Effects of romosozumab on low bone mineral density or osteoporosis in postmenopausal women: a systematic review
Introduction
Osteoporosis is characterized by reduced bone mass and deterioration of the skeletal microstructural (1). It is predicted that about 14 million American people aged 50 years or older will be with osteoporosis by 2020 (2). As shown in the investigating, nearly 13 million Japanese women have osteoporosis (3). Despite all this, most people do not receive regular anti-osteoporosis therapy around the globe. Even suffering fracture, more than 75% of patients are absent from pharmacologic treatment for osteoporosis (4).
Sclerostin, encoded by the gene SOST, inhibits the Wnt and bone morphogenetic protein signaling pathways, thereby decreases bone formation to regulate bone turnover (5,6). Some investigators found that a genetic deficiency of sclerostin would increase bone mass and bone strength (7). Moreover, mice with the sclerostin gene deleted increases bone formation compared with controls (8). Animal study has demonstrated that administration of an antibody to sclerostin caused enhanced bone mass (9). Romosozumab binding sclerostin results in elevated bone formation and decreased bone resorption. As romosozumab blocks sclerostin activity, it results in allowing for increased Wnt signaling (10,11).
Alendronate can inhibit bone resorption and is used as first-line drug treating osteoporosis. In a trial, postmenopausal women treated with alendronate had less risk of fractures than those treated with placebo (12). To be different from alendronate, bone-forming therapy increases bone mass by stimulating bone formation. Teriparatide is one of bone-forming agents and works by improving remodeling activity (13). Despite teriparatide boosts trabecular bone mineral density (BMD), the promoting in bone resorption during up to 24 months of therapy has an association with a reduce in volumetric BMD (vBMD) of the cortical bone at the hip (14).
In the past several years, many studies about the effect of romosozumab on osteoporosis were published. Postmenopausal women with osteoporosis using romosozumab therapy for 12 months followed by alendronate caused a significantly decreased risk of fracture compared with that with treatment of alendronate alone (15). It was found that vertebral and femoral strength enhanced more with treatment of romosozumab than that with treatment of teriparatide at month 12 (16). Moreover, in women with postmenopausal osteoporosis, romosozumab resulted in gains in hip BMD which was not observed with treatment of teriparatide (17). Thus, we systematically review the effect of romosozumab in postmenopausal women with low BMD or osteoporosis.
Methods
Search trials
We searched the PubMed, Cochrane library, and EMBASE databases from the inception dates till March 12, 2019, using the keywords romosozumab, postmenopausal women, and osteoporosis to identify published RCTs meeting inclusion criteria. Only studies restricted to English with the full text available were included.
Inclusion criteria
Studies were selected according to the following inclusion criteria: (I) RCTs comparing romosozumab, romosozumab + alendronate, or romosozumab + denosumab with placebo, alendronate, teriparatide, or placebo + denosumab; (II) trials enrolling postmenopausal women with low BMD or osteoporosis; (III) trials providing data of BMD or bone formation marker PINP and bone resorption marker CTX. Exclusion criteria were (I) randomized trials without a placebo or treatment group; (II) a history of vertebral fracture or a fracture of the wrist, humerus, hip; (III) a history of metabolic bone disease; (IV) current hypercalcemia, hypocalcemia, hyperparathyroidism or hypoparathyroidism; (V) participants if they had recent use of a medication affecting bone metabolism.
Risk-of-bias assessments
The methodological quality for the included RCTs was evaluated independently by 2 researchers on the basis of Cochrane risk-of-bias criteria, and each quality item was graded as low risk, high risk, or unclear risk. The 7 items used to assess bias in each study included the randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
Data extraction
Country of researches, publish year, number of subjects, intervening measure, mean age, outcome (BMD, bone formation marker PINP and bone resorption marker CTX, adverse events), time of follow-up were comprised. Two researchers independently extracted the information by using a data extraction form from each study. Disagreements were resolved by discussion with another researcher until a consensus was reached.
Synthesis of results
A narrative synthesis was conducted under specific headings according to the framework by the Economic Social and Research Council. The narrative synthesis process was exploring relationships within and between the extracted data to find out how different studies contributed to the specific headings. Accurate findings from included studies were grouped together and reported by using appropriate descriptive statistics.
