
Anatomic anterior cruciate ligament reconstruction for adolescent patients with open physis
Introduction
In the past, anterior cruciate ligament (ACL) injuries in patients with open physes were mainly managed non-operatively or by suture repair, which too frequently resulted in unsuccessful outcomes (1,2). At that time, the diagnostic possibilities were inferior to current standards, and the consequences of pediatric ACL tears and the iatrogenic risk of pediatric ACL reconstruction had not deeply been evaluated yet. Many pediatric ACL injuries were diagnosed late, so that the orthopedic surgeons dealt predominantly with a negative selection of ACL-injured children, who presented with secondary meniscal tears and cartilage lesions. Today, pediatric ACL reconstruction (ACLR) is considered a safe procedure with low complication rates, provided that surgery is performed correctly (3,4). However, the outcome after ACLR in children and adolescents is poorer in comparison to adults, which suggests to carefully evaluate each patient before confirming the indication for surgery and to avoid treating all pediatric ACL injuries surgically on a systematic basis (3). Indication for surgery needs to be tailored to each patient, and should be based on clinical factors like functional instability, associated injuries, remaining knee growth and patient expectations.
Recently, international guidelines on the management of pediatric ACL injuries have been published by the International Olympic Committee (IOC), in partnership with international scientific organizations like ESSKA, AOSSM, SLARD, APKASS and ISAKOS (5,6). Non-operative treatment with a structured rehabilitation program has shown to be successful in some patients (7). However, a strong association between the delay of surgery and the occurrence of meniscus and cartilage lesions has been reported, suggesting that an uncontrolled non-operative treatment may be detrimental to the intra-articular soft tissue structures (1,2,8-10). Therefore, since the young ACL-deficient knee can evolve with patient’s growth, regular follow-up visits with clinical investigation, magnetic resonance imaging (MRI) and laxity testing are indicated (11). Pediatric ACL surgery is highly specialized, due to the specific anatomy of children’s knees and its serious complication potential (12-17). Surgical results are good, but seem to be less predictable than in adults (18-22). Furthermore, there are not enough high-quality outcome studies after surgical treatment (3). The goal of this article is to provide a concise overview on the state of the art of pediatric ACL injuries.
Epidemiology
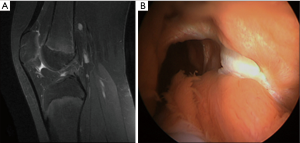
ACL tears in children and adolescents with open growth plates (Figure 1) account for less than 5% of all ACL injuries (24-26). They do rarely occur before the age of nine and three out of four are sports injuries (20). The number of pediatric ACL tears seems to be rising over the years. Between 1994 and 2013 a mean annual increase of pediatric ACL tears of 2.3% was reported in the USA (27), whereas in Victoria, Australia, the overall annual rate of hospital-treated ACL injuries increased by 147.8% between 2005 and 2015 (28). This dramatic increase is caused by the physicians’ improved clinical and diagnostic skills (27-29), the growing popularity of high-risk sports in children and adolescents (5,28,30,31), and potentially also the decreasing motor skills in this young population (32). In a non-athletic adult population, the incidence of ACL injuries is approaching the 0.1% rate (33-35). Data from populations of young athletes are rare. In a sports school including young athletes from various sports disciplines, we identified an incidence approaching the 1% rate (non-published data).
Clinical presentation
Usually, the ACL tear it is a consequence of a noncontact injury involving cutting, pivoting or rapid deceleration. Valgus twisting is the most common injury mechanism, but pure internal rotation of the tibia on the femur and hyperextension of the knee can also be involved.
In an acute setting, ACL injuries are often associated with intense pain and haemarthrosis, which can impede a precise examination. The non-injured knee should always be tested in order to assess physiologic laxity, often more important in skeletally immature patients than in adults. The assessment of the range of motion can show loss of extension in case of displaced bucket-handle meniscal tears, chondral or osteochondral fragments or arthrofibrosis. The loss of flexion can be caused by the haemarthrosis. Joint line tenderness and positive McMurray, Apley or Childress tests can suggest a meniscal tear. Localized pain on the tibial or femoral collateral ligaments attachment sites, associated with positive varus or valgus stress tests can be signs of associated ligamentous injuries. The clinical examination should also include the evaluation of the extensor mechanism, patellar tracking and stability, the posterior cruciate ligament (PCL) and the collateral ligaments. The ACL is evaluated with the Lachman test, the anterior drawer and the pivot shift test, which is not always easy to assess in pediatric patients. Since clinical symptoms and presentations may vary at different time point after injury, tests should be repeated at every follow-up visit. To confirm and document the diagnosis, the pivot shift test should be done systematically also in the operating room under general anesthesia in case of surgery. Lower extremity alignment and limb length should also be documented. In the absence of fractures, patellar dislocations and congenital meniscus lesions need to be ruled out as main differential diagnosis.
Imaging
Standard radiographs including anteropostetior (AP), strict lateral and skyline views are the first diagnostic workup in severe knee injuries in children. MRI is mandatory in case of suspected ACL tear; however, identification of this injury is more difficult in children as compared to adults, with a sensitivity of 62% and a specificity of 90% in children under the age of 12 (36,37). From 12 to 16 years, sensitivity and specificity increase to 78% respectively 96% (38). Secondary MRI features like subchondral bone bruise are less frequently identified in children, owing to the inherent increased laxity of pediatric knees.
