Reverse for fracture: indications, techniques, and outcomes
Introduction
Fractures of the proximal humerus account for nearly 5% of all fracture types, and are increasing in frequency with our aging population (1). A majority of these fractures are nonsurgical, however, elderly patients are more prone to have more complex fracture patterns, more comminution and poor bone quality compared to their younger counterparts. Surgical options include open reduction internal fixation (ORIF) and hemiarthroplasty (HHR), and recently, reverse shoulder arthroplasty (RSA). Historically, the results of ORIF and HHR have been disappointing, unpredictable and fraught with complications.
ORIF of complex proximal humerus fractures in the elderly population is challenging. Risk factors for failure in surgical cases include advanced age, comminution, osteoporosis and female sex. Unfortunately, a majority of the patients that sustain this injury have those characteristics. Despite the introduction of locking plate technology for osteosynthesis over the past 15 years, multiple studies have shown a high failure rate to include, osteonecrosis, screw cutout/penetrance and loss of fixation (2-4). Multiple complications have also been noted in those treated with ORIF.
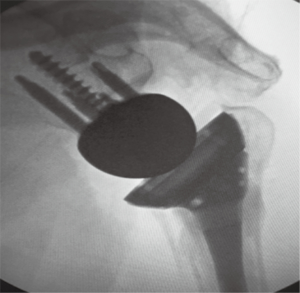
Neer introduced HHR in patients not amenable to ORIF as a means to achieve pain relief and improved function (5). Pain relief was found to be good, however, many studies showed unpredictable results in regards to function and range of motion (6). HHR outcomes are bimodal and are reliant upon the healing of the tuberosities (6-8). Multiple variables also include prosthetic height and version, tuberosity reduction and underlying patient biology. Due to these multiple variables and uncertainty of outcomes after HHR for fracture, the RSA has been advocated as an additional treatment modality. Recent studies have shown that RSA has become the treatment of choice for displaced complex proximal humerus fractures in the elderly (9). It has been felt that more reliable, consistent and predictable results are often achieved with the use of RSA in the setting of complex proximal humerus fractures in the elderly (10). Tuberosity healing is not a requirement for good clinical outcomes with RSA, but healing of the tuberosities does lead to superior clinical outcomes (11). The purpose of this article is to review the indications of RSA for fracture, the technical aspects of the procedure, and the outcomes of surgically treated fractures with this prosthesis up to this date (Figure 1).
Indications for RSA for fracture
RSA was originally described for use in the cuff deficient shoulder with pseudoparalysis and in the oncologic patient with proximal humerus tumors. Indications have substantially expanded since its reintroduction to the United States in 2004. In regards to fractures, RSA can be indicated for acute complex three and four part proximal humerus fractures in patients physiologically above the age of 65–70, fracture sequelae, and revision from failed ORIF and HHR. Relative indications include fracture with associate characteristics to include poor tuberosity bone with osteoporosis and comminution, pre-existing rotator cuff tear and/or arthritis (12). Absolute contraindications include permanent axillary nerve palsy, global brachial plexopathy and deltoid dysfunction. Relative contraindications include partial deltoid dysfunction, acromial or scapular spine fracture that may displace or affect deltoid tensioning and implant stability, associated glenoid fractures that compromise baseplate stability, significant medical comorbidities or inability to comply with postoperative restrictions. Extreme caution should be exercised in open fractures due to infection concerns (12).
RSA has been used successfully in the scenario of complex proximal humerus fractures in the elderly because it is less dependent upon a functioning rotator cuff or tuberosity healing to provide reliable pain relief, forward elevation and improved function (10). Implant longevity remains a concern; few salvage options are available in the face of RSA failure. Long-term outcomes are not well defined and are evolving. Shoulder surgeons currently restrict the use of RSA in the carefully selected elderly patient with complex proximal humerus fractures for these above reasons.
