Surgical considerations to avoid adverse mechanics
Introduction
Hip resurfacing arthroplasty (HRA) continues to divide surgical opinion. Surgeon’s deciding to introduce this procedure into their practice need to understand what risks and benefits it offers over conventional hip arthroplasty, the importance of patient selection, implant selection, surgical approach and; the pitfalls that ought to be avoided. There are several presumed advantages that make HRA the intuitive arthroplasty option particularly for the young active patient (Table 1) (1,2).

Full table
The advantages are not evident in all cases, as incorrect implant selection, implant positioning and patient selection may all lead to a poor outcome. The formation of adverse soft-tissue reactions around the Metal on metal hip resurfacing arthroplasty (MoMHRA) has caused considerable concern internationally. These reactions have been termed Adverse Reactions to Metal Debris (ARMD) and have been shown to have a strong association with increased wear (3). The wear debris is associated with soft-tissue necrosis and nonspecific foreign-body macrophage response coupled with a variable adaptive or specific immune response. Patient, implant and surgical factors have all been found to contribute to the wear process.
The aim is to review factors that are potentially controllable by the surgeon to optimize surgical outcome and reduce the risk of femoral component failure (femoral neck fracture or aseptic loosening) as these reasons for revisions can often be avoided by adequate component positioning and knowledge of the vascular anatomy. Understanding the importance of component orientation as well as the association between head-neck ratio (HNR) alterations occurring secondary to the resurfacing procedure and subsequent risk of ARMD (4-6).
Patient and implant selection
Patient selection
Identifying appropriate patients to perform a HRA is a factor that can reduce the incidence of ARMD formation as well as risk of early femoral component failure. Beaulé et al. established a set of risk factors for early failure after metal on metal hip resurfacing: Surface Arthroplasty Risk Index (SARI) (7). This encompassed femoral bone quality, history of previous hip surgery, component sizing and activity level where a score greater than 3 was associated with a 12-fold increase in having early signs of failure (7). These identified risk factors were also found by others and also included female gender, small size, hip dysplasia, and in women age less than 40 (8). In young women, hip dysplasia is the predominant reason for development of premature arthritis (9). In females below 60 years undergoing a HRA, 32% had a primary diagnosis of DDH compared to only 6% in men (9). It is thought that both the acetabulum and femoral components size and component orientation have an influence on the high failure rates reported in the dysplastic cohort. Although cup inclination and anteversion were within the acceptable range in many cases, excess femoral anteversion from minor hip dysplasia was overlooked, leading to excess combined anteversion, edge loading and high wear (9). Because dysplastic hips tend to have smaller sized components, the arc of cover or contact patch rim distance is at risk range.
Many failures may simply be related to the smaller sized components as a smaller size has been shown to have higher ion levels and are theoretically more likely to edge load since component size influences the acetabular component’s arc of cover (10). Increased edge loading will lead to increased wear and may lead to increase ARMD risk (11). Femoral heads below 44 mm had a five-fold increased revision risk in comparison to femoral heads above 55 mm (12). Although the majority (80%) of ARMD are associated with high volume wear a minority of are associated with low wear and a prominent immune response (3). A proportion of patients will therefore be likely to develop an ARMD regardless of methods used to prevent significant wear. Innate and adaptive immune responses to metal wear are seen in periprosthetic histological tissue in patients with both elevated and non-elevated metal ion levels (13). Although metal ion levels are elevated in most cases of ARMD, the finding of a normal metal ion level does not exclude this diagnosis (13). This should be explained to the patient during the consent process. It is important to note that to-date no preclinical testing has been identified to identify patients at increased risk to developing a predominantly immune, ARMD-type response in the presence expected wear.
Implant selection
The Birmingham Hip Resurfacing (BHR) (Midland Medical Technologies), and the Conserve Plus (C+) hip resurfacing (Wright Medical), were both released in 2007 and remain two of the most popular HRAs on the market. Since their introduction the Articular Surface Replacement (ASR; DePuy, Warsaw) and the Durom Acetabular Component (Zimmer Inc.) have both been launched and now no longer manufactured due to high failure rates. CORIN is the other design, CORMET 2000 that received FDA approval which also has a cementless femoral component fixation option.
