubMed]
- Hamada M, Shino K, Horibe S, et al. Changes in Cross-Sectional Area of Hamstring Anterior Cruciate Ligament Grafts as a Function of Time Following Transplantation. Arthroscopy 2005;21:917-22. [Crossref] [PubMed]
- Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med 1993;21:176-85. [Crossref] [PubMed]
- Tomita F, Yasuda K, Mikami S, et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy 2001;17:461-76. [Crossref] [PubMed]
- Shimizu K, Yoshiya S, Kurosaka M, et al. Change in the cross-sectional area of a patellar tendon graft after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 2007;15:515-21. [Crossref] [PubMed]
- Kaeding CC, Aros B, Pedroza A, et al. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction. Sports Health 2011;3:73-81. [Crossref] [PubMed]
- Araujo P, van Eck CF, Torabi M, et al. How to optimize the use of MRI in anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2013;21:1495-501. [Crossref] [PubMed]
- Takenaga T, Yoshida M, Albers M, et al. Preoperative sonographic measurement can accurately predict quadrupled hamstring tendon graft diameter for ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Sommer C, Friederich NF, Müller W. Improperly placed anterior cruciate ligament grafts: correlation between radiological parameters and clinical results. Knee Surg Sports Traumatol Arthrosc 2000;8:207-13. [Crossref] [PubMed]
- Ferretti M, Ekdahl M, Shen W, et al. Osseous Landmarks of the Femoral Attachment of the Anterior Cruciate Ligament: An Anatomic Study. Arthroscopy 2007;23:1218-25. [Crossref] [PubMed]
- Colvin A, Sharma C, Parides M, et al. What is the best femoral fixation of hamstring autografts in anterior cruciate ligament reconstruction?: a meta-analysis. Clin Orthop Relat Res 2011;469:1075-81. [Crossref] [PubMed]
- Jisa KA, Williams BT, Jaglowski JR, et al. Lack of consensus regarding pretensioning and preconditioning protocols for soft tissue graft reconstruction of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 2016;24:2884-91. [Crossref] [PubMed]
- Austin JC, Phornphutkul C, Wojtys EM. Loss of Knee Extension After Anterior Cruciate Ligament Reconstruction: Effects of Knee Position and Graft Tensioning. J Bone Joint Surg Am 2007;89:1565. [PubMed]
- Suomalainen P, Moisala A-S, Paakkala A, et al. Double-Bundle versus Single-Bundle Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2011;39:1615-22. [Crossref] [PubMed]
- Järvelä T, Moisala AS, Sihvonen R, et al. Double-Bundle Anterior Cruciate Ligament Reconstruction Using Hamstring Autografts and Bioabsorbable Interference Screw Fixation. Am J Sports Med 2008;36:290-7. [Crossref] [PubMed]
- Crain EH, Fithian DC, Paxton EW, et al. Variation in anterior cruciate ligament scar pattern: does the scar pattern affect anterior laxity in anterior cruciate ligament-deficient knees? Arthroscopy 2005;21:19-24. [Crossref] [PubMed]
- Dodds JA, Arnoczky SP. Anatomy of the anterior cruciate ligament: a blueprint for repair and reconstruction. Arthroscopy 1994;10:132-9. [Crossref] [PubMed]
- Tie K, Chen L, Hu D, et al. The difference in clinical outcome of single-bundle anterior cruciate ligament reconstructions with and without remnant preservation: A meta-analysis. Knee 2016;23:566-74. [Crossref] [PubMed]
- Naraoka T, Kimura Y, Tsuda E, et al. Is Remnant Preservation Truly Beneficial to Anterior Cruciate Ligament Reconstruction Healing? Clinical and Magnetic Resonance Imaging Evaluations of Remnant-Preserved Reconstruction. Am J Sports Med 2017;45:1049-58. [Crossref] [PubMed]
- Murray MM, Flutie BM, Kalish LA, et al. The Bridge-Enhanced Anterior Cruciate Ligament Repair (BEAR) Procedure. Orthop J Sports Med 2016;4:2325967116672176 [Crossref] [PubMed]
- Abouljoud MM, Everhart JS, Sigman BO, et al. Risk of Retear Following Anterior Cruciate Ligament Reconstruction Using a Hybrid Graft of Autograft Augmented With Allograft Tissue: A Systematic Review and Meta-analysis. Arthroscopy 2018;34:2927-35. [Crossref] [PubMed]
Cite this article as: Golan EJ, Meredith SJ, Nakamura T, Rothrauff BB, Fu FH. Anatomic reconstruction of anterior cruciate ligament: concept, indication and its efficacy. Ann Joint 2019;4:9.