General therapeutic considerations (strategy)
Goals of pediatric ACL tears treatment are to restore a stable, well-functioning knee, enabling an active lifestyle, to reduce the impact of existing meniscal or chondral pathology, the risk of further degenerative joint changes and the need for future surgery and minimize the risk of growth disturbances and deformity. Surgery for pediatric ACL tears is indicated in case of associated meniscus or cartilage lesions, recurrent, symptomatic giving way and unacceptable restriction in participation to sports or recreational activities.
Acute ACL surgery is rarely indicated. Associated meniscus lesions or cartilage injuries may support the need of an acute ACL reconstruction, especially in case of a dislocated bucket-handle meniscus tear or a large osteochondral flake fracture. Physicians should bear in mind that a patient referred for a dislocated meniscus bucket handle tear may have an underlying, previously undiagnosed ACL injury. ACL reconstruction may be considered in the presence of no or only minor swelling and synovitis; of capital importance are, an experienced surgical environment and fully informed young patients and parents. They need to be aware of the complication potential and the need for a close clinical follow-up until the end of the growth period. Because of their unfavorable results with respect to recurrent injuries, isolated meniscus repair without associated or subsequent ACL reconstruction cannot be recommended.
In all other cases, bracing and a home-based rehabilitation program are encouraged. This can be a short-term option to delay surgery until skeletal maturity is reached or a permanent treatment if no further disease progression is observed. For isolated ACL injuries, a structured rehabilitation program should be applied and priority should be given to regain free range of motion and a pain free, non-swollen knee. In non-operated knees, physical activity can be regained progressively over a 3 to 6 months’ period (5). Return to level 1 sports (sports with frequent pivoting and contact, e.g., soccer, handball, basketball) (39) should be considered with caution. In children with lower ambitions, changing physical activity to level 2 sports (mostly individual sports with less frequent pivoting than level I sports, e.g., racket sports, alpine skiing, snowboarding, gymnastics and aerobics) (39) is considered a safer option.
If a decision for a longer-term nonoperative treatment is chosen, we perform a systematic follow up with annual MRI’s to evaluate the meniscal status as well as the development of the PCL angle. The PCL angle is determined as the angle between the lines drawn through the central portion of the tibial and femoral insertions of the PCL. A PCL angle of <105° is considered suggestive of an ACL injury due to a chronic anterior drawer of the tibia in relation to the femur (11,36). Progression of meniscal lesions and PCL angle on MRI indicate the decompensation of an ACL-deficient knee with a chronic anterior drawer (Figures 2,3), which warrants surgical treatment. Likewise, lateral monopodial stance radiographs at 15° of flexion of the injured and contralateral knee may be used to evaluate side-to-side differences in spontaneous anterior drawer (40).
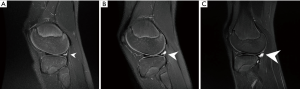
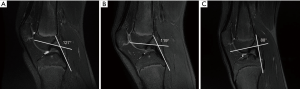
Remaining functional instability with recurrent giving way episodes despite the structured rehabilitation program is another indication for surgery. Surgeons must be aware that younger children may not recognize or describe them as such and specific questioning may therefore be required. In the surgical decision-making process, the child’s individual needs and maturation process, including remaining growth based on skeletal age determination must be considered. Likewise, ethical standards to guide shared decision making must be taken into consideration in ambitious young athletes (41).
Knee growth, maturation and preoperative planning
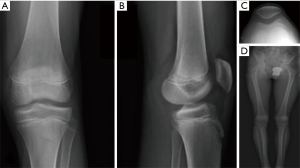
Systematic preoperative planning is mandatory in young individuals. Standard radiographs should include AP, lateral and patellofemoral views, as well as an AP view in 45° of knee flexion (Figure 4). Determination of the skeletal age and the remaining growth potential by using X-rays of the left hand and wrist and the Greulich and Pyle tables is recommended. Additional methods such as pelvic or elbow X-rays can complement the Greulich and Pyle method and can be more accurate in certain patients during puberty (42,43). Newer MRI based methods of bone age assessment will need further validation (44).
Lower limb standing radiographs should be obtained for documentation of alignment as well as to rule out preoperatively existing limb length discrepancies. If available, low-dose irradiation long leg standing radiographs (e.g., with the EOS system) should be preferred to standard techniques (45).
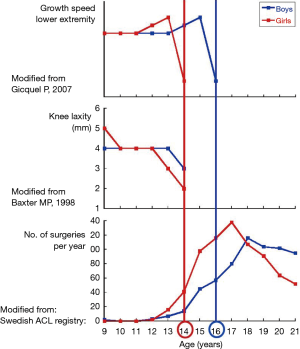
Knowledge of the remaining knee growth is of utmost importance. In this respect, the knee growth and maturation chart (Figure 5), can be a useful tool (47). It is based on the growth speed of the physeal plates at the knee and the skeletal age and allows differentiating between three different periods: (I) a first, prepubertal phase, in which the growth potential of the distal femoral and the proximal tibial physis are still high. The end of this phase occurs approximately at the age of 13 in girls and 15 in boys. At this stage, pediatric surgical techniques should be considered; (II) a second, pubertal phase, with a decreasing physeal growth potential, the duration of which approximates 1 year (13–14 years old in girls and 15–16 years old in boys). Pediatric surgical techniques are also recommended at this stage, because growth plate injuries still can cause significant growth abnormalities; (III) the final adult phase starts at 14 years old in girls and 16 years old in boys. At this moment, growth plate closure has occurred at the distal femur and the proximal tibia and adult procedures can be used.