The ASR system resulted in poor early/mid-term survivorship in both independent centre studies as well as in the national registry data (14-16). The incidence rates for ARMD revision between the BHR, the ASR and the Conserve Plus prostheses demonstrated a ten-fold increase in the incidence of AMRD with the ASR (17). These findings subsequently led to the withdrawal of the ASR resurfacing system. The failure of the implant is felt to be primary related to the characteristics of the acetabular component, particularly the ability to prevent deflection and the lower head coverage (subtended angle), which has been implicated in the ASR and other sub-hemispherical designs (18). Lower clearance, as seen with the ASR, may also increase wear and subsequent failure (19). The Durom Acetabular Component was removed from the market due to a high incidence of early failure (20) but without a clearly described reason for failure. The BHR results on mid/long-term survivorship continue to be promising with data in male patients being reported regularly over 96% at 10 years (21), 98% at 10 years (22). A study in Japan with an average population age of 52 with a mix of male and female patients recorded a 96.5% survival at 10 years and 93.6% at 15 years (23). In England and Wales national joint registry (NJR) 2018, the revision rate at 14 years was 11% (16). The Conserve Plus (C+) hip resurfacing also has good survival results with five-year survival was 94.5% (24) and at 10 years survival has been shown to be at 89% with no revisions in acetabular components with a diameter greater than 46 mm (25). The NJR 2018 results found the survival to be 84% at 14 years but this included a 37% population of females (16). The CORMET hybrid resurfacing analysis reveals good 10 year survivorship with Kaplan-Meier survivor analysis showing 93% at nine to ten years and with survivorship in the osteoarthritis subgroup reaching 96.7% (26) in a single surgeon analysis 11-year survival was 93% (27). The fully uncemented CORMET results have shown a 95% survivorship at four to five years (28).
Pre-operative surgical planning is always advised particularly when performing a HRA. In the case of performing a BHR, in 2015 there was a voluntary removal from the market of femoral head sizes 46 mm and below. It is advised that a minimum of a 50 mm femoral head size should be used but size 48 mm heads continue to be manufactured in case intraoperative downsizing is needed (29). Similarly, Conserve Plus is no longer available in sizes <48 mm (30). If a patient is potentially going to be close to this cut-off size they should be informed and consented for a THA.
Surgical factors to minimise wear
Once patient selection has been optimized surgeons should aim to minimize wear of MoMHRA with appropriate implant selection and surgical technique. Three important surgical factors to observe include edge loading, impingement and HNR.
Edge loading
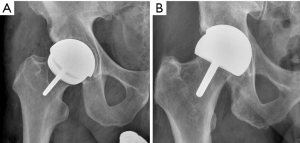
Edge loading should be avoided, to facilitate low wear. This can be achieved with the correct orientation of the femoral and acetabular components preventing contact area between the two components at the edge of the acetabular component. The incidence of ARMD occurring when the acetabular component is correctly positioned (45°±10° inclination and 20°±10° anteversion) is four times lower, than when not optimally positioned (4). Positioning the component in the optimum position allows an entrainment wedge to form aiding the separation of the two articulating surfaces with a thin layer of joint lubricant. If film lubrication is disrupted, MOM implants will exhibit increased wear due to the inability to form the tribolayer (31). This typically occurs during edge loading, preventing the entrainment wedge effect and fluid entrainment (18). Furthermore, finite element modelling has shown edge-loading can double contact stresses (32). Figure 1 shows correct acetabular positioning and excessive inclination that will result in edge loading occurring. In vivo evaluation of edge loading supports it as an important mechanism leading to localised excessive wear. There is significantly longer duration and greater magnitude of force compared to the MoMHRA hips without ARMD during activities of daily living (33). Both the femoral head and acetabular component have six independent degrees of freedom, three rotational and three translational. Rotational or translational mal-positioning of implants could theoretically result in edge loading. Rotational mal-positioning can result in a wear rate of 1 to 5 mm3/million cycles, whilst translational mal-positioning can increase wear by 10 to 100 mm3/million cycles (34-36). Rotational mal-positioning leading to edge loading (1° edge loading) occurs when the acetabular component is positioned with excessive inclination or inappropriate version. Translational mal-positioning occurs when impingement or joint laxity would lead to levering out of femoral head and contra-coup edge loading occurs (2° edge loading).