Results
Studies retrieved and characteristics
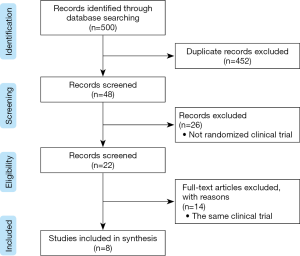
Initially, the search strategy identified 500 titles and abstracts. After excluding duplicates, 48 titles and abstracts were screened. Ultimately, 8 studies met the inclusion criteria and were included in Figure 1. Among these studies, six trials included were romosozumab compared with placebo, three trials included were romosozumab compared with teriparatide and one trial included was romosozumab compared with alendronate (Table 1). Overall, all included studies were randomized, controlled trial and were judged to be of good quality.
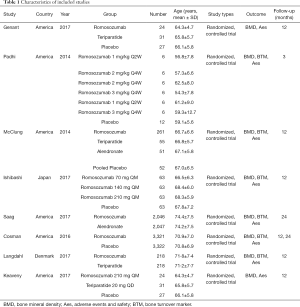
Full table
Romosozumab compared with placebo
BMD
It was shown that romosozumab significantly increased BMD from baseline compared with placebo (18). Particularly, several studies have suggested that BMD of the lumbar spine was increased from baseline in comparison with placebo (2,11,16,19,20). McClung et al. (19) found that the results had statistical difference whether dose frequency (monthly or every 3 months) or dose level (140 or 210 mg). Ishibashi et al. (20) discovered that dose-dependent effect of romosozumab on BMD were existed in postmenopausal Japanese women with osteoporosis. It was also observed that BMD of the total hip and femoral neck was increased compared with placebo group in varying degrees (11,16,19). However, in another study, no significant difference was found in the risk of nonvertebral fracture at 24 months in both romosozumab group and placebo group (11).
Bone turnover marker
Padhi et al. (2) found that the serum level of the bone-formation marker PINP was increased from baseline at all romosozumab doses. Moreover, serum concentration of PINP of the subjects using romosozumab was significantly higher than that in the placebo group. McClung et al. and Cosman et al. (11,19) discovered that the level of P1NP increased rapidly in the early time (month 1 or day 14), then declined to baseline level (between months 2 and 6 or at 12 months). Almost all studies (2,11,19,20) included on this topic showed that the level of the bone-resorption marker CTX decreased from baseline early, remaining below placebo and below baseline through the whole follow-up period, while it was an exception for women with dose of 1 mg/kg Q2W (2).
Romosozumab compared with teriparatide
BMD
The researchers (16-18) found that the BMD of the lumbar spine, femoral neck and total hip was increased in both romosozumab group and teriparatide group at month 6 and 12. These changes were more for romosozumab group than that for teriparatide group.
Bone turnover marker
Langdahl et al. (17) discovered that the level of P1NP increased rapidly in the romosozumab group, with an initial rise that was significantly higher than that in the teriparatide group, while the level of P1NP was significantly lower in patients in the romosozumab group than that in the teriparatide group measured between months 3 and 12. CTX decreased rapidly in the initial time, then increased to baseline by month 3, with the level remaining near baseline at month 12. Similarly, the concentration of CTX was significantly lower in patients in the romosozumab group than that in the teriparatide group measured between months 3 and 12.
Romosozumab compared with alendronate
BMD
Patients using romosozumab had greater gains in BMD from baseline measured at all the time points than patients who received alendronate alone. Therapy with romosozumab followed by alendronate resulted in lower risk of new vertebral fractures and nonvertebral fracture than alendronate alone over a period of 24 months (15).
Bone turnover marker
Romosozumab increased the level of P1NP and decreased the level of CTX within 12 months. After the transition to alendronate, the level of P1NP and β-CTX declined and remained below baseline level at 36 months. The level of P1NP and β-CTX of patients using alendronate alone decreased within 1 month and remained below baseline at 36 months (15).