Surgical techniques
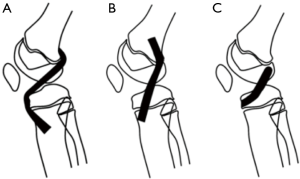
Many ACL reconstruction techniques have been described in children and adolescents. Their goal is to reproduce ACL anatomy the best possible and to reduce iatrogenic complications to a minimum. Due to the presence of the distal femoral and proximal tibial growth plates, anatomic graft placement is difficult to obtain in children with the currently available techniques (48). With respect to tibial and femoral tunnel placement, surgical techniques can be divided into three categories (Figure 6): (I) transphyseal procedures, in which the tunnels are drilled through the growth plates—this is the author’s preferred technique, even in prepubertal and pubertal patients—(II) epiphyseal techniques, in the growth plate is not injured, since the tunnels are located in the tibial and femoral epiphysis, and (III) extraepiphyseal techniques, in which the graft is placed around the growth plate. The graft can be placed in different techniques both on the tibial and the femoral side. Every surgical strategy bear potential for specific complications. To increase the safety and reproducibility of the surgical procedures and minimize the risk of affecting future normal growth, general surgical guidelines have been established (49) (Table 1).

Full table
The different graft types which are used in adults may also be used with some modifications in children. Hamstring grafts are the most popular (49,50). In some cases, their diameter can be too small to reproduce a graft diameter of 6–8 mm (51). Unfortunately, preoperative determination of the graft diameter is unreliable and therefore they may be reinforced with other tendon material (e.g., by a quadriceps strip) if this situation would occur unexpectedly during surgery (51,52). In order to prevent injuring the growth plate of the tibial tuberosity which can be the cause of a recurvatum knee in case of growth arrest, it is important not to harm the tibial periosteal attachment of the hamstrings. Unlike the adult harvesting technique, it is recommended to detach the hamstrings tendons proximal to their bony insertion site, leaving the tibial attachment site intact. Quadriceps and patellar tendon grafts remain a valid option also in pediatric ACL reconstruction: in this case, it is recommended to harvest them without a bone block. If a bone block is part of the technique (e.g., in an epiphyseal procedure), the placement of the block through the growth plate must be absolutely avoided, since it can cause early growth plate fusion. The iliotibial band is a further option as a graft material, especially if an extraepiphyseal, extraarticular technique is performed (53). Care should be taken to inform the patient on potential cosmetic (large incision) and harvesting site problems (pain). A general consensus in the IOC recommendations has been expressed against the use of cadaver allografts in immature children, which should be avoided due to poor clinical outcomes (5). Living-donor hamstring tendon allografts may have certain advantages over the cadaver allografts but they raise ethical questions and long-term outcomes still need to be assessed (54,55). The use of synthetic graft material is prohibited in pediatric ACL reconstruction, since it may cause significant growth arrest.
Some authors differentiate their specific pediatric ACL reconstruction technique according to the amount of knee growth remaining. They recommended extraphyseal reconstruction techniques for very young patients, transphyseal reconstruction for older patients, and partial transphyseal procedures in between (51,56,57). The background for this strategy is based on the theoretical age-related risk of growth arrest represented by the principle that the extent of a possible deformity is inversely proportional to the patient’s age. However, it was noticed that the probability for growth abnormalities to occur existed mainly in adolescents during the last year before knee physeal closure (20). This may be related to the high capacity of the growth plate to break small epiphyso-metaphyseal bone bridges spontaneously in young children, a capacity which is slowing down with the maturation process (58). In others words, the amount of potential growth deformity is minor in older children, but the risk for a growth arrest to occur may be much higher. For this reason, we believe that when a patient’s knee is close to skeletal maturity, delayed reconstruction may be considered (59). At the other end of the spectrum, in the prepubertal phase, there is sufficient evidence that transphyseal techniques are safe (60), provided that the technical recommendations for these procedures are respected (49).
In order to minimize the risk of growth disturbance, Kocher advocated a physeal-sparing combined intra-articular and extra-articular reconstruction with an autologous iliotibial band in prepubescent (Tanner stage 1 or 2) children with a large amount of growth remaining (21,22). In pubescent adolescents with growth remaining (Tanner stage 3), they recommended a transphyseal hamstring graft technique with extracortical fixation (22). A similar technique is used by the authors on a routine basis even in prepubescent children (Figure 7) (61). It does not differ drastically from ACL reconstructions in the adult patient. The diameter of the graft usually varies between 6 and 8 mm. To minimize the drill injury in prepubescent children under the age of 10, a transtibial technique is preferred for the femoral tunnel. It allows creating a more perpendicular femoral tunnel in relation to the distal femoral physis. In children older than 10, with still significant knee growth remaining, the femoral tunnel is drilled through the anteromedial portal after having placed the knee in maximal flexion. While this induces a greater drill injury, it allows obtaining a more anatomic placement of the femoral graft. The use of a femoral drill guide with a 5-mm or even a 7-mm offset can be considered to prevent a blowout of the posterior cortex of the femur and to avoid injury of the perichondral structures (Figures 8,9). On the tibial side, care must be taken to position the tunnel entrance more medially as it is done in adults. It helps to protect the apophysis of the tibial tuberosity (located laterally to the tunnel entry point) as well as to avoid subsequent development of a growth arrest with a secondary recurvatum deformity (62). An arthroscopically assisted technique which combines a transphyseal drilling on the tibial side and an intraepiphyseal drilling on the femoral side has been proposed by Henry et al. (9). In this technique, a quadriceps tendon graft with a trapezoidal bone block is used. A pin is inserted under fluoroscopic guidance to ensure that the femoral tunnel is drilled parallel and at a safe distance from the physis. An outside-in technique is then used and the graft is introduced. The bone block is impacted press-fit in the femoral tunnel. Double tibial fixation is obtained by combining by an extracortical staple and a biodegradable screw in the tunnel which is placed distal to the tibial physis. The so-called Clocheville technique is an example of a nonanatomic, extraphyseal technique, in which the mid-third of the patellar tendon is used, without bone blocks (18,24,63). Instead of these, a periosteal flap is harvested at both the tibial and the patellar insertion sites. The femoral tunnel is positioned proximally to the growth plate, whereas on the tibial side, the graft is fixed at the epiphysis in a bone trough, which has a depth of 1 cm. This procedure has been used for many years, especially in very young, prepubertal children, although it is technically more demanding than the arthroscopic single tunnel technique.