Impingement
In 2006 it was reported that up to 56% of hips prior to surgery who underwent a HRA had an abnormal offset ratio (37). The two main groups making up this percentage were patients with osteonecrosis or osteoarthritis, both these conditions have been associated with impingement in the periarthritis state (38,39). Femoral acetabular impingement (FAI) is a common cause of arthritis believed to result from a lack of femoral head-neck offset in the anterolateral region of the femoral head-neck junction. This must be recognised and addressed during the HRA procedure or ongoing impingement may occur (40). Painful impingement of HRA is a known cause for revision, it will also lead to increased wear. The reduced HNR of HRAs, relative to THAs, coupled with the younger patient with greater range of movement (ROM) during normal activities render acetabular component position of great importance to avoid impingement and edge loading. Impingement will lever out the femoral head leading to translational mal-positioning and as such contra-coup edge loading. These effects can be minimised by appropriate femoral component placement (8). The concept of combined version was first described with the McKibbin index in relation to instability of newborns hips (41). This provided the understanding that the effects of femoral and acetabular anteversion may be additive or may offset each other. Combined acetabular and femoral anteversion for a THA should be 45° or less, to avoid edge loading and posterior impingement and high enough (>20°) to prevent anterior impingement and posterior edge loading (42). Impingement can also be caused by decreased offset or by medial or superior translation of the centre of acetabular component; factors that the surgeon may be able to optimize (43,44).
Unlike THA, positioning of the femoral component to prevent impingement is limited due to the lack of modularity and preservation of the native femoral neck. Thus the HNR following hip resurfacing is less compared to a THR. To reduce the occurrence of impingement, it is important to remodel the head/neck junction by removing osteophytes and performing an anterior femoral osteochondroplasty in order to restore head sphericity, adequate head-neck contour and sufficient anterior offset (37). In addition, translation of the femoral component can be undertaken to improve anterior offset and therefore decrease the risk of anterior impingement but potentially results in decreased posterior offset. Due to the limited capacity of changing the femoral version, appropriate acetabular positioning is important to aim towards preventing impingement.
Component size decision & HNR
HNR is defined as the femoral head diameter divided by the femoral neck diameter. A decrease in HNR may lead to a decrease in functional ROM (45) and will increase risk of impingement and hence wear. Most hips undergoing resurfacing have an abnormal femoral head/neck offset, which is best assessed in the sagittal plane (6). Surgeons should aim to use as large of a component as possible, considering acetabular anatomy and acetabular bone preservation. In order to achieve this, after surgical dislocation of the femoral head, surgeons should use a femoral sizing ring placed over the articular surface and make a note of the femoral head size. Implanting as large of a component as possible, i.e., one as similar as possible to native femoral head diameter, would increase HNR, decrease impingement risk and in turn reduce risk of edge loading and increased wear. Most surgeons would measure the diameter of the femoral head and the widest diameter of the femoral neck in order to decide on femoral component size, which would in turn dictate acetabular component size too. Thus, a surgeon has to put as large of a component as possible in order to improve HNR and mechanics, respecting however the acetabular bone stock and ensuring no excessive reaming takes place to accommodate for a larger diameter acetabular component. Relative to the femoral head size, it is most usual that the femoral component will be of similar size to the native femoral head or 2 mm less.
Surgical factors to avoid femoral neck fracture
Femoral neck fracture is an early-term failure mode with an incidence reported in early studies up to 12% (45,46) but in more recent literature this has fallen to 1.1% (47). Patient and surgical technique related risk factors for femoral neck fracture have been described, including gender (48), proximal femoral bone quality, vascular compromise (49), prosthesis placement (48) and cementation. Other recognized failure modes include component loosening (50,51), avascular necrosis (AVN) of the femoral head (49) and painful impingement (52).
Stress loading proximal femur
It is generally agreed that maintenance of bone in the proximal aspect of the femur is desirable and is of importance if the need for revision arises as preservation of femoral bone stock to support an implant during revision becomes especially important. One of the proposed advantages of HRA has been the theoretical ease of conversion to THA. This is thought to be the case due to the normal proximal femoral loading preventing stress shielding and the preservation of the native femoral neck and intramedullary canal architecture (49,53).
