
Canine osteosarcoma: where human and canine research intersect
Introduction
Osteosarcoma (OSA) is the most common malignant bone tumor in children with peak incidence during the 2nd decade (1,2). The treatment and outcomes of OSA have largely remained the same over the past several decades with 5-year event-free survival (EFS) of 60–70% for localized disease despite many previous clinical and mechanistic studies to identify novel drug targets (1-3). Many of these studies have utilized cell lines, overutilized primary tumors that do not reflect the original patient disease, or orthotopic mouse models that may not accurately recapitulate human disease (1,4). There is now enthusiasm in comparative oncology and evaluating disease in naturally occurring models, and for OSA, dogs provide an exemplary model of naturally occurring disease that is analogous to human disease.
Canine osteosarcoma
Osteosarcoma is the most common primary bone tumor diagnosed in dogs and is primarily a disease of older dogs with the median/mean age of affected dogs in most studies being > eight years of age (5-8). A bimodal presentation has been appreciated, however, and a second peak of OSA in young dogs under the age of three has been demonstrated. Large breed dogs tend to be overrepresented, with breeds such as the great Dane, rottweiler, golden retriever, Labrador retriever and German shepherd dog being regularly diagnosed (7-10). Most cases of canine OSA manifest in the appendicular skeleton (11), although axial locations, such as the skull, mandible and maxilla and the extracranial flat and irregular bones (i.e., vertebra, rib, sternum, scapula, or pelvis) are also regularly described (12-15); additionally, extraskeletal OSA has been documented in several companion animal reports (16). While small breed dogs have a lower likelihood of developing OSA, they are more likely to develop axial OSA than their large breed counterparts (17).
Osteosarcoma has a characteristic radiographic appearance; appendicular lesions in dogs usually occur in the metaphyseal region and have a mixed osteolytic and osteoproductive radiographic appearance (Figure 1). The most common tumor locations are the distal radius and proximal humerus, but it is not unusual to diagnose OSA in other appendicular locations. Routine staging includes three-view thoracic radiographs to evaluate for pulmonary metastatic disease (Figure 1). Similar to human disease (1), micrometastatic disease is thought to be present in nearly all dogs diagnosed with appendicular OSA, but gross pulmonary metastasis is uncommonly detected at the time of initial diagnosis. Pulmonary and bony metastases occur with approximately equal frequency at the time of initial diagnosis, although metastasis to other locations is possible. Abdominal ultrasound may also be performed to check for metastatic disease (5), particularly in cases with pelvic limb neoplasia, lymphadenopathy, or atypical presentation/clinical findings. Similarly, lymph node aspirates should be performed when lymphadenopathy is noted on physical examination or imaging studies.
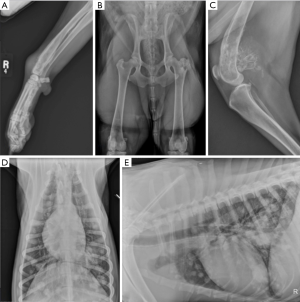
In human OSA, staging analysis identifies metastatic disease in approximately 15–20% of patients (1) and this portends a poor prognosis (18,19). Similarly, approximately 10% of dogs will have radiographically identifiable metastasis at the time of diagnosis; however, this number downplays the overall metastatic potential of OSA in dogs as greater than 90% of dogs will die of pulmonary metastatic disease after treatment of the primary tumor (20-22). Computed tomography (CT) is being increasingly used to assess canine patients for the presence of thoracic metastases (23). While traditional, high-quality radiography is able to detect pulmonary metastases that are approximately 7–9 mm in size, CT has a reported sensitivity for detection of lesions as small as 1 mm. Despite this improved detection of disease, the clinical relevance of smaller metastatic lesions with regard to survival times is uncertain in dogs.
A cytologic or histologic diagnosis of OSA can be pursued via fine-needle aspiration or bone biopsy. In one study evaluating the use of ultrasound-guidance, a diagnostic sample was obtained in 89% of dogs (24). Of the diagnostic samples, cytology indicated sarcoma, with a high sensitivity (97%) and specificity (100%) (24). Pre-treatment biopsy of a suspected OSA lesion is rarely performed in dogs due to the characteristic changes noted on radiographs.
Currently, the recommended approach to treatment of dogs with OSA is aggressive local control of the tumor with surgery or radiation and systemic chemotherapy. A review of the available treatments is below. Additionally, new innovative treatments are being investigated with a goal of benefitting both canine and human patients.
For dogs with appendicular osteosarcoma that are treated with amputation alone, the median survival time (MST) is less than six months (20,21), and the one- and two-year survival rates are only 11.5% and 2% respectively (21). Amputation followed with chemotherapy significantly improves prognosis, with median survival times approximately twice as long (25). Longer survival times are associated with periosteal or parosteal OSA, small stature, and dogs developing surgical site infections after a limb-sparing surgery (8,26,27). Negative prognostic indicators include elevated serum ALP, dogs developing OSA when they are less than five years old, metastatic disease (bone, lymph node or lung), increased tumor necrosis, proximal humeral location, higher grade and larger tumor size (8,28-35).
In one study evaluating a histologic scoring system for canine OSA, nine distinct subtypes were noted based on the type of matrix (osteoid, cartilage and fibrous tissue) present. The osteoblastic subtype predominated being noted in >50% of samples. Furthermore, vascular invasion was common as >70% of primary tumors in that study demonstrated this phenomenon (33). Most of the OSA samples in that study demonstrated characteristics expected of aggressive tumors such as severe to extreme cellular pleomorphism, varying numbers of mitoses and necrosis (33).