History and physical exam
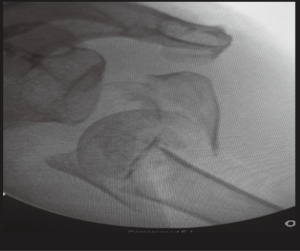
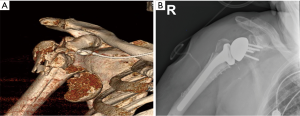
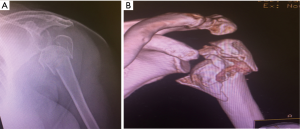
Initial evaluation should include a detailed history and physical, general health assessment, previous shoulder surgeries, independence, cognition and functional demands (12). Images include standard 3 view radiographs and CT scanning with 3D reconstructions. CT scanning can assist in evaluating additional fractures involving the glenoid, scapula, acromion and the degree of osteoporosis (13) (Figure 2).
Neurological exam is important to asses for axillary nerve and brachial plexus function. If axillary nerve function appears to be compromised preoperatively, electromyography should be obtained because an intact deltoid and axillary nerve are necessary for performing RSA in the fracture setting.
Authors’ preferred surgical technique
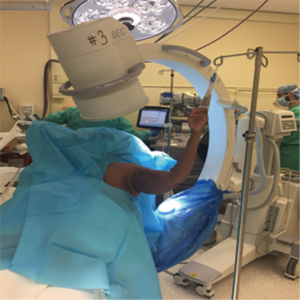
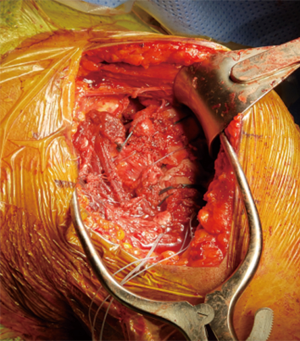
Patients are positioned in the beach chair position on a standard operative bed; the patient is intubated with general anesthesia without regional block. The arm and proximal shoulder girdle is prepped and draped in a sterile fashion with the arm draped free to allow for full adduction and external rotation. Fluoroscopy is brought over the ipsilateral shoulder at the head of the table; the monitor is across the bed (Figure 3). This allows for appropriate images for the procedure (Figure 4). Appropriate lead and hoods are utilized for protection during the case. The arm is placed on a padded sterile mayo stand (Figure 5). The deltopectoral approach is preferred and the cephalic vein is taken laterally. The superior 1–2 cm of the pectoralis major insertion is released from the humerus; the subacromial and subdeltoid space are then bluntly developed and all bursa can be removed. The clavipectoral fascia is opened along the lateral muscular border of the conjoint tendon and a finger is swept underneath to palpate the axillary nerve. A deep retractor is then placed. The biceps tendon is found underneath the pectoralis major tendon and released through the rotator interval to the supraglenoid tubercle with a Mayo scissors; the biceps (if present) is then tenotomized at the level of the interval proximally. The rotator interval can guide us to our tuberosities. A portion of the supraspinatus is released off of the greater tuberosity and both the greater and lesser tuberosity are released and mobilized with a Cobb elevator and scissors. The humeral head is then identified and removed, bone is saved for later cancellous autograft. The greater tuberosity is tagged as per Boileau (14) to obtain control for tuberosity reconstruction; two no. 5 Ethibond sutures and two no. 2 Fiberwire sutures are placed at the tendon bone junction around the tuberosity in alternating fashion (Figure 6). A tagging suture of no.1 Vicryl is placed around the subscapularis. A portion of the latissimus is released off the humeral calcar to allow for better shaft exposure. The deep retractors are removed and the arm is abducted onto a mayo stand and then our attention is directed towards glenoid exposure.