Importance of neck shaft angle (NSA)
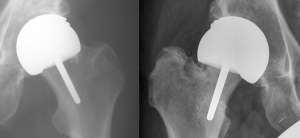
Several retrospective reviews have correlated errors in surgical technique with the risk of periprosthetic femoral neck fracture (48,49,53,54). These include notching of the superior aspect of the femoral neck, a varus position of the femoral component, and inadequate coverage of the reamed femoral head. The importance of the NSA has been shown in several studies (48,54). The surgeon should tend towards a valgus position of the femoral component. In the coronal plane the aim should be to place the femoral component into a relative valgus angle of 5–10 degrees to minimize tensile stress at the superior bone junction (55,56). The tensile stress of the superior neck has been calculated to decrease by up to 31% whilst walking with a change from 140 degrees to a more varus 130 degrees. If the femoral component angle is less than 130 degrees, the risk of adverse outcome is increased by a factor of over 6 (57). The valgus positioning should not be to the extent that it causes notching in the superior neck in order to prevent fracture of the femoral neck (Figure 2).
Surgical approach
Understanding the anatomy of the hip is not only important for implant positioning but also the preservation of blood supply to the femoral neck particularly the MFCA to prevent osteonecrosis and consequently aseptic loosening. The rate of AVN following uncomplicated dislocation of the hip and fracture-dislocation of the hip are significantly different. After an uncomplicated dislocation treated non-operatively the incidence of AVN is up to 11% (52,58) while in fracture-dislocation treated operatively, it rises to 31% (48,50,58,59). The only significant difference between these two groups may be the iatrogenic trauma to the medial femoral circumflex artery (MFCA) and/or its peripheral anastomoses. Protection of vascular structures during HRA is essential in order to prevent osteonecrosis and consequently aseptic loosening. In this centre a direct anterior surgical approach is favoured as the pelvic position is more reliable when the patient is in the supine position, leading to more consistent orientation of the acetabular component (60,61). The approach also reduces the soft tissue trauma to the hip as it does not require muscle detachments from the bone (62). The Posterior approach for HRA has also been shown to result in a potential vascular insult to the femoral head, with posterior zones more affected than the anterior zones (63). A modified posterior approach, unlike the standard extended approach, may be utilized as this does not significantly compromise the blood supply to the head (64).
Biomechanics of hip resurfacing vs. total hip
Patients following unilateral hip resurfacing do have some degree of gait asymmetry between the operated hip and the unoperated side in long term (65). However, there are a number of factors that may contribute to this observation including the fact the patient may have had an abnormal gait that contributed to the original pathology. However, it is of interest that patients who undergo a unilateral HRA do have recorded biomechanical characteristics that could allow their gait pattern to closely replicate what takes place in the non-diseased state compared to patients that have undergone a THA (66). To that effect, HRA has been shown to preserve a more normal weight acceptance and patients have been reported to reach a higher walking speed with better hip flexion relative to their unoperated leg compared to patients who have undergone a unilateral THA (67).
Summary
Surgical considerations to avoid adverse mechanics:
- Understand the importance of patient selection and patient education;
- Select a surgical implant with a good long-term outcome;
- Aim to use as large of a component as possible, considering acetabular anatomy and acetabular bone preservation;
- Understand the vascular anatomy of the femoral neck with importance of the MFCA;
- Cup inclination should be 45°±10°;
- When performing a HRA using a posterior approach Cup anteversion should be 20°±10°;
- NSA should have mild valgus alignment with avoidance of varus positioning;
- Medialize the acetabular cup to ensure containment of the component but do not excessively medialize the acetabular cup as it will decrease native offset and contribute to impingement—this is a factor that cannot be corrected on the femoral component due to the lack of modularity;
- Regular post-operative monitoring should be undertaken with an emphasis on clinical review.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Hip Resurfacing for the Young Arthritic Hip”. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.10.05). The series “Hip Resurfacing for the Young Arthritic Hip” was commissioned by the editorial office without any funding or sponsorship. GG as the unpaid Guest Editor of the series and served as an unpaid editorial board member of Annals of Joint from May 2019 to Apr 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shimmin A, Beaule PE, Campbell P. Metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am 2008;90:637-54. [Crossref] [PubMed]
- Dowding C, Dobransky JS, Kim PR, et al. Metal on Metal Hip Resurfacing in Patients 45 Years of Age and Younger at Minimum 5-Year Follow-Up. J Arthroplasty 2018;33:3196-200. [Crossref] [PubMed]
- Grammatopoulos G, Pandit H, Kamali A, et al. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J Bone Joint Surg Am 2013;95:e81 [Crossref] [PubMed]
- Grammatopoulos G, Pandit H, Glyn-Jones S, et al. Optimal acetabular orientation for hip resurfacing. J Bone Joint Surg Br 2010;92:1072-8. [Crossref] [PubMed]
- Grammatopoulos G, Pandit H, Murray DW, et al. The relationship between head-neck ratio and pseudotumour formation in metal-on-metal resurfacing arthroplasty of the hip. J Bone Joint Surg Br 2010;92:1527-34. [Crossref] [PubMed]
- Beaulé PE, Harvey N, Zaragoza E, et al. The femoral head/neck offset and hip resurfacing. J Bone Joint Surg Br 2007;89:9-15. [Crossref] [PubMed]
- Beaulé PE, Dorey FJ, Le Duff MJ, et al. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin Orthop Relat Res 2004;87-93. [Crossref] [PubMed]
- Glyn-Jones S, Pandit H, Kwon YM, et al. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg Br 2009;91-12:1566-74.