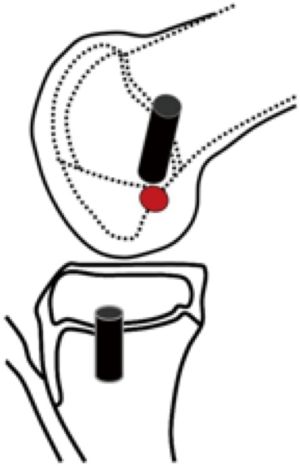
Recent anatomical studies suggested a role of the anterolateral ligament of the knee in common knee instability patterns (64). Certain authors suggest therefore lateral extra-articular tenodesis as a method of ACL graft protection and knee stabilization for adult patients with high-grade pivot shift test, in chronic ACL rupture, young patients, pivoting activities, and patients undergoing medial meniscus repair (65). There is currently no consensus regarding the use of this technique in skeletally immature patients.
Growth plate injury and potential complications
Growth disturbances after ACL reconstruction are underreported (50). They evolve throughout the entire process of remaining growth. The type and amount of the deformity are proportional to the localization and the size of the initial growth plate injury as well as the patient’s remaining growth potential.
Growth disturbances after ACL reconstruction can be categorized into three groups (66). Growth arrest (A) is caused by a localized injury of the growth plate, which activates the formation of a transphyseal bone bridge. If this growth arrest is located at the periphery of the physis, it may lead to axial deformities. If it is located in the center of the growth plate, it can lead to symmetrical leg length discrepancies. In very young children the growth plate can create distraction forces even after the creation of a transphyseal bone bridge, so that a spontaneous breakage of the bridge may occur. Bone bridge formation can be prevented if a soft tissue graft crosses the growth plate injury. Transphyseal placement of a bone block, hardware or synthetic graft placement should be avoided to avoid sudden growth arrest. Peripheral growth plate injuries to the distal femoral plate can also be provoked using a transphyseal technique if the tunnel is too large or if there is a posterior blow-out with subsequent injury of the perichondral structures of the growth plate (Ranvier zone and perichondral ring of Lacroix). Growth plate injuries cannot be avoided using epiphyseal technique. The former will be much larger as compared to those possible with a transphyseal technique: asymmetric growth in these cases can be much more severe in comparison to an arrest caused by transphyseal drilling; for this reason, tunnel drilling should always be performed under fluoroscopy to ensure that the femoral tunnel is located distally to the growth plate. When considering extraphyseal (over the top) techniques, extreme care is necessary to avoid an excessive rasping of the over the top position. This surgical maneuver is used to obtain a better graft adhesion, but may cause injuries to the perichondral structures and lead to axial malalignment. Since injuries using this technique occur in a posterolateral position, a growth arrest at the femoral tunnel site will lead to a deformity in valgus and flexion. In these cases, the amount of deformity can be predicted anticipating the remaining growth allows. On the tibial side, peripheral injuries may be caused if the tibial tuberosity apophysis is damaged, either during harvesting of the hamstring tendons or if the tibial tunnel entrance is created in a too anterior position: a recurvatum of the proximal tibia will result from a growth arrest in this region.
Focal physeal disruptions after transphyseal ACL reconstruction in adolescents with open physis were evaluated by Yoo et al. In 5 of 43 adolescent patients’ disruption was observed in MRI, although without any clinical consequences, so that the authors concluded that transphyseal techniques are not harmless and should not be used in young children (58). In contrast to this conclusion, we believe that in younger children those focal bone bridges will break easily, so that these patients will bear a lower risk of epiphysiodesis as compared to adolescents (59). In any case, the risks of a procedure and its consequences are concepts which cannot be assimilated: in fact, adolescents are at a higher risk of epiphysiodesis, but this has, in their age, limited clinical consequences in terms of a disturbance in future limb growth. On the other hand, the risk of epiphysiodesis in young children is low, but this even has sometimes dramatic clinical consequences if a physeal bridge persists and continues to develop until the end of growth.
The second type of growth abnormality is an overgrowth process (type B: boost). Boosts occur mainly in very young children and are likely to be caused by a local hypervascularization, which stimulates the physeal growth process. The growth disturbance will become apparent within a limited period of 2 years following surgery. It usually has a symmetric form and may lead to a moderate leg length discrepancy. McIntosh et al. reported a leg length discrepancy of less than 10 mm in 15 out of 16 patients. Only 1 patient had the operated limb 15 mm longer than the healthy limb (67). As compared to a full growth arrest, the clinical impact of overgrowth is usually low. Nevertheless, the need of a percutaneous epiphysiodesis has been reported because of a provisional leg discrepancy around two centimeters in an 8-year-old child at the time of ACL reconstruction (66). A tibial valgus deformity can sometimes also occur, due to asymmetrical overgrowth. This is similar to a posttraumatic genu valgum, the deformity observed after metaphyseal pediatric proximal tibial fractures. After an initial progressive increase of the deformity, a spontaneous correction can however occur, a close follow-up with nonoperative treatment of the deformity is therefore recommended (66).