Molecular similarity
The similarity in the clinical presentation of canine and human OSA underscores the importance of performing genetic evaluations to compare these two diseases. Due to the expedited clinical course in dogs, if genetic similarities are identified, the canine genetic profile could potentially be utilized to help establish therapeutic or prognostic targets. Several studies have identified genetic alterations that occur in OSA samples, and there exists some commonality between dogs and humans. However, translating these similarities into improved diagnostics and outcomes is still elusive.
In one study comparing canine and pediatric OSA, the genetic signatures of these species clustered together and were indistinguishable (36). In a separate study the comparison of human and canine samples demonstrated that data derived from canine OSA samples may help to group human OSA into molecular subtypes which, in turn, could have a clinical impact (37). More recently, the genetic risk factors associated with the initiation and progression of OSA in a cohort of dogs of different breeds was evaluated as an initial step towards using the dog as a model for human OSA (38). In that study, 33 genomic regions were found to be associated with OSA and several potentially causative genes and pathways were identified (38).
Transcriptome analysis was recently performed in an effort to compare human, mice and canine OSA cell lines (39). Interestingly, dog and mouse OSA samples both correlated with the human OSA samples, but this correlation did not hold for non-OSA tumors (39). These types of findings open the door for further investigation into ways that canine and human OSA can be investigated for mutual benefit.
Many different tumor suppressor pathways and proto-oncogenes are being investigated in canine OSA, and a full review of these are beyond the scope of this article; the reader is encouraged to investigate articles dedicated to this topic (22,40). One particular protein, TP53, involved in a tumor suppressor pathway has demonstrated potential when comparisons between canine and human OSA are made. P53 protein is an important regulator of cell replication, and mutations of p53 lead to an increase in replicative capability of tumor cells (41). TP53 gene mutations have been noted to be common in canine OSA (34), and a strong homology seems to exist between dog and human OSA (42). Additionally, human and canine OSA patients seem to have p53 mutations at similar frequencies (40).
Treatment
Current protocols for treatment of human OSA rely on neoadjuvant chemotherapy, with subsequent surgical resection, followed by adjuvant chemotherapy. The most accepted chemotherapy regimen includes methotrexate, doxorubicin, and cisplatin, given in 2 preoperative cycles and 4 postoperative cycles. Surgical resection is performed with the intent of obtaining tumor-free margins while maintaining the best functional outcome as possible and most often can be done in limb-sparing method. Necrosis in the resected tumor has often been considered a major prognosticator for outcome. Unfortunately, as shown in the EURAMOS-1 study, modifying postoperative chemotherapy for poor-responders has not led to any improvement in survival outcomes for these patients (1,43).
Surgical treatment
Contrary to the surgical management of human OSA, amputation is considered the gold standard surgical option for the management of appendicular OSA in canine patients (27). The decision to pursue limb amputation is typically difficult for owners; however, dogs generally recover well from surgery with good ambulation and high owner satisfaction. Tumors of the thoracic limb are generally treated by forequarter amputation. Forequarter amputation including excision of the scapula is preferred by most surgeons due to the ease of the surgical procedure, the likelihood of wide margins around the disease, and improved cosmetic appearance when the scapula is removed, as muscle atrophy over this bone can be unsightly. Tumors of the scapula or ulna may be treated by partial removals (14,44); however, evaluation of the extent of disease with advanced imaging (i.e., CT) is always recommended. Additional stabilization may be required to improve function, but most dogs tolerate these procedures well.
Pelvic limb tumors are most commonly treated with coxofemoral disarticulation; this surgical technique is generally preferred over a mid-shaft femoral amputation due to cosmesis, ease of technique, and wider surgical margins. Tumors of the proximal femur may be treated by en bloc excision of the acetabulum in addition to amputation or hemipelvectomy. Pelvic tumors can also be treated by partial or total hemipelvectomy (45). In dogs, hemipelvectomy with amputation is tolerated similarly to amputation alone.
Recovery after amputation is generally rapid, with most dogs ambulating within a few days of surgery. Perioperative analgesia is essential and may be provided with a wound soaker catheter or with systemically administered medications. Complications of these surgeries include seroma formation, and less commonly dehiscence, hemorrhage, and infection.
Limb-sparing surgery is described for appendicular OSA in dogs, but appropriate case selection and client education is essential. In general, limb-sparing surgery is reserved for cases in which a dog is unable to tolerate amputation due to orthopedic or neurologic comorbidities or when owners have declined amputation. Several limb-sparing techniques have been developed for dogs, but currently, the major location where these surgeries is pursued is the distal radius as other locations have proven challenging when evaluating functional outcome post-operatively; dogs tend to tolerate pancarpal arthrodesis quite well, likely owing to the high success rate for this procedure in radial OSA cases (27,46,47). Significant complications have been noted with limb-sparing surgery in dogs and include primarily infection, implant failure, and local recurrence (48). Infection rates have been documented to exceed 70% in some studies, and this high rate of infection is likely due to a variety of factors such as impaired vascular supply, minimal soft tissue coverage, usage of implants, and subsequent administration of chemotherapy (48).
Limb-sparing surgery was first demonstrated to be feasible in dogs with OSA in the late 1980s (46). The earliest limb-sparing techniques in dogs involved the placement of cortical allografts after the resection of the tumor (27). In these cases, the tumor and adjacent tissue would be removed en bloc, and a pre-sized cortical allograft would be utilized to fill the gap left from the tumor removal. A limb-sparing bone plate is attached to the cortical allograft as well as the proximal native radius and a metacarpal bone resulting in a pancarpal arthrodesis (Figure 2).