- McMinn DJW, Daniel J, Ziaee H, et al. Indications and results of hip resurfacing. Int Orthop 2011;35:231-7. [Crossref] [PubMed]
- De Haan R, Pattyn C, Gill HS, et al. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br 2008;90:1291-7. [Crossref] [PubMed]
- Kovochich M, Fung ES, Donovan E, et al. Characterization of wear debris from metal-on-metal hip implants during normal wear versus edge-loading conditions. J Biomed Mater Res B Appl Biomater 2018;106:986-96. [Crossref] [PubMed]
- Prosser GH, Yates PJ, Wood DJ, et al. Outcome of primary resurfacing hip replacement: evaluation of risk factors for early revision. Acta Orthop 2010;81:66-71. [Crossref] [PubMed]
- Grammatopoulos G, Munemoto M, Pollalis A, et al. Correlation of serum metal ion levels with pathological changes of ARMD in failed metal-on-metal-hip-resurfacing arthroplasties. Archives of Orthopaedic and Trauma Surgery 2017;137:1129-37. [Crossref] [PubMed]
- Langton DJ, Jameson SS, Joyce TJ, et al. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br 2010;92:38-46. [Crossref] [PubMed]
- Langton DJ, Jameson SS, Joyce TJ, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br 2011;93:1011-6. [Crossref] [PubMed]
- NJR. National Joint Registry England and Wales. Annual Report 2018. Available online: https://www.hqip.org.uk/resource/national-joint-registry-15th-annual-report-2018/
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br 2011;93:164-71. [Crossref] [PubMed]
- Campbell P, Beaulé PE, Ebramzadeh E, et al. The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res 2006;35-46. [Crossref] [PubMed]
- Jameson SS, Baker PN, Mason J, et al. Independent predictors of revision following metal-on-metal hip resurfacing: a retrospective cohort study using National Joint Registry data. J Bone Joint Surg (Br) 2012;94:746-54. [Crossref] [PubMed]
- Naal FD, Pilz R, Munzinger U, et al. High revision rate at 5 years after hip resurfacing with the Durom implant. Clin Orthop Relat Res 2011;469:2598-604. [Crossref] [PubMed]
- Treacy RB, McBryde CW, Shears E, et al. Birmingham hip resurfacing: a minimum follow-up of ten years. J Bone Joint Surg Br 2011;93:27-33. [Crossref] [PubMed]
- Coulter G, Young DA, Dalziel RE, et al. Birmingham hip resurfacing at a mean of ten years: Results from an independent centre. J Bone Joint Surg Br 2012;94:315-21. [Crossref] [PubMed]
- Uemura K, Takao M, Hamada H, et al. Long-term results of Birmingham hip resurfacing arthroplasty in Asian patients. J Artif Organs 2018;21:117-23. [Crossref] [PubMed]
- Zylberberg AD, Nishiwaki T, Kim PR, et al. Clinical results of the conserve plus metal on metal hip resurfacing: an independent series. J Arthroplasty 2015;30:68-73. [Crossref] [PubMed]
- Amstutz HC, Le Duff MJ, Campbell PA, et al. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten year follow-up. J Bone Joint Surg Am 2010;92:2663-71. [Crossref] [PubMed]
- Spencer RF. Evolution in Hip Resurfacing Design and Contemporary Experience with an Uncemented Device. J Bone Joint Surg Am 2011;93:84-8. [Crossref] [PubMed]
- Gross TP, Liu F, Webb LA. Clinical Outcome of the Metal-on-Metal Hybrid Corin Cormet 2000 Hip Resurfacing System: An up to 11-Year Follow-Up Study. J Arthroplasty 2012;27:533-538.e1. [Crossref] [PubMed]
- Spencer RF. Hip resurfacing—UK experience. Eighth Symposium on Joint Preserving and Minimally Invasive Surgery of the Hip; 2010 Jun 10-12; Ottawa, Canada.