The third type of growth disturbance is a deceleration of the remaining growth (type C: decelerate), which may be caused by a so-called “tenoepiphysiodesis” effect due to an excessive graft tension across the physis (13). Up to date, it is not yet clear which is the exact amount of graft tension necessary to cause such an abnormality in humans. Animal studies have demonstrated that it should not exceed 80 N. The use of a non-biological, synthetic graft is expected to produce the same effect. The mechanism underlying this growth abnormality is called Hueter-Volkmann principle, which affirms that an excessive pressure directed on the growth plate reduces the longitudinal growth, and vice versa.
To detect in a timely manner any possible growth abnormality, a much stricter post-operative follows up is recommended for children, as compared to adults: clinical and radiological controls are mandatory until the end of the growth. If a permanent growth abnormality is discovered, the cause of which is clearly identified (i.e., transphyseal hardware or bone block placement), immediate surgical revision is recommended. A Langenskiöld procedure (soft-tissue interposition) or an additional epiphysiodesis may be considered. On the other hand, if operative revision is not considered immediately, a correction osteotomy using specific plates or Ilizarov fixators can be necessary at the end of the growth period. Fortunately, the incidence of these complications is extremely low, especially if surgery was properly performed. Nevertheless, before surgery the children and their parents must be informed on their possible occurrence, even in experienced hands.
Rehabilitation and return to sports
Rehabilitation guidelines differ for prepubescent children and adolescents who are close to skeletal maturity. Whereas adolescents may follow rehabilitation and return-to-sports-principles and guidelines which are intended for adults, specific considerations have been recommended in the IOC consensus for children. In general terms, rehabilitation is similar irrespective of the surgical technique, although more carefully handled than in adults. Weight bearing is allowed from the beginning, with an exception for associated cartilage repair procedures or some types of meniscal repair (e.g., radial tear or meniscus root repair). In case of an associated meniscus repair, an extension brace is usually recommended over a period of 6 weeks. Motion must be started early on to avoid arthrofibrosis (68).
Functional tests, criteria to evaluate movement quality and return to sports criteria have not been validated in children so far. A recent multicenter investigation from the French Arthroscopy Society analyzing graft signal intensity after pediatric ACLR in 126 prepubescent children (skeletal age <13.5 years in girls and <15.5 years in boys) revealed that return to sports should be more conservative in children in comparison to adults. Investigators looked at graft changes on MRI up to 2 years’ post-surgery (69). They found an absence of signal normalization of the ACL graft with inhomogeneous signal-to-noise quotient and lower Howell grades than those which can be found in adults.
Results, clinical outcomes and first registries
Providing a complete overview of clinical results after pediatric ACL surgery would be beyond the scope of this article. Therefore, this chapter was restricted to two major reviews. The first analyzed the quality of published studies and the second analyzes the clinical results and complications.
Due to widespread methodological deficiencies of many of the studies on the treatment of pediatric ACL injuries, Moksnes et al. advised caution when interpreting results (3). No randomized controlled trials and just few prospective cohort studies are currently available in the literature (7,70). Operated patients considered in these studies could represent a negative selection of all ACL injured skeletally immature patients inducing a potential bias. The methodological quality of 31 studies investigating the outcome of the treatment of ACL injuries in skeletally immature individuals was recently evaluated by the Coleman Methodology Score, which can range from 0 to 100 (maximum) (71). The authors identified only four studies with a score of 60 or more (maximum 64), so that they concluded defining as low current treatment evidence of ACL injuries in children.
Fifty-five articles with 935 patients (median age 13 years) were included in a meta-analysis of case series (level of evidence IV) of pediatric patients undergoing ACL reconstruction by Frosch et al. The median follow-up of 40 months (range 14 to 89 months), after which leg-length discrepancies or axial malalignments were documented in 1.8% of the cases (60). Excellent or good function, indicated by an International Knee Documentation Committee grade A or B, was obtained in 84.2% of all knees, and the average Lysholm scores was 96.3. Approximately 5% of re-ruptures occurred. The risk of leg-length differences or axial malalignment was significantly lower in transphyseal reconstructions when compared with physeal-sparing techniques. However, the risk of recurrent tears was higher for the transphyseal reconstructions (4.2% vs. 1.4%). The authors concluded suggesting the need of randomized controlled trials to clarify these issues in the management of ACL injuries in children and adolescents.
In order to overcome the intrinsic limits of regional case studies and to provide a stronger basis to scientific knowledge, two registries have been developed, which monitor the outcomes of pediatric ACL treatment. The PLUTO (Pediatric ACL: Understanding Treatment Outcomes) is a multi-center, prospective cohort study started in 2016 under the lead of the Boston Children’s Hospital, which aim is to evaluate the safety and effectiveness of non-operative treatment, as well as four operative treatments including transphyseal, partial transphyseal, and physeal-sparing techniques (72). The PAMI (Paediatric Anterior Cruciate Ligament Monitoring Initiative) is a recently started pan-European system for the collection and analysis of data from orthopaedic surgeons who are treating children and adolescents with ACL injuries, aimed at collecting short-, medium- and long-term clinical outcome of conservative and surgical treatment and at proposing internationally accepted treatment guidelines (50).