Adverse events and safety
Among the 36 subjects using romosozumab, two subjects developed neutralizing antibodies during the study (18). Injection-site reactions were detected in several studies. Injection-site reactions (mostly mild in severity) were observed more frequently with romosozumab than with placebo, but no dose-response relationship was observed (2). Likewise, it was reported in 187 patients (5.2%) using romosozumab and in 104 (2.9%) using placebo over the 12-month period (15). In the first 12 months, injection-site reactions were reported in more patients using romosozumab [90 of 2,040 patients (4.4%)] than in those using alendronate [53 of 2,014 patients (2.6%)] (20). Otherwise, injection site reactions were reported by 17 (8%) patients with romosozumab and 6 (3%) patients with teriparatide (11). Many adverse events, involving hematochezia, acute blood loss anemia, severe coronary artery disease was not regarded to be associated with study treatment (18,19). Serious adverse events were reported in 14% in patients with placebo (7 of 50), 8% in patients with alendronate (4 of 51), 9% in patients with teriparatide (5 of 54), and 7% in patients with romosozumab (17 of 255) (2).
Discussion
It was found that romosozumab significantly improved BMD by using DXA from baseline and decreased the risk of fracture compared with placebo (2,11,16,18,19,20). In the patients with romosozumab, the level of bone turnover marker PINP increased from baseline, while the level of CTX declined from baseline compared with placebo (2,11,19,20). The changes of BMD from baseline were greater for romosozumab than those for teriparatide (16-18). Both the level of PINP and CTX were significantly increased in patients in the teriparatide group than those in the romosozumab group at months 3 and 12. The level of PINP was higher than the baseline at all the time points and the level of CTX was nearly equivalent to the baseline (17). Patients who received alendronate alone had less gains in BMD from baseline than patients who received romosozumab plus alendronate at all measured sites (15). Romosozumab increased the concentrations of P1NP, whereas alendronate reduced the level of PINP. Both romosozumab and alendronate resulted in the decreased level of CTX from baseline (15). No fatal adverse event was found in the studies of therapy with romosozumab. Seriously adverse of hypersensitivity took place in 7 patients in the romosozumab group in the first 12 months (20). Common adverse events mainly involved neutralizing antibodies developed, injection site reactions.
From the results of the included studies, we knew that alendronate and teriparatide could be good drugs for treatment of osteoporosis. However, alendronate may decrease the level of PINP when it inhibits bone resorption and teriparatide may increase CTX when it promotes bone formation. Romosozumab increased bone formation and reduced bone resorption, the effect of which was also superior to alendronate and teriparatide. In animal test, some researchers (21) examined the effect of romosozumab on bone quality in ovariectomized cynomolgus monkeys. In accordance with the included studies, the result showed that it was increased in bone mass, architecture, and bone strength. In general, the occurrence rate of adverse events was rare with the treatment of romosozumab. Otherwise, the study of Chouinard et al. (22) suggested that romosozumab would not generate a carcinogenic risk to humans. Nevertheless, serious cardiovascular events using romosozumab were a little more than those using alendronate and teriparatide. And this disadvantage will influence physicians to prescribe romosozumab and may remain obstacles to accept the new therapy for patients (23).
Our review firstly systematically showed the effect of romosozumab on osteoporosis and preventing fractures compared with various kinds of drugs or placebo. Compared with the study of Liu et al. (24), our review had more advantages such as the enlarged the enrolled subjects. Because the increased participants would improve the reliability of treatment effect of romosozumab. Except evaluating the changes of BMD, we also paid attention to the variation of bone turnover markers involved PINP and CTX. In addition, assessment of adverse events and safety could not be ignored for any drugs, therefore we review all the descriptive adverse events and safety in the included studies.
There are several limitations of our systematic review. Firstly, we have not analyzed quantificationally the outcome by different doses of romosozumab compared with different drugs at various time points as the studies were still lack. Secondly, most of the included studies derived from Europe and the United States, which resulted in some bias of research participants. Thirdly, due to the short space, we did not present all results of every study and showed the main information in each paper.
Conclusions
Romosozumab increased BMD greater compared with alendronate, teriparatide, or placebo. In addition, romosozumab markedly increased bone formation markers and continually decreased bone resorption markers. However, the cardiovascular effects were main adverse events for romosozumab and the incidence was a bit higher than that with treatment of alendronate or teriparatide. It requires more researches of romosozumab as a therapy for patients with osteoporosis to validate the treatment outcome.