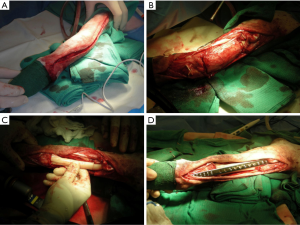
Due to the challenges associated with bone harvesting and the maintenance of bone banks, an endoprosthesis was developed. The earliest design of the endoprosthesis was a construct of 316L surgical steel that was pre-made to a length of 122 mm and included two machined holes. A limb-sparing plate was specifically designed to be used with the endoprosthesis and had 24 holes; proximal holes were made to accommodate 3.5 or 4.5 mm cortical screws for the radius and 2.7 or 3.5 mm cortical screws for the metacarpal bone.
Studies have compared the biomechanical and clinical outcome associated with cortical allograft and endoprosthesis (27,49). The biomechanical comparison was performed as a cadaveric study in which a limb-sparing surgery was performed on canine forelimbs with either cortical allograft or steel endoprosthesis; the authors also assessed whether ulnar salvage was important biomechanically (49). Limbs reconstructed with an endoprosthesis were biomechanically superior to limbs reconstructed with a cortical allograft, however, no significant differences in stiffness or energy to failure between the two groups were noted (49). The ulna was not needed for stability, which allows for surgeons to be more aggressive in attempting to achieve a surgical margin as the ulna can be removed (49). In the clinical study, construct failure was noted in 40% of dogs in both the cortical allograft and endoprosthesis groups; however the mode of failure differed between groups (27). In the cortical allograft group, screw loosening or breakage was noted in the radiocarpal or metacarpal bones. In the endoprosthesis group, screw loosening or breakage occurred in the proximal aspect of the radius. Overall, limb function was determined to be good to excellent in 75% of dogs with no significant difference between the two groups (27). After results of the above studies, a second-generation steel endoprosthesis was developed with the goal of addressing some of the identified issues of the first generation endoprosthesis (48). In a recent study, no significant difference in complication severity, frequency, or time to complication was noted, however, when the two constructs were compared (48).
Other techniques for limb-sparing in dogs have tried to utilize native bone. One such technique, called an ulnar rollover transposition has been described in a few studies (50,51). For this technique, the affected section of radius is removed. Two ulnar osteotomies are performed to create a segment of ulnar that is approximately the same length as the removed section of radius. The goal is to preserve the caudal interosseous artery and vein as well as several of the muscular attachments to improve acceptance of the ulna into the new site. The ulnar graft is rolled into the location where the radial tumor was removed, between the remaining radius and the carpus. A dynamic compression plate is then placed spanning from the remaining segment of radius to the fourth metacarpal bone with two screws also being inserted into the ulnar graft (51). The initial study on the technique demonstrated graft viability and good to excellent function post-operatively in three dogs. In a more recent study describing outcomes in 26 dogs, viability of the graft was relatively high at 85% in those limbs with known outcome (50). However, similar to other limb-sparing techniques, the complication rate with ulnar rollover transposition appears to be high (50). A similar technique in which the manus is translated laterally to allow the ulna to fill in the defect left by the resection of radial OSA has also been described (52). The goal with this procedure is to laterally translate the manus to place the proximal surface of the radiocarpal bone in contact with the distal aspect of the ulna. Bone plates are then placed on the cranial surface of the remaining radius and extending to the dorsal surface of the third metacarpal bone as well as from the lateral aspect of the ulna to the cranial surface of the fourth metacarpal bone (52). The median amount of radius removed was 54% with one dog undergoing removal of 94% of the radius (52).
Other techniques that have been utilized include distraction osteogenesis (53) and autografts that were vascularized (51), irradiated (54) and pasteurized (55). Most recently, three-dimensional printing of endoprostheses has been described (56,57). Early results are promising but further investigation is needed. In one dog, a three-dimensional printed scaffolding was investigated. In this case, no complications associated with the scaffold or surgery occurred and significant improvement was noted in limb function and quality of life (56).
The development of a surgical-site infection (SSI) has been reported to prolong survival times in dogs undergoing limb-sparing surgery in several studies (26,27,58). This was first reported by Thrall et al., in which cases developing infections associated with the allograft had better local control rates and improved survival times (59). In 2005, Lascelles et al. reported on 47 dogs that were treated with limb-sparing surgery and adjuvant chemotherapy. Of the 47 dogs, 32 (68%) developed SSIs, and those dogs were determined to have a survival advantage over dogs not developing an SSI (26). Additionally, dogs with infection were about half as likely to have metastasis diagnosed (26). In a study comparing dogs undergoing cortical allograft limb-sparing and dogs undergoing limb-sparing with a steel endoprosthesis, the median survival time was demonstrated to be longer in those dogs developing infection (685 days) vs. those that did not (289 days). However, there was not a significant difference in the overall infection rate between the two groups, which was an interesting finding as it was thought that the steel endoprosthesis cases may be less likely to develop infection post-operatively due to a lack of allogeneically-induced foreign body reaction (27). In a more recent study evaluating dogs that survived greater than one year after histopathologic disease of OSA, a similar finding to the above studies was found (58). The 20 dogs that developed SSI in that study had significantly longer survival times after 1 year. The median survival time of dogs in the SSI group after 1 year was 180 days (range 25 to 1,899 days) compared to 28 days (range, 8 to 282 days) in the dogs that did not develop a SSI (58). The potential of an SSI to impact median survival time was recently evaluated in a group of dogs that underwent amputation with no form of limb-sparing (60). Neither the disease-free interval or the median survival time were affected when dogs with SSIs were compared to dogs without SSIs in that cohort (60). Interestingly, some human OSA studies have also reported a similar finding of increased survival in patients with SSI after limb salvage reconstructions (61,62). The role of SSI in improving survival outcomes leads to speculation of an auto-immunotherapy mechanism and adds weight to the continued efforts of immunotherapy research in this disease with potential translation to both canine and human patients (63).