- Available online: http://www.smith-nephew.com/bhr/
- Conserve Plus -information from surgical technique 2016. Available online: http://osimplantes.com.br/produtos/files/microport/quadril/Conserve%20Plus%20-%20Tecnica.pdf
- Anissian HL, Stark A, Gustafson A, et al. Metal-on-metal bearing in hip prosthesis generates 100-fold less wear debris than metal-on-polyethylene. Acta Orthop Scand 1999;70:578-82. [Crossref] [PubMed]
- Mellon SJ, Kwon YM, Glyn-Jones S, et al. The effect of motion patterns on edge-loading of metal-on-metal hip resurfacing. Med Eng Phys 2011;33:1212-20. [Crossref] [PubMed]
- Kwon YM, Mellon SJ, Monk P, et al. In vivo evaluation of edge-loading in metal-on-metal hip resurfacing patients with pseudotumours. Bone Joint Res 2012;1:42-9. [Crossref] [PubMed]
- Leslie IJ, Williams S, Isaac G, et al. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop Relat Res 2009;467:2259-65. [Crossref] [PubMed]
- Williams S, Stewart TD, Ingham E, et al. Metal-on-metal bearing wear with different swing phase loads. J Biomed Mater Res B Appl Biomater 2004;70:233-9. [Crossref] [PubMed]
- Williams S, Leslie I, Isaac G, et al. Tribology and wear of metal-on-metal hip prostheses: influence of cup angle and head position. J Bone Joint Surg Am 2008;90:111-7. [Crossref] [PubMed]
- Beaulé PE, Poitras P. Femoral component sizing and positioning in hip resurfacing arthroplasty. Instr Course Lect 2007;56:163-9. [PubMed]
- Kloen P, Leunig M, Ganz R. Early lesions of the labrum and acetabular cartilage in osteonecrosis of the femoral head. J Bone Joint Surg Br 2002;84:66-9. [Crossref] [PubMed]
- Ganz R, Parvizi J, Leunig M, et al. Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin Orthop Relat Res 2003;112-20. [PubMed]
- Wiadrowski TP, McGee M, Cornish BL, et al. Peripheral wear of wagner resurfacing hip arthroplasty acetabular components. J Arthroplasty 1991;6:103-7. [Crossref] [PubMed]
- McKibbin B. Anatomical factors in the stability of the hip joint in the newborn. J Bone Joint Surg Br 1970;52:148-59. [Crossref] [PubMed]
- Daniel J. Incidence and risk factors for pseudotumours in a series of 3014 metal-on-metal resurfacings. Orthopaedic Research Society Annual Meeting. New Orleans, USA, 2010.
- Kurtz WB, Ecker TM, Reichmann WM, et al. Factors affecting bony impingement in hip arthroplasty. J Arthroplasty 2010;25:624-34.e1. [Crossref] [PubMed]
- Kluess D, Zietz C, Lindner T, et al. Limited range of motion of hip resurfacing arthroplasty due to unfavorable ratio of prosthetic head size and femoral neck diameter. Acta Orthop 2008;79:748-54. [Crossref] [PubMed]
- Shimmin AJ, Back D. Femoral neck fractures following Birmingham hip resurfacing: a national review of 50 cases. J Bone Joint Surg Br 2005;87:463-4. [Crossref] [PubMed]
- Capello WN, Ireland PH, Trammell TR, et al. Conservative total hip arthroplasty: a procedure to conserve bone stock. Part I: analysis of sixty-six patients. Part II: analysis of failures. Clin Orthop Relat Res 1978;59-74. [PubMed]
- Matharu GS, McBryde CW, Revell MP, et al. Femoral neck fracture after Birmingham Hip Resurfacing Arthroplasty: prevalence, time to fracture, and outcome after revision. J Arthroplasty 2013;28:147-53. [Crossref] [PubMed]
- Richards CJ, Giannitsios D, Huk OL, et al. Risk of periprosthetic femoral neck fracture after hip resurfacing arthroplasty: valgus compared with anatomic alignment. A biomechanical and clinical analysis. J Bone Joint Surg Am 2008;90:96-101. [Crossref] [PubMed]