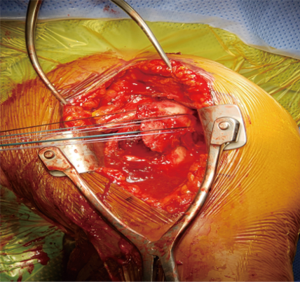
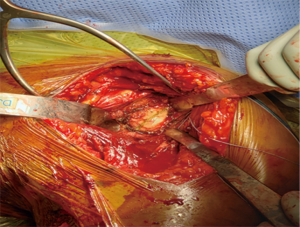
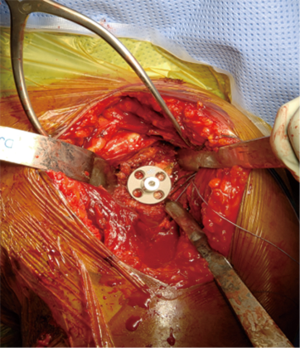
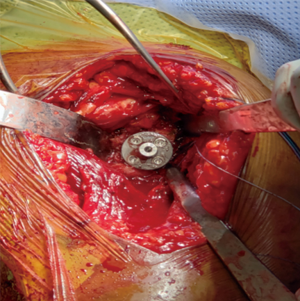
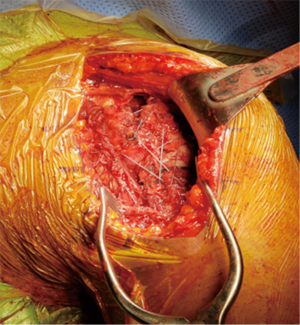
A retractor is placed posteroinferiorly along the glenoid, retracting the shaft and a Hohmann retractor is placed posterosuperiorly. The capsule and subscapularis/lesser tuberosity is then properly mobilized with scissors and the capsule is identified medially at the muscle junction and developed with a scissors. The axillary nerve is palpated in this interval and protected. The anterior and inferior capsule is released from the subscapularis and an anterior glenoid retractor is placed protecting the axillary nerve and neurovascular structures. The biceps is excised, a circumferential labral excision, anterior capsulectomy and inferior release are performed with bovie electrocautery taking care to stay on the glenoid rim and expose the glenoid neck (15). In cases of fracture sequelae, a posterior release is also generously performed. The anterior glenoid retractor is then repositioned. Glenoid exposure is more easily achieved in the fracture setting than with a standard RSA because of the soft tissue and capsular injury from the recent trauma and tuberosity fracture (Figure 7). The baseplate should be inserted based upon the technique recommended for the selected device (Figure 8). The author has utilized a variety of glenosphere designs including Grammont style and lateral center of rotation spheres. We attempt for at least 3 screws, using the “3-column concept” (16). Currently, the superior screw is kept short (18–22 mm) to avoid postoperative scapular spine fracture. The most inferior screw is drilled centrally and not along the scapular pillar (17). Four screws are typically used (Figure 9). Glenosphere size is based upon patient gender, size and anatomy. Females typically receive a smaller size; larger females and males typically receive a larger size. The selected glenosphere is then inserted on the baseplate (Figure 10). Retractors are then removed and attention is then to preparation of the humerus.
Humeral preparation is performed with the arm adducted and extended, a Deltoid retractor is utilized to expose the humerus; anterior glenoid retractors are maintained. The humeral shaft is serially reamed until cortical chatter is detected. Two drill holes are then placed, one lateral to the bicipital groove and 2 cm distal to the fracture and one anterior to the groove in the same fashion. Two no.2 Fiberwire sutures are placed in Criss cross fashion through these holes and tagged with a hemostat for later tuberosity reconstruction. The trial implant is placed in 20–30 degrees of humeral retroversion based upon the patients forearm. The bicipital groove has been noted to be an unreliable landmark for stem positioning (18). If there is no calcar comminution, the medial portion of the stem typically sits on the calcar for proper height. A jig may also be utilized for height and rotational maintenance during the procedure. Proper rotation and height of the humeral implant aids in tensioning and tuberosity fixation and healing. The humeral stem and liners are then trialed with attention to ease of reduction, tension and stability of the prosthesis, soft tissue tension and ability to repair the tuberosities. Fluoroscopy is brought in at this point to assess stability and tuberosity placement. Tensioning is at the discretion of the surgeon; the appropriate tension is unknown. In cases of uncomplicated fractures, the smallest polyethylene liner can be chosen. Over lengthening should be avoided; restoration of humeral length is important in minimizing postoperative instability (19). Soft tissue tension is adequate when dislocation is difficult to achieve with axial and lateral forces.