Chemotherapeutic treatment
Over the last 25 years, the major impact on prognosis in dogs with OSA has been the addition of adjuvant chemotherapy in the absence of detectable metastatic disease (8,64). However, the approach to chemotherapy administration in dogs differs from human patients, in that chemotherapy in canine OSA patients is generally dosed in a manner and timeline which minimizes the chance of chemotherapy-induced complications. Because of this, chemotherapy administered to dogs with OSA is generally well-tolerated with minimal toxicity (21). Additionally, drugs such as cisplatin and methotrexate, which are regularly utilized in human patients with OSA are not part of the typical OSA protocols in companion animals. High dose methotrexate has not been fully studied in dogs as it is labor intense and costly. While cisplatin, which has a higher toxicity profile, appears to be superior in human OSA, it has not shown a survival advantage over carboplatin in dogs.
In general, chemotherapy protocols with a single agent platinum drug, or alternating carboplatin and doxorubicin have been the most regularly investigated (20,64-69). The alternating protocol has not been shown to be superior to single agent protocols in several studies (64,68). A few studies have made an effort to assess whether a single-agent carboplatin protocol is similarly effective to an alternating protocol of carboplatin and doxorubicin (6). In the first, five protocols were compared: carboplatin administered for 4 or 6 cycles, doxorubicin administered every 14 or 21 days for 5 cycles and alternating carboplatin and doxorubicin (6). After statistically comparing these different protocols, none were demonstrated to provide a significant reduction in the risk of metastasis or death; however, the group of dogs receiving single agent carboplatin for 6 doses had a lower proportion of adverse events as compared to the other protocols (6). A more recent study compared the administration of six doses of carboplatin to three doses each of carboplatin and doxorubicin on an alternating schedule (7). The dogs in the carboplatin alone group had a significantly longer disease-free interval (425 days) as compared to dogs receiving alternating carboplatin and doxorubicin (134 days) (7).
While the addition of chemotherapy to the treatment protocol of dogs with OSA without detectable metastatic disease seems to prolong median survival time, the impact of chemotherapy in the treatment of metastatic disease is less well-understood. Traditional drugs used in dogs with OSA have been shown to be mostly ineffective (70). Recently, ifosfamide was administered to a group of dogs with OSA with visible metastatic disease (71). While only 17/19 dogs were available for response assessment, the results were disappointing in that an 11.8% response rate was noted (71). The drug was mostly well-tolerated, but the median survival time from the first dose was only 95 days (71). Similarly, the use of a small molecule receptor tyrosine kinase inhibitor (toceranib) demonstrated minimal response in dogs with macroscopic pulmonary metastasis (72).
Aerosolized chemotherapy has been considered as a treatment modality for dogs with pulmonary metastatic disease as well due to the potential to increase local concentration of the drug and decrease systemic toxicity (73,74). Aerosolized gemcitabine was administered to 20 dogs with OSA via a compressor with nebulizer. The administrations were well-tolerated as side effects were minimal; in particular, arterial blood gas and alveolar-arterial gradients did not vary from base line in any dog and no gastrointestinal toxicity was reported (73). When the quality of life was deemed to be diminished or unacceptable, the dog was humanely euthanatized and sections of the lungs were obtained for evaluation. Histologically, minimal airway and lung toxicity were noted secondary to the gemcitabine; however, necrosis was noted intra-tumorally in all dogs opening the door for further investigation in dogs in the future (73). In a separate study where 10 of 28 dogs were diagnosed with OSA, dogs received inhaled doxorubicin and/or paclitaxel (74). The response rate was low at 25%, but again, no systemic toxicity was noted. Paclitaxel was also tolerated locally in the lung, but about 50% of dogs receiving doxorubicin experienced an intermittent, non-productive cough (74).
Immunotherapeutics are now transforming the landscape in the treatment of many types of human cancers, and multiple modalities are being explored in veterinary cases as well. The activation of macrophages and monocytes is an attractive option when considering therapies for micrometastatic disease in dogs with OSA (75,76). Macrophages and monocytes can be activated by liposome-encapsulated immunomodulating agents to target neoplastic cells for destruction (75). One such agent that has been investigated in several studies of canine OSA is liposome-encapsulated muramyl tripeptide-phosphatidylethanolamine (L-MTP-PE) (75-78). When administered intravenously in dogs, L-MTP-PE is well-tolerated, with the major side effect being a minor increase in body temperature (75,76). The earliest study of the use of L-MTP-PE in dogs with OSA was performed in a randomized, double-blinded fashion. These dogs first underwent limb amputation and were then randomized into either a group receiving L-MTP-PE or a group receiving a placebo (76). Dogs receiving L-MTP-PE survived significantly longer (222 days) as compared to dogs receiving placebo (77 days) (76). Also of note, in the L-MTP-PE group, four dogs were still alive and free of metastasis >1 year after surgery (76).
Follow-up studies began to evaluate the impact of chemotherapy in dogs undergoing treatment with L-MTP-PE (75,78). In the first study, the addition of L-MTP-PE was evaluated in dogs undergoing amputation of the primary tumor followed by four treatments with cisplatin (75). After completion of chemotherapy, dogs were randomized into either a group receiving a placebo or L-MTP-PE. Dogs receiving L-MTP-PE had a significantly longer median disease-free interval and median survival time as compared to dogs receiving placebo (75). In two randomized, double-blind clinical trials published a year later, dogs receiving L-MTP-PE (after amputation and cisplatin) were again shown to survive longer than dogs receiving a placebo (78). Administering L-MTP-PE concurrently with cisplatin did not demonstrate a survival advantage, however (78). L-MTP-PE has also been studied in human OSA with reported improvement in EFS in both localized and metastatic disease (3,79,80). Findings remain somewhat controversial and while L-MTP-PE (mifamurtide) is approved in Europe, it was denied approval by the US FDA (1).