Conclusions
The knowledge of pediatric ACL injuries and their treatment has made significant progress over the last 3 decades. Over the years, specific pediatric surgical techniques were developed and optimized. They proved to be successful and safe if used in a technically correct way. Nowadays, although pediatric techniques are used on a larger scale, surgery still maintains challenging aspects, especially due to the specific characteristics of the pediatric patient. Surgery-related complications occur, but their frequency has dropped to an acceptably low level (<2%). In the last decade, attention to nonoperative treatment also increased, which helped refining the indications for conservative treatment. It is estimated that 30–50% of patients could benefit from nonoperative treatment, whereas others may develop rapidly secondary soft-tissue injuries requiring surgery. A close follow up of pediatric patients is therefore recommended. As pediatric ACL injuries are increasingly recognized and as physicians are confronted with many different situations at different evolutionary stages, continuous progress is now required to select the correct treatment at the right moment for the appropriate patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takeshi Muneta) for the series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.06.02). The series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mizuta H, Kubota K, Shiraishi M, et al. The conservative treatment of complete tears of the anterior cruciate ligament in skeletally immature patients. J Bone Joint Surg Br 1995;77:890-4. [Crossref] [PubMed]
- Millett PJ, Willis AA, Warren RF. Associated injuries in pediatric and adolescent anterior cruciate ligament tears: Does a delay in treatment increase the risk of meniscal tear? Arthroscopy 2002;18:955-9. [Crossref] [PubMed]
- Moksnes H, Engebretsen L, Risberg MA. The current evidence for treatment of ACL injuries in children is low: a systematic review. J Bone Joint Surg Am 2012;94:1112-9. [Crossref] [PubMed]
- Peterson DC, Ayeni OM. Pediatric anterior cruciate ligament reconstruction outcomes. Curr Rev Musculoskelet Med 2016;9:339-47. [Crossref] [PubMed]
- International Olympic Committee Pediatric ACL Injury Consensus Group. 2018 International Olympic Committee Consensus Statement on Prevention, Diagnosis, and Management of Pediatric Anterior Cruciate Ligament Injuries. Orthop J Sports Med 2018;6:2325967118759953 [PubMed]
- Seil R, Theisen D, Moksnes H, et al. ESSKA partners and the IOC join forces to improve children ACL treatment. Knee Surg Sports Traumatol Arthrosc 2018;26:983-4. [Crossref] [PubMed]
- Moksnes H, Engebretsen L, Eitzen I, et al. Functional outcomes following a non-operative treatment algorithm for anterior cruciate ligament injuries in skeletally immature children 12 years and younger. A prospective cohort with 2 years follow-up. Br J Sports Med 2013;47:488-94. [Crossref] [PubMed]
- Dumont GD, Hogue GD, Padalecki JR, et al. Meniscal and chondral injuries associated with pediatric anterior cruciate ligament tears: relationship of treatment time and patient-specific factors. Am J Sports Med 2012;40:2128-33. [Crossref] [PubMed]
- Henry J, Chotel F, Chouteau J, et al. Rupture of the anterior cruciate ligament in children: early reconstruction with open physes or delayed reconstruction to skeletal maturity? Knee Surg Sports Traumatol Arthrosc 2009;17:748-55. [Crossref] [PubMed]
- Lawrence JTR, Argawal N, Ganley TJ. Degeneration of the knee joint in skeletally immature patients with a diagnosis of an anterior cruciate ligament tear: is there harm in delay of treatment? Am J Sports Med 2011;39:2582-7. [Crossref] [PubMed]
- Cucchi D, Mouton C, Dor, et al. Paediatric ACL tear. In: Tapasvi S, Shekhar S. Knee Arthroscopy: A Case Repository. New Delhi, India: Jaypee Brothers Medical Publishers (P) Ltd., 2019.
- Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament in skeletally immature patients. A preliminary report. J Bone Joint Surg Am 2003;85:1255-63. [Crossref] [PubMed]
- Edwards TB, Greene CC, Baratta RV, et al. The effect of placing a tensioned graft across open growth plates. A gross and histologic analysis. J Bone Joint Surg Am 2001;83:725-34. [Crossref] [PubMed]
- Hudgens JL, Dahm DL. Treatment of anterior cruciate ligament injury in skeletally immature patients. Int J Pediatr 2012;2012:932702 [Crossref] [PubMed]
- Kocher MS, Saxon HS, Hovis W, et al. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and The ACL Study Group. J Pediatr Orthop 2002;22:452-7. [Crossref] [PubMed]
- Seil R, Pape D, Kohn D. The risk of growth changes during transphyseal drilling in sheep with open physes. Arthroscopy 2008;24:824-33. [Crossref] [PubMed]
- Stadelmaier DM, Arnoczky SP, Dodds J, et al. The effect of drilling and soft tissue grafting across open growth plates. A histologic study. Am J Sports Med 1995;23:431-5. [Crossref] [PubMed]
- Bonnard C, Fournier J, Babusiaux D, et al. Physeal-sparing reconstruction of anterior cruciate ligament tears in children: results of 57 cases using patellar tendon. J Bone Joint Surg Br 2011;93:542-7. [Crossref] [PubMed]
- Cassard X, Cavaignac E, Maubisson L, et al. Anterior cruciate ligament reconstruction in children with a quadrupled semitendinosus graft: preliminary results with minimum 2 years of follow-up. J Pediatr Orthop 2014;34:70-7. [Crossref] [PubMed]
- Chotel F, Bonnard C, Accadbled F, et al. Résultats et facteurs pronostiques de la reconstruction du LCA sur genou en croissance. À propos d’une série multicentrique de 102 cas. Rev Chir Orthop 2007;93:131-8. [Crossref]
- Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg Am 2005;87:2371-9. [PubMed]
- Kocher MS, Smith JT, Zoric BJ, et al. Transphyseal anterior cruciate ligament reconstruction in skeletally immature pubescent adolescents. J Bone Joint Surg Am 2007;89:2632-9. [Crossref] [PubMed]
- Seil R, Chotel F. ACL Injuries in Children. In: Doral M, Karlsson J. Sports Injuries. Copenhagen, Denmark: Springer 2014:1-17.