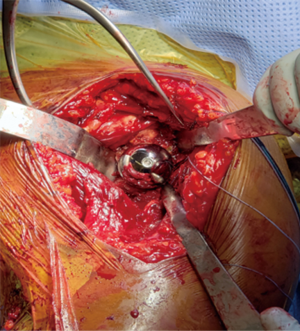
Final humeral component implantation is then performed. Humeral stems utilized in RSA for fracture include cemented standard stems, cemented fracture specific stems and uncemented stems. A fracture specific cemented stem has been universally favored and utilized; the author has no experience with uncemented humeral stems in this scenario. A cement restrictor is placed in the humerus and the stem is cemented in 20–30 degrees of retroversion based upon the forearm and proper height. A jig may be utilized. The four sutures from the greater tuberosity are placed through the medial fin of the prosthesis prior to final reduction and cement hardening (20). The final polyethylene liner is then impacted without issue and the RSA is then reduced and stability checked. Techniques that are utilized include the ability to internally rotate the arm, amount of forward flexion, shuck testing and anterior drawer testing with the arm extended, adducted and externally rotated. Conjoint tendon tension can also be checked. The arm is then placed on a Mayo stand and tuberosity reconstruction is undertaken. The greater tuberosity is repaired to the stem with the two no. 5 Ethibond horizontal sutures and bone grafted with cancellous humeral head autograft and checked under fluoroscopic guidance to allow for proper positioning (Figure 11). The two no. 2 Fiberwire sutures are then placed horizontally around the lesser tuberosity and tied down compressing the fragment to the prosthesis (Figure 12). Pointed reduction forceps can be utilized if needed. Repair of the lesser tuberosity is surgeon dependent and prosthesis dependent. The humeral shaft sutures are then placed in a cuff stich, figure of eight fashion through the tuberosities and cuff to give vertical fixation of the construction (Figure 13). This allows additional rotation stability to the prevent tuberosity fixation failure. The axillary nerve is then palpated, the biceps are tenodesis to the pectoralis tendon stump and the wound is closed in standard fashion over a suction drain to prevent hematoma formation. The patient is placed in an abduction sling and the drain is removed postoperative day number 1.
Postoperative rehabilitation
There has been no universal protocol in the literature that has validated superiority for one postop protocol compared to others. The current protocol utilized after RSA for complex proximal humerus fractures includes a sling with an abduction pillow in neutral rotation for up to 6 weeks; the patient is allowed elbow/wrist and hand motion immediately. The patient is kept non-weight bearing on the affected extremity for 6 weeks; no heavy push/pull is allowed for up to 12 weeks. At 6 weeks the sling is removed and full active and passive range of motion in all planes is allowed and encouraged. Gentle deltoid reeducation is started at this point. A home-based program is taught and encouraged; limited formal physical therapy is necessary. The patient is allowed to use the shoulder as tolerated. At 12 weeks resistance is allowed with unrestricted overhead activities and use of the arm is permitted. Gradual pain relief and functional improvement can be expected over a period of 1 year.
Outcomes
Clinical outcomes have been invariably good for RSA in the setting of proximal humerus fractures. Gallinet and associates (21) and Sirveaux and coworkers (22) compared HHR and RSA for proximal humerus fractures demonstrated a more reliable restoration of overhead function in the RSA group as opposed to HHR. Garrigues et al. (23) noted an improvement in ASES scores for RSA for fracture on average of 81.1 (range of 75–88) compared to 47.4 (range, 30–81) for HHR. Ross et al. (24) had 21 patients who underwent RSA for fracture followed at a mean of 54 months. Similar improvements in Constant scores (mean, 70.9) and ASES scores (mean, 89.3) that were noted in the literature. These however were all retrospective or comparative studies. In regards to range of motion, studies have shown forward elevation improvement up to 145 degrees and external rotation to 27 degrees (11,21,22,25-27). Chalmers et al. (28) in a retrospective cohort study showed that RSA for fracture achieved better forward elevation at 1 year compared to ORIF and HHR. Recently, the ReShAPE trial (29), a multicenter randomized and observational study has been initiated to assess the effectiveness of RSA for complex proximal humerus fractures in hopes to guide treatment. Some studies have shown that RSA for proximal humerus fractures has allowed for decreased pain medication requirements, earlier independence and equivalent to improved costs compared to other treatment options (30).