Interleukin 2 (IL-2) has the potential to modulate the immune response to cause targeting of cancer cells, and for this reason therapeutic use of IL-2 is being investigated (25,81,82). Due to a narrow therapeutic index and potential for side effects, the aerosolized administration of IL-2 presents an attractive option to investigate. The use of aerosolized IL-2 as an immunotherapeutic has particular potential in treating pulmonary metastatic disease as local concentrations can be increased and systemic side effects can ideally be minimized. A case series described nebulized IL-2 liposome therapy, in a cohort of dogs diagnosed with pulmonary metastases or primary lung carcinoma (83). Of that group, two of four dogs with metastatic pulmonary OSA had complete regression of metastatic disease and regression was stable for more than 12 months and more than 20 months, respectively, in those two cases (83). Additionally, toxicity associated with IL-2 therapy was considered minimal (83). IL-2 as a stimulant of NK cells in the treatment of lung metastasis is also a target of study for human OSA. This has been evaluated in mouse model in vivo studies with demonstrated augmented NK cell killing of pulmonary nodules (84).
A landmark study was published in 2016 describing the administration of highly attenuated, recombinant Listeria monocytogenes expressing a chimeric human HER2/neu fusion protein (ADXS31-164) to dogs with OSA who had undergone amputation or limb-sparing surgery plus adjuvant chemotherapy (85). The objectives of that study “were to determine the safety of ADXS31-164 and its ability to generate HER2/neu-specific immunity in dogs with spontaneous osteosarcoma following amputation and adjuvant carboplatin chemotherapy” and “to determine whether ADXS31-164, administered in the setting of minimal residual disease, would prevent metastatic disease and prolong overall survival (85).” Dogs undergoing either amputation or limb-sparing surgery who also received four doses of carboplatin chemotherapy were enrolled. Toxicity associated with the drug administration was considered transient and low-grade. This study demonstrated that an antigen-specific IFNγ response was generated against the intracellular domain of HER2/neu in 15/18 dogs within 6 months of treatment secondary to ADXS31-164 administration (85). Impressively, 1-, 2-, and 3-year survival rates for dogs treated with ADXS31-164 were 77.8%, 67%, and 56%, respectively (85). Future work is underway to further investigate the findings of this promising study.
Radiation therapy
Radiation therapy (RT), administered in different forms, can be used to treat local disease in canine OSA cases. Full course curative intent RT is not routinely pursued in dogs due to the cost, side effects to adjacent tissue, and lack of benefit when compared with standard surgical treatment (86). Palliative RT may also be pursued for pain relief in cases where surgery is not possible or owners do not elect for aggressive therapy. Generally, palliative RT involves the delivery of several large doses of radiation in 2-4 fractions (87). Decrease in inflammation, slowing of osteolysis, and reduction in tumor size are all benefits associated with palliative radiation therapy. Over 50% of patients respond to therapy, with onset of pain relief in 1–2 weeks and improvement lasting approximately 2–3 months (87).
Stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) has been described in the management of canine appendicular OSA in several studies (88-91), and more recently, in the treatment of vertebral OSA (92). The development of fractures after SRS/SRT in dogs is a major concern, thus appropriate patient selection is of the utmost importance. The region of bone particularly affected by tumor has been shown to be an important factor in the determination of bone fracture after radiotherapy; dogs with subchondral bone involvement had a median time to fracture of 4.2 months as compared to dogs without subchondral bone involvement which had a median time to fracture of 16.3 months (91).
Due to the concern for fracture secondary to OSA and compounding with RT, stabilization was performed concurrently with SRT in one study (88). In that cohort using SRT in combination with stabilization utilizing a bone plate or interlocking nail, the complication rate was shown to be exceptionally high; major complications (e.g., infection, fracture) were reported to occur in approximately 90% of dogs (88). In a separate study, six dogs with pathologic fracture either prior to or after SRS were treated with internal fixation (89). Infection and implant failure rates were high, but limb function was considered good when implants were stable, and infections were subclinical (58).
Three studies have combined intra-arterial chemotherapy with radiation therapy in the treatment of canine OSA (93-95). In an appendicular OSA study, client-owned dogs were treated with intra-arterial cisplatin (2 doses, 21 days apart) and the majority also received radiation therapy (95). Median survival time in these dogs was 9.3 months. The authors noted that the survival time for these dogs was longer than would be expected for amputation alone, suggesting that there was a survival benefit to the intra-arterial chemotherapy (95). Dogs with >75% tumor necrosis had significantly lower recurrence rates at 1 year (15%) versus dogs with <75% tumor necrosis (65%) (95). In a separate study comparing different treatments for canine OSA including intra-arterial chemotherapy alone and intra-arterial chemotherapy with radiation therapy, the percent of tumor necrosis was 49.1% and 83.7%, respectively, for those treatment categories (94). That study demonstrated that a radiation dose of 28.1 Gy caused 80% tumor necrosis when combined with intra-arterial chemotherapy versus a dose of 42.2 Gy when radiation was the sole therapy (94). Interestingly for human patients, the established tumor necrosis goal in resected specimens after the standard neoadjuvant chemotherapeutic regimen with methotrexate, cisplatin, and doxorubicin is >90%. Another canine study documented a median survival time of 6.7 months in dogs receiving intra-arterial cisplatin chemotherapy in conjunction with radiation therapy as an alternative to amputation or limb-sparing surgery (93). Intra-arterial chemotherapy has also been utilized in combination with limb-sparing surgeries (46,57).