- Parkkari J, Pasanen K, Mattila VM, et al. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9-year follow-up. Br J Sports Med 2008;42:422-6. [Crossref] [PubMed]
- Shea KG, Grimm NL, Ewing CK, et al. Youth sports anterior cruciate ligament and knee injury epidemiology: who is getting injured? In what sports? When? Clin Sports Med 2011;30:691-706. [Crossref] [PubMed]
- Seil R, Kohn D. Les ruptures du ligament croisé antérieur chez l’enfant. Bull Soc Sci Med Grand Duche Luxemb 2000;1:39-53. [PubMed]
- Beck NA, Lawrence JTR, Nordin JD, et al. ACL Tears in School-Aged Children and Adolescents Over 20 Years. Pediatrics 2017;139:e20161877 [Crossref] [PubMed]
- Shaw L, Finch CF. Trends in Pediatric and Adolescent Anterior Cruciate Ligament Injuries in Victoria, Australia 2005-2015. Int J Environ Res Public Health 2017;14:e599 [Crossref] [PubMed]
- Dodwell ER, Lamont LE, Green DW, et al. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med 2014;42:675-80. [Crossref] [PubMed]
- Astur DC, Cachoeira CM, da Silva Vieira T, et al. Increased incidence of anterior cruciate ligament revision surgery in paediatric verses adult population. Knee Surg Sports Traumatol Arthrosc 2018;26:1362-6. [Crossref] [PubMed]
- Werner BC, Yang S, Looney AM, et al. Trends in Pediatric and Adolescent Anterior Cruciate Ligament Injury and Reconstruction. J Pediatr Orthop 2016;36:447-52. [Crossref] [PubMed]
- Myer GD, Faigenbaum AD, Ford KR, et al. When to initiate integrative neuromuscular training to reduce sports-related injuries and enhance health in youth? Curr Sports Med Rep 2011;10:155-66. [Crossref] [PubMed]
- Renström PA. Eight clinical conundrums relating to anterior cruciate ligament (ACL) injury in sport: recent evidence and a personal reflection. Br J Sports Med 2013;47:367-72. [Crossref] [PubMed]
- Granan LP, Forssblad M, Lind M, et al. The Scandinavian ACL registries 2004-2007: baseline epidemiology. Acta Orthop 2009;80:563-7. [Crossref] [PubMed]
- Granan LP, Bahr R, Lie SA, et al. Timing of anterior cruciate ligament reconstructive surgery and risk of cartilage lesions and meniscal tears: a cohort study based on the Norwegian National Knee Ligament Registry. Am J Sports Med 2009;37:955-61. [Crossref] [PubMed]
- Lee K, Siegel MJ, Lau DM, et al. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology 1999;213:697-704. [Crossref] [PubMed]
- Trivedi V, Mishra M, Verma D. Pediatric ACL Injuries: A Review of Current Concepts. Open Orthop J 2017;11:378-88. [Crossref] [PubMed]
- Kocher MS, DiCanzio J, Zurakowski D, et al. Diagnostic performance of clinical examination and selective magnetic resonance imaging in the evaluation of intraarticular knee disorders in children and adolescents. Am J Sports Med 2001;29:292-6. [Crossref] [PubMed]
- Grindem H, Eitzen I, Engebretsen L, et al. Nonsurgical or Surgical Treatment of ACL Injuries: Knee Function, Sports Participation, and Knee Reinjury: The Delaware-Oslo ACL Cohort Study. J Bone Joint Surg Am 2014;96:1233-41. [Crossref] [PubMed]
- Dejour H, Bonnin M. Tibial translation after anterior cruciate ligament rupture. Two radiological tests compared. J Bone Joint Surg Br 1994;76:745-9. [Crossref] [PubMed]
- Ardern CL, Ekås GR, Grindem H, et al. Prevention, diagnosis and management of paediatric ACL injuries. Br J Sports Med 2018;52:1297-8. [Crossref] [PubMed]
- Canavese F, Charles YP, Dimeglio A, et al. A comparison of the simplified olecranon and digital methods of assessment of skeletal maturity during the pubertal growth spurt. Bone Joint J 2014;96-B:1556-60. [Crossref] [PubMed]
- Canavese F, Charles YP, Dimeglio A. Skeletal age assessment from elbow radiographs. Review of the literature. Chir Organi Mov 2008;92:1-6. [Crossref] [PubMed]
- Dvorak J, George J, Junge A, et al. Age determination by magnetic resonance imaging of the wrist in adolescent male football players. Br J Sports Med 2007;41:45-52. [Crossref] [PubMed]
- Wybier M, Bossard P. Musculoskeletal imaging in progress: the EOS imaging system. Joint Bone Spine 2013;80:238-43. [Crossref] [PubMed]
- Gicquel P, Giacomelli M, Karger C, et al. Développement embryonnaire et croissance normale du genou. Rev Chir Orthopédique 2007;(93):3S100-102.