Despite the initial enthusiasm for RSA in proximal humerus fractures, long-term follow-up studies in regards to longevity is lacking. Midterm results utilizing this prosthesis have shown a high incidence of scapular notching, neurologic complications and component loosening.
Complications
Complication rates of RSA for fracture can be up to 40% in the literature (31). These complications include scapular notching, instability, neurologic injury, component loosening, acromial/scapular fractures, infection, hematoma and complex regional pain syndromes. Management of these complications can be challenging; instability is the most common currently reported complication at 4.7%, and revision rates in even the most skilled of hands can be up to 15% (32). Technical aspects of the procedure do require a steep learning curve (33); increasing familiarity with RSA, proper indications and further research and improved implant designs hope to lead to a decrease in complications.
Summary
RSA for certain complex proximal humerus fractures in the elderly has become a successful option compared to previous surgical treatments. Short to mid-term results have been promising; good results can be achieved without tuberosity healing and more predictable results and pain relief can be obtained. RSA for proximal humerus fractures has already outpaced HHR in patients above 65 in the USA who require surgical treatment of these injuries (34). Appropriate surgical experience, careful preoperative planning and attention to technical details can lead to satisfactory results. Prospective studies and long-term follow-up are necessary prior to advocating the widespread use of RSA for proximal humerus fractures in the elderly.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Joseph A. Abboud) for the series “Evolving Trends in Reverse Shoulder Arthroplasty” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.02.05). The series “Evolving Trends in Reverse Shoulder Arthroplasty” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72:365-71. [Crossref] [PubMed]
- Jost B, Spross C, Grehn H, et al. Locking plate fixation of fractures of the proximal humerus: analysis of complications, revision strategies and outcome. J Shoulder Elbow Surg 2013;22:542-9. [Crossref] [PubMed]
- Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures J Bone Joint Surg Am 2008;90:233-40. [corrected]. [Crossref] [PubMed]
- Ricchetti ET, Warrender WJ, Abboud JA. Use of locking plates in the treatment of proximal humerus fractures. J Shoulder Elbow Surg 2010;19:66-75. [Crossref] [PubMed]
- Neer CS. Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am 1970;52:1090-103. [Crossref] [PubMed]
- Antuña SA, Sperling JW, Cofield RH. Shoulder hemiarthroplasty for acute fractures of the proximal humerus: a minimum five-year follow-up. J Shoulder Elbow Surg 2008;17:202-9. [Crossref] [PubMed]
- Robinson CM, Page RS, Hill RM, et al. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am 2003;85-A:1215-23. [Crossref] [PubMed]
- Sirveaux F, Navez G, Favard L, et al. The Multi-centere Study. Available online: https://www.researchgate.net/publication/283405176_The_multi-centere_study
- Savin DD, Zamfirova I, Iannotti J, et al. Survey study suggests that reverse total shoulder arthroplasty is becoming the treatment of choice for four-part fractures of the humeral head in the elderly. Int Orthop 2016;40:1919-25. [Crossref] [PubMed]
- Bufquin T, Hersan A, Hubert L, et al. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br 2007;89:516-20. [Crossref] [PubMed]
- Gallinet D, Adam A, Gasse N, et al. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:38-44. [Crossref] [PubMed]
- Jobin CM, Galdi B, Anakwenze OA, et al. Reverse Shoulder Arthroplasty for the Management of Proximal Humerus Fractures. J Am Acad Orthop Surg 2015;23:190-201. [Crossref] [PubMed]