Summary
Osteosarcoma is naturally occurring in dogs with striking similarity to human disease in clinical course, genetics, and treatment strategies. While the benefits of comparing OSA in these species is obvious, there are inherent challenges with conducting clinical trials in canine patients. Dogs are considered pets, and in many cases, family members, which may lead to challenges in treatment decision-making for their caregivers. Therefore, risks and potential benefits for canine patients must be taken into account during study design, in order to assure regulatory approval and accrual. Similarly, informed owner consent is absolutely essential. Additionally, the financial burden of treatment of OSA in dogs generally falls on the caregiver, and inherent limitations may exist due to the cost of care. However, the understanding that advances in the management of OSA in dogs also has the potential to benefit humans has greatly progressed the opportunity to conduct high-quality, impactful clinical trials in recent years.
Current and future studies will likely continue to explore immunotherapeutic approaches including immunomodulation and adoptive immune cell transfer as well as targeting treatment resistant cell populations such as cancer stem cells. Furthermore, tumor microenvironment studies will be of interest in further understanding tumorigenesis and metastagenesis. Through comparative studies, new targets, new strategies, and novel therapeutics can be developed in collaborative fashion with hope of improving outcomes for both human and canine patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.11.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harrison DJ, Schwartz CL. Osteogenic sarcoma: systemic chemotherapy options for localized disease. Curr Treat Options Oncol 2017;18:24. [Crossref] [PubMed]
- Luetke A, Meyers PA, Lewis I, et al. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev 2014;40:523-32. [Crossref] [PubMed]
- Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23:2004-11. [Crossref] [PubMed]
- Jacques C, Renema N, Lezot F, et al. Small animal models for the study of bone sarcoma pathogenesis: characteristics, therapeutic interests and limitations. J Bone Oncol 2018;12:7-13. [Crossref] [PubMed]
- Sacornrattana O, Dervisis NG, McNiel EA. Abdominal ultrasonographic findings at diagnosis of osteosarcoma in dogs and association with treatment outcome. Vet Comp Oncol 2013;11:199-207. [Crossref] [PubMed]
- Selmic LE, Burton JH, Thamm DH, et al. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med 2014;28:554-63. [Crossref] [PubMed]
- Skorupski KA, Uhl JM, Szivek A, et al. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol 2016;14:81-7. [Crossref] [PubMed]
- Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med 1996;10:76-81. [Crossref] [PubMed]
- Story AL, Boston SE, Kilkenny JJ, et al. Evaluation of weight change during carboplatin therapy in dogs with appendicular osteosarcoma. J Vet Intern Med 2017;31:1159-62. [Crossref] [PubMed]
- Sapierzyński R, Czopowicz M. The animal-dependent risk factors in canine osteosarcomas. Pol J Vet Sci 2017;20:293-8. [Crossref] [PubMed]
- Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 2014;55:69-85. [Crossref] [PubMed]
- Gold R, Oliveira F, Pool R. Zygomatic arch parosteal osteosarcoma in dogs and a cat. Vet Pathol 2019;56:274-6. [Crossref] [PubMed]
- Kruse MA, Holmes ES, Balko JA, et al. Evaluation of clinical and histopathologic prognostic factors for survival in canine osteosarcoma of the extracranial flat and irregular bones. Vet Pathol 2013;50:704-8. [Crossref] [PubMed]
- Montinaro V, Boston SE, Buracco P, et al. Clinical outcome of 42 dogs with scapular tumors treated by scapulectomy: a Veterinary Society of Surgical Oncology (VSSO) retrospective study (1995-2010). Vet Surg 2013;42:943-50. [Crossref] [PubMed]
- Selmic LE, Lafferty MH, Kamstock DA, et al. Outcome and prognostic factors for osteosarcoma of the maxilla, mandible, or calvarium in dogs: 183 cases (1986-2012). J Am Vet Med Assoc 2014;245:930-8. [Crossref] [PubMed]
- Duffy D, Selmic LE, Kendall AR, et al. Outcome following treatment of soft tissue and visceral extraskeletal osteosarcoma in 33 dogs: 2008-2013. Vet Comp Oncol 2017;15:46-54. [Crossref] [PubMed]
- Rebhun RB, Kass PH, Kent MS, et al. Evaluation of optimal water fluoridation on the incidence and skeletal distribution of naturally arising osteosarcoma in pet dogs. Vet Comp Oncol 2017;15:441-9. [Crossref] [PubMed]
- Kaste SC, Pratt CB, Cain AM, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer 1999;86:1602-8. [Crossref] [PubMed]
- Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am 1997;44:973-89. [Crossref] [PubMed]
- Bacon NJ, Ehrhart NP, Dernell WS, et al. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma: 50 cases (1999-2006). J Am Vet Med Assoc 2008;232:1504-10. [Crossref] [PubMed]
- Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988). J Am Vet Med Assoc 1992;200:995-9. [PubMed]
- Varshney J, Scott MC, Largaespada DA, et al. Understanding the osteosarcoma pathobiology: A comparative oncology approach. Vet Sci 2016;3: [Crossref] [PubMed]
- Oblak ML, Boston SE, Woods JP, et al. Comparison of concurrent imaging modalities for staging of dogs with appendicular primary bone tumours. Vet Comp Oncol 2015;13:28-39. [Crossref] [PubMed]