- Baxter MP. Assessment of normal pediatric knee ligament laxity using the genucom. J Pediatr Orthop 1988;8:546-50. [Crossref] [PubMed]
- McCarthy MM, Tucker S, Nguyen JT, et al. Contact stress and kinematic analysis of all-epiphyseal and over-the-top pediatric reconstruction techniques for the anterior cruciate ligament. Am J Sports Med 2013;41:1330-9. [Crossref] [PubMed]
- Seil R, Weitz FK, Pape D. Surgical-experimental principles of anterior cruciate ligament (ACL) reconstruction with open growth plates. J Exp Orthop 2015;2:11. [Crossref] [PubMed]
- Moksnes H, Engebretsen L. The ESSKA paediatric anterior cruciate ligament monitoring initiative. Knee Surg Sports Traumatol Arthrosc 2016;24:680-7. [Crossref] [PubMed]
- Pennock AT, Ho B, Parvanta K, et al. Does Allograft Augmentation of Small-Diameter Hamstring Autograft ACL Grafts Reduce the Incidence of Graft Retear? Am J Sports Med 2017;45:334-8. [Crossref] [PubMed]
- Momaya AM, Beicker C, Siffri P, et al. Preoperative Ultrasonography Is Unreliable in Predicting Hamstring Tendon Graft Diameter for ACL Reconstruction. Orthop J Sports Med 2018;6:2325967117746146 [Crossref] [PubMed]
- Micheli LJ, Rask B, Gerberg L. Anterior cruciate ligament reconstruction in patients who are prepubescent. Clin Orthop 1999;40-7. [Crossref] [PubMed]
- Goddard M, Bowman N, Salmon LJ, et al. Endoscopic Anterior Cruciate Ligament Reconstruction in Children Using Living Donor Hamstring Tendon Allografts. Am J Sports Med 2013;41:567-74. [Crossref] [PubMed]
- Heath EL, Salmon LJ, Cooper R, et al. 5-Year Survival of Pediatric Anterior Cruciate Ligament Reconstruction With Living Donor Hamstring Tendon Grafts. Am J Sports Med 2019;47:41-51. [Crossref] [PubMed]
- Engebretsen L, Svenningsen S, Benum P. Poor results of anterior cruciate ligament repair in adolescence. Acta Orthop Scand 1988;59:684-6. [Crossref] [PubMed]
- Mohtadi N, Grant J. Managing anterior cruciate ligament deficiency in the skeletally immature individual: a systematic review of the literature. Clin J Sport Med 2006;16:457-64. [Crossref] [PubMed]
- Yoo WJ, Kocher MS, Micheli LJ. Growth plate disturbance after transphyseal reconstruction of the anterior cruciate ligament in skeletally immature adolescent patients: an MR imaging study. J Pediatr Orthop 2011;31:691-6. [Crossref] [PubMed]
- Chotel F, Seil R. Growth disturbances after transphyseal ACL reconstruction in skeletally immature patients: who is more at risk? Young child or adolescent? J Pediatr Orthop 2013;33:585-6. [Crossref] [PubMed]
- Frosch KH, Stengel D, Brodhun T, et al. Outcomes and risks of operative treatment of rupture of the anterior cruciate ligament in children and adolescents. Arthroscopy 2010;26:1539-50. [Crossref] [PubMed]
- Wilmes P, Lorbach O, Chotel F, et al. Ersatzplastik des vorderen Kreuzbandes bei offenen Wachstumsfugen. Arthroskopie 2009;22:35-44. [Crossref]
- Shea KG, Appel P, Pfeiffer R. ACL injuries in paediatric and adolescent patients. Sports Med 2003;33:455-71. [Crossref] [PubMed]
- Robert H, Bonnard C. The possibilities of using the patellar tendon in the treatment of anterior cruciate ligament tears in children. Arthroscopy 1999;15:73-6. [Crossref] [PubMed]
- Claes S, Vereecke E, et al. Anatomy of the anterolateral ligament of the knee. J Anat 2013;223:321-8. [Crossref] [PubMed]
- Sonnery-Cottet B, Vieira TD, Ouanezar H. Anterolateral Ligament of the Knee: Diagnosis, Indications, Technique, Outcomes. Arthroscopy 2019;35:302-3. [Crossref] [PubMed]
- Chotel F, Henry J, Seil R, et al. Growth disturbances without growth arrest after ACL reconstruction in children. Knee Surg Sports Traumatol Arthrosc 2010;18:1496-500. [Crossref] [PubMed]
- McIntosh AL, Dahm DL, Stuart MJ. Anterior cruciate ligament reconstruction in the skeletally immature patient. Arthroscopy 2006;22:1325-30. [Crossref] [PubMed]
- Nwachukwu BU, McFeely ED, Nasreddine A, et al. Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents. J Pediatr Orthop 2011;31:811-7. [Crossref] [PubMed]
- Pauvert A, Robert H, Gicquel P, et al. MRI study of the ligamentization of ACL grafts in children with open growth plates. Orthop Traumatol Surg Res 2018;104:S161-7. [Crossref] [PubMed]
- Geffroy L, Lefevre N, Thevenin-Lemoine C, et al. Return to sport and re-tears after anterior cruciate ligament reconstruction in children and adolescents. Orthop Traumatol Surg Res 2018;104:S183-8. [Crossref] [PubMed]
- Coleman BD, Khan KM, Maffulli N, et al. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports 2000;10:2-11. [Crossref] [PubMed]
- Pediatric ACL: Understanding Treatment Options (PLUTO) NCT02772770. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02772770
Cite this article as: Seil R, Cucchi D, Ninulescu C, Dor J, Mouton C. Anatomic anterior cruciate ligament reconstruction for adolescent patients with open physis. Ann Joint 2019;4:31.