- Schneider R. Imaging of osteoporosis. Rheum Dis Clin North Am 2013;39:609-31. [Crossref] [PubMed]
- Boileau P, Walch G, Krishnan S. Tuberosity osteosynthesis and hemiarthroplasty for four-part fractures of the proximal humerus. Tech Shoulder Elbow Surg 2000;1:96-109. [Crossref]
- Hatzidakis AM, Norris TR, Boileau P. Reverse shoulder arthroplasty: indications, techniques, and results. Tech Shoulder Elbow Surg 2005;6:135-9. [Crossref]
- Humphrey CS, Kelly JD, Norris TR. Optimizing glenosphere position and fixation in reverse shoulder arthroplasty, part two: the three-column concept. J Shoulder Elbow Surg 2008;17:595-601. [Crossref] [PubMed]
- Parsons BO, Gruson KI, Accousti KJ, et al. Optimal rotation and screw positioning for initial glenosphere baseplate fixation in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:886-91. [Crossref] [PubMed]
- Balg F, Boulianne M, Boileau P. Bicipital groove orientation: considerations for the retroversion of a prosthesis in fractures of the proximal humerus. J Shoulder Elbow Surg 2006;15:195-8. [Crossref] [PubMed]
- Lädermann A, Walch G, Lubbeke A, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:336-41. [Crossref] [PubMed]
- Frankle MA, Ondrovic LE, Markee BA, et al. Stability of tuberosity reattachment in proximal humeral hemiarthroplasty. J Shoulder Elbow Surg 2002;11:413-20. [Crossref] [PubMed]
- Gallinet D, Clappaz P, Garbuio P, et al. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res 2009;95:48-55. [Crossref] [PubMed]
- Sirveaux F, Roche O, Molé D. Shoulder arthroplasty for acute proximal humerus fracture. Orthop Traumatol Surg Res 2010;96:683-94. [Crossref] [PubMed]
- Garrigues GE, Johnston PS, Pepe MD, et al. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics 2012;35:e703-8. [Crossref] [PubMed]
- Ross M, Hope B, Stokes A, et al. Reverse Shoulder Arthroplasty for the treatment of three-part and four-part proximal humeral fractures in the elderly. J Shoulder Elbow Surg 2015;24:215-22. [Crossref] [PubMed]
- Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br 2010;92:535-9. [Crossref] [PubMed]
- Klein M, Juschka M, Hinkenjann B, et al. Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma 2008;22:698-704. [Crossref] [PubMed]
- Lenarz C, Shishani Y, McCrum C, et al. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient? Early observations. Clin Orthop Relat Res 2011;469:3324-31. [Crossref] [PubMed]
- Chalmers PN, Slikker W III, Mall NA, et al. Reverse total shoulder arthroplasty for acute proximal humeral fracture: comparison to open reduction-internal fixation and hemiarthroplasty. J Shoulder Elbow Surg 2014;23:197-204. [Crossref] [PubMed]
- Smith GC, Bateman E, Cass B, et al. Reverse Shoulder Arthroplasty for the treatment of Proximal humeral fractures in the Elderly (ReShAPE trial): study protocol for a multicenter combined randomised controlled and observational trial. Trials 2017;18:91. [Crossref] [PubMed]
- Wolfensperger F, Grüninger P, Dietrich M, et al. Reverse shoulder arthroplasty for complex fractures of the proximal humerus in elderly patients: impact on the level of independency, early function, and pain medication. J Shoulder Elbow Surg 2017;26:1462-8. [Crossref] [PubMed]
- Zumstein MA, Pinedo M, Old J, et al. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2011;20:146-57. [Crossref] [PubMed]
- Cheung E, Willis M, Walker M, et al. Complications of reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011;19:439-49. [Crossref] [PubMed]
- Kempton LB, Ankerson E, Wiater JM. A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res 2011;469:2496-504. [Crossref] [PubMed]
- Rajaee SS, Yalamanchili D, Noori N, et al. Increasing Use of Reverse Total Shoulder Arthroplasty for Proximal Humerus Fractures in Elderly Patients. Orthopedics 2017;40:e982-9. [Crossref] [PubMed]
Cite this article as: Kazanjian JE. Reverse for fracture: indications, techniques, and outcomes. Ann Joint 2019;4:19.