- Britt T, Clifford C, Barger A, et al. Diagnosing appendicular osteosarcoma with ultrasound-guided fine-needle aspiration: 36 cases. J Small Anim Pract 2007;48:145-50. [Crossref] [PubMed]
- Rodriguez CO Jr. Using canine osteosarcoma as a model to assess efficacy of novel therapies: can old dogs teach us new tricks? Adv Exp Med Biol 2014;804:237-56. [Crossref] [PubMed]
- Lascelles BD, Dernell WS, Correa MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol 2005;12:1073-83. [Crossref] [PubMed]
- Liptak JM, Dernell WS, Ehrhart N, et al. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg 2006;35:518-33. [Crossref] [PubMed]
- Boerman I, Selvarajah GT, Nielen M, et al. Prognostic factors in canine appendicular osteosarcoma - a meta-analysis. BMC Vet Res 2012;8:56. [Crossref] [PubMed]
- Hillers KR, Dernell WS, Lafferty MH, et al. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986-2003). J Am Vet Med Assoc 2005;226:1364-7. [Crossref] [PubMed]
- Ehrhart N, Dernell WS, Hoffmann WE, et al. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990-1996). J Am Vet Med Assoc 1998;213:1002-6. [PubMed]
- Garzotto CK, Berg J, Hoffmann WE, et al. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med 2000;14:587-92. [Crossref] [PubMed]
- Coyle VJ, Rassnick KM, Borst LB, et al. Biological behaviour of canine mandibular osteosarcoma. A retrospective study of 50 cases (1999-2007). Vet Comp Oncol 2015;13:89-97. [Crossref] [PubMed]
- Kirpensteijn J, Kik M, Rutteman GR, et al. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol 2002;39:240-6. [Crossref] [PubMed]
- Kirpensteijn J, Kik M, Teske E, et al. TP53 gene mutations in canine osteosarcoma. Vet Surg 2008;37:454-60. [Crossref] [PubMed]
- Schmidt AF, Nielen M, Klungel OH, et al. Prognostic factors of early metastasis and mortality in dogs with appendicular osteosarcoma after receiving surgery: an individual patient data meta-analysis. Prev Vet Med 2013;112:414-22. [Crossref] [PubMed]
- Paoloni M, Davis S, Lana S, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics 2009;10:625. [Crossref] [PubMed]
- Scott MC, Sarver AL, Gavin KJ, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone 2011;49:356-67. [Crossref] [PubMed]
- Karlsson EK, Sigurdsson S, Ivansson E, et al. Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B. Genome Biol 2013;14:R132. [Crossref] [PubMed]
- Scott MC, Temiz NA, Sarver AE, et al. Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression, and survival in osteosarcoma. Cancer Res 2018;78:326-37. [Crossref] [PubMed]
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res 2007;27:155-64. [PubMed]
- O'Brien MG, Straw RC, Withrow SJ, et al. Resection of pulmonary metastases in canine osteosarcoma: 36 cases (1983-1992). Vet Surg 1993;22:105-9. [Crossref] [PubMed]
- Nasir L, Argyle DJ, McFarlane ST, et al. Nucleotide sequence of a highly conserved region of the canine p53 tumour suppressor gene. DNA Seq 1997;8:83-6. [Crossref] [PubMed]
- Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol 2016;17:1396-408. [Crossref] [PubMed]
- Sivacolundhu RK, Runge JJ, Donovan TA, et al. Ulnar osteosarcoma in dogs: 30 cases (1992-2008). J Am Vet Med Assoc 2013;243:96-101. [Crossref] [PubMed]
- Bray JP, Worley DR, Henderson RA, et al. Hemipelvectomy: outcome in 84 dogs and 16 cats. A Veterinary Society of Surgical Oncology retrospective study. Vet Surg 2014;43:27-37. [Crossref] [PubMed]
- LaRue SM, Withrow SJ, Powers BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc 1989;195:1734-44. [PubMed]
- Kuntz CA, Asselin TL, Dernell WS, et al. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg 1998;27:417-22. [Crossref] [PubMed]
- Mitchell KE, Boston SE, Kung M, et al. Outcomes of limb-sparing surgery using two generations of metal endoprosthesis in 45 dogs with distal radial osteosarcoma. A Veterinary Society of Surgical Oncology retrospective study. Vet Surg 2016;45:36-43. [Crossref] [PubMed]
- Liptak JM, Ehrhart N, Santoni BG, et al. Cortical bone graft and endoprosthesis in the distal radius of dogs: a biomechanical comparison of two different limb-sparing techniques. Vet Surg 2006;35:150-60. [Crossref] [PubMed]
- Séguin B, O'Donnell MD, Walsh PJ, et al. Long-term outcome of dogs treated with ulnar rollover transposition for limb-sparing of distal radial osteosarcoma: 27 limbs in 26 dogs. Vet Surg 2017;46:1017-24. [Crossref] [PubMed]
- Séguin B, Walsh PJ, Mason DR, et al. Use of an ipsilateral vascularized ulnar transposition autograft for limb-sparing surgery of the distal radius in dogs: an anatomic and clinical study. Vet Surg 2003;32:69-79. [PubMed]
- Séguin B, Walsh PJ, Ehrhart EJ, et al. Lateral manus translation for limb-sparing surgery in 18 dogs with distal radial osteosarcoma in dogs. Vet Surg 2019;48:247-56. [Crossref] [PubMed]
- Ehrhart N. Longitudinal bone transport for treatment of primary bone tumors in dogs: technique description and outcome in 9 dogs. Vet Surg 2005;34:24-34. [Crossref] [PubMed]
- Boston SE, Duerr F, Bacon N, et al. Intraoperative radiation for limb sparing of the distal aspect of the radius without transcarpal plating in five dogs. Vet Surg 2007;36:314-23. [Crossref] [PubMed]
- Morello E, Vasconi E, Martano M, et al. Pasteurized tumoral autograft and adjuvant chemotherapy for the treatment of canine distal radial osteosarcoma: 13 cases. Vet Surg 2003;32:539-44. [Crossref] [PubMed]
- Choi S, Oh YI, Park KH, et al. New clinical application of three-dimensional-printed polycaprolactone/beta-tricalcium phosphate scaffold as an alternative to allograft bone for limb-sparing surgery in a dog with distal radial osteosarcoma. J Vet Med Sci 2019;81:434-9. [Crossref] [PubMed]
- Séguin B, Pinard C, Lussier B, et al. Limb-sparing in dogs using patient-specific, 3 dimensional-printed endoprosthesis for distal radial osteosarcoma: a pilot study. Vet Comp Oncol 2020;18:92-104. [PubMed]
- Culp WT, Olea-Popelka F, Sefton J, et al. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997-2008). J Am Vet Med Assoc 2014;245:1141-6. [Crossref] [PubMed]
- Thrall DE, Withrow SJ, Powers BE, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: a dose response assay. Int J Radiat Oncol Biol Phys 1990;18:1351-7. [Crossref] [PubMed]
- Hans EC, Pinard C, van Nimwegen SA, et al. Effect of surgical site infection on survival after limb amputation in the curative-intent treatment of canine appendicular osteosarcoma: a Veterinary Society of Surgical Oncology retrospective study. Vet Surg 2018;47:E88-96. [Crossref] [PubMed]
- Chen YU, Xu SF, Xu M, et al. Postoperative infection and survival in osteosarcoma patients: Reconsideration of immunotherapy for osteosarcoma. Mol Clin Oncol 2015;3:495-500. [Crossref] [PubMed]
- Jeys LM, Grimer RJ, Carter SR, et al. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol 2007;14:2887-95. [Crossref] [PubMed]
- Sottnik JL. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother 2010;59:367-78. [Crossref] [PubMed]
- Phillips B, Powers BE, Dernell WS, et al. Use of single-agent carboplatin as adjuvant or neoadjuvant therapy in conjunction with amputation for appendicular osteosarcoma in dogs. J Am Anim Hosp Assoc 2009;45:33-8. [Crossref] [PubMed]
- Berg J, Weinstein MJ, Schelling SH, et al. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb-sparing surgery: 22 cases (1987-1990). J Am Vet Med Assoc 1992;200:2005-8. [PubMed]
- Frimberger AE, Chan CM, Moore AS. Canine osteosarcoma treated by post-amputation sequential accelerated doxorubicin and carboplatin chemotherapy: 38 cases. J Am Anim Hosp Assoc 2016;52:149-56. [Crossref] [PubMed]
- Kent MS, Strom A, London CA, et al. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med 2004;18:540-4. [Crossref] [PubMed]
- Bailey D, Erb H, Williams L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med 2003;17:199-205. [Crossref] [PubMed]
- Straw RC, Withrow SJ, Richter SL, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med 1991;5:205-10. [Crossref] [PubMed]
- Boston SE, Ehrhart NP, Dernell WS, et al. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985-2004). J Am Vet Med Assoc 2006;228:1905-8. [Crossref] [PubMed]
- Batschinski K, Dervisis NG, Kitchell BE. Evaluation of ifosfamide salvage therapy for metastatic canine osteosarcoma. Vet Comp Oncol 2014;12:249-57. [Crossref] [PubMed]
- Kim C, Matsuyama A, Mutsaers AJ, et al. Retrospective evaluation of toceranib (Palladia) treatment for canine metastatic appendicular osteosarcoma. Can Vet J 2017;58:1059-64. [PubMed]
- Rodriguez CO Jr, Crabbs TA, Wilson DW, et al. Aerosol gemcitabine: preclinical safety and in vivo antitumor activity in osteosarcoma-bearing dogs. J Aerosol Med Pulm Drug Deliv 2010;23:197-206. [Crossref] [PubMed]
- Hershey AE, Kurzman ID, Forrest LJ, et al. Inhalation chemotherapy for macroscopic primary or metastatic lung tumors: proof of principle using dogs with spontaneously occurring tumors as a model. Clin Cancer Res 1999;5:2653-9. [PubMed]
- MacEwen EG, Kurzman ID, Helfand S, et al. Current studies of liposome muramyl tripeptide (CGP 19835A lipid) therapy for metastasis in spontaneous tumors: a progress review. J Drug Target 1994;2:391-6. [Crossref] [PubMed]
- MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst 1989;81:935-8. [Crossref] [PubMed]
- Kurzman ID, Cheng H, MacEwen EG. Effect of liposome-muramyl tripeptide combined with recombinant canine granulocyte colony-stimulating factor on canine monocyte activity. Cancer Biother 1994;9:113-21. [Crossref] [PubMed]
- Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res 1995;1:1595-601. [PubMed]
- Mori K, Ando K, Heymann D. Liposomal muramyl tripeptide phosphatidyl ethanolamine: a safe and effective agent against osteosarcoma pulmonary metastases. Expert Rev Anticancer Ther 2008;8:151-9. [Crossref] [PubMed]
- Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children's Oncology Group. J Clin Oncol 2008;26:633-8. [Crossref] [PubMed]
- Khanna C, Hasz DE, Klausner JS, et al. Aerosol delivery of interleukin 2 liposomes is nontoxic and biologically effective: canine studies. Clin Cancer Res 1996;2:721-34. [PubMed]
- Khanna C, Waldrep JC, Anderson PM, et al. Nebulized interleukin 2 liposomes: aerosol characteristics and biodistribution. J Pharm Pharmacol 1997;49:960-71. [Crossref] [PubMed]
- Khanna C, Anderson PM, Hasz DE, et al. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer 1997;79:1409-21. [Crossref] [PubMed]
- Guma SR, Lee DA, Yu L, et al. Natural killer cell therapy and aerosol interleukin-2 for the treatment of osteosarcoma lung metastasis. Pediatr Blood Cancer 2014;61:618-26. [Crossref] [PubMed]
- Mason NJ, Gnanandarajah JS, Engiles JB, et al. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res 2016;22:4380-90. [Crossref] [PubMed]
- Walter CU, Dernell WS, LaRue SM, et al. Curative-intent radiation therapy as a treatment modality for appendicular and axial osteosarcoma: a