Acknowledgments
The authors thank their colleagues at the Department of Orthopedics, Huzhou Central Hospital and Medical College of Zhejiang University.
Funding: This work was supported by grants from the Science and Technology Program of Zhejiang Province (2017C37119).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.12.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 2014;25:2359-81. [Crossref] [PubMed]
- Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol 2014;54:168-78. [Crossref] [PubMed]
- Yoshimura N, Nakamura K. Epidemiology of Locomotive Organ Disorders and Symptoms: An Estimation Using the Population-Based Cohorts in Japan. Clin Rev Bone Miner Metab 2016;14:68-73. [Crossref] [PubMed]
- Wilk A, Sajjan S, Modi A, et al. Post-fracture pharmacotherapy for women with osteoporotic fracture: analysis of a managed care population in the USA. Osteoporos Int 2014;25:2777-86. [Crossref] [PubMed]
- Graeff C, Campbell GM, Peña J, et al. Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone 2015;81:364-9. [Crossref] [PubMed]
- Krause C, Korchynskyi O, de Rooij K, et al. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 2010;285:41614-26. [Crossref] [PubMed]
- Loots GG, Kneissel M, Keller H, et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 2005;15:928-35. [Crossref] [PubMed]
- Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 2008;23:860-9. [Crossref] [PubMed]
- Li X, Warmington KS, Niu QT, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 2010;25:2647-56. [Crossref] [PubMed]
- Ellies DL, Viviano B, McCarthy J, et al. Bone density ligand, Sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 2006;21:1738-49. [Crossref] [PubMed]
- Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 2016;375:1532-43. [Crossref] [PubMed]
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996;348:1535-41. [Crossref] [PubMed]
- Papapoulos S, Makras P. Selection of antiresorptive or anabolic treatments for postmenopausal osteoporosis. Nat Clin Pract Endocrinol Metab 2008;4:514-23. [Crossref] [PubMed]
- Borggrefe J, Graeff C, Nickelsen TN, et al. Quantitative computed tomographic assessment of the effects of 24 months of teriparatide treatment on 3D femoral neck bone distribution, geometry, and bone strength: results from the EUROFORS study. J Bone Miner Res 2010;25:472-81. [Crossref] [PubMed]
- Saag KG, Petersen J, Brandi ML, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 2017;377:1417-27. [Crossref] [PubMed]
- Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater Gains in Spine and Hip Strength for Romosozumab Compared With Teriparatide in Postmenopausal Women With Low Bone Mass. J Bone Miner Res 2017;32:1956-62. [Crossref] [PubMed]
- Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017;390:1585-94. [Crossref] [PubMed]
- Genant HK, Engelke K, Bolognese MA, et al. Effects of Romosozumab Compared With Teriparatide on Bone Density and Mass at the Spine and Hip in Postmenopausal Women With Low Bone Mass. J Bone Miner Res 2017;32:181-7. [Crossref] [PubMed]
- McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412-20. [Crossref] [PubMed]
- Ishibashi H, Crittenden DB, Miyauchi A, et al. Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: A phase 2 study. Bone 2017;103:209-15. [Crossref] [PubMed]
- Ominsky MS, Boyd SK, Varela A, et al. Romosozumab Improves Bone Mass and Strength While Maintaining Bone Quality in Ovariectomized Cynomolgus Monkeys. J Bone Miner Res 2017;32:788-801. [Crossref] [PubMed]
- Chouinard L, Felx M, Mellal N, et al. Carcinogenicity risk assessment of romosozumab: A review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol 2016;81:212-22. [Crossref] [PubMed]
- Khosla S. Bone diseases: Romosozumab - on track or derailed. Nat Rev Endocrinol 2017;13:697-8. [Crossref] [PubMed]
- Liu Y, Cao Y, Zhang S, et al. Romosozumab treatment in postmenopausal women with osteoporosis: a meta-analysis of randomized controlled trials. Climacteric 2018;21:189-95. [Crossref] [PubMed]
Cite this article as: Chen W, Yang H, Jiang X. Effects of romosozumab on low bone mineral density or osteoporosis in postmenopausal women: a systematic review. Ann Joint 2020;5:18.