- Deuel CR, Jamali AA, Stover SM, et al. Alterations in femoral strain following hip resurfacing and total hip replacement. J Bone Joint Surg Br 2009;91:124-30. [Crossref] [PubMed]
- Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br 2008;90:436-41. [Crossref] [PubMed]
- Amstutz HC, Le Duff MJ. Eleven years of experience with metal-on-metal hybrid hip resurfacing: a review of 1000 conserve plus. J Arthroplasty 2008;23:36-43. [Crossref] [PubMed]
- Lavigne M, Boddu Siva Rama KR, Roy A, et al. Painful Impingement of the Hip Joint After Total Hip Resurfacing: A Report of Two Cases. J Arthroplasty 2008; [Crossref] [PubMed]
- Ong KL, Day JS, Kurtz SM, et al. Role of Surgical Position on Interface Stress and Initial Bone Remodeling Stimulus around Hip Resurfacing Arthroplasty. J Arthroplasty 2009;24:1137-42. [Crossref] [PubMed]
- Schnurr C, Nessler J, Meyer C, et al. Is a valgus position of the femoral component in hip resurfacing protective against spontaneous fracture of the femoral neck? J Bone Joint Surg Br 2009;91:545-51. [Crossref] [PubMed]
- Kukla C, Gaebler C, Pichl RW, et al. Predictive geometric factors in a standardized model of femoral neck fracture. Experimental study of cadaveric human femurs. Injury 2002;33:427-33. [Crossref] [PubMed]
- Jolley MN, Salvati EA, Brown GC. Early results and complications of surface replacement of the hip. J Bone Joint Surg Am 1982;64:366-77. [Crossref] [PubMed]
- Beaulé PE, Amstutz HC. Orientation of the femoral component in surface arthroplasty of the hip. J Bone Joint Surg Am 2005;87:1162. [PubMed]
- Gautier E, Ganz K, Krügel N, et al. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br 2000;82:679-83. [Crossref] [PubMed]
- Kwon YM, Ostlere SJ, McLardy-Smith P, et al. "Asymptomatic" Pseudotumors After Metal-on-Metal Hip Resurfacing Arthroplasty Prevalence and Metal Ion Study. J Arthroplasty 2011;26:511-8. [Crossref] [PubMed]
- Benoit B, Gofton W, Beaulé PE. Hueter anterior approach for hip resurfacing: assessment of the learning curve. Orthop Clin North Am 2009;40:357-63. [Crossref] [PubMed]
- Grammatopoulos G, Gofton W, Cochran M, et al. Pelvic positioning in the supine position leads to more consistent orientation of the acetabular component after total hip arthroplasty. Bone Joint J 2018;100-B:1280-8. [Crossref] [PubMed]
- Kreuzer S, Leffers K, Kumar S. Direct Anterior Approach for Hip Resurfacing: Surgical Technique and Complications. Clin Orthop Relat Res 2011;469:1574-81. [Crossref] [PubMed]
- Alsheri M, Bali K, Railton P, et al. Anatomic study on the blood supply to the femoral head following hip resurfacing using the posterior approach. Hip Int 2019;29:558-63. [Crossref] [PubMed]
- Steffen RT, De Smet KA, Murray DW, et al. A modified posterior approach preserves femoral head oxygenation during hip resurfacing. J Arthroplasty 2011;26:404-8. [Crossref] [PubMed]
- Resende RA, Kirkwood RN, Rudan JF, et al. How symmetric are metal-on-metal hip resurfacing patients during gait? Insights for the rehabilitation. J Biomech 2017;58:37-44. [Crossref] [PubMed]
- Nantel J. Gait patterns after total hip arthroplasty and surface replacement arthroplasty. Arch Phys Med Rehabil 2009;90:463-9. [Crossref] [PubMed]
- Gerhardt DMJM, Mors TGT, Hannink G, et al. Resurfacing hip arthroplasty better preserves a normal gait pattern at increasing walking speeds compared to total hip arthroplasty. Acta Orthop 2019;90:231-6. [Crossref] [PubMed]
Cite this article as: Nicol G, Vanbiervliet J, Grammatopoulos G. Surgical considerations to avoid adverse mechanics. Ann Joint 2020;5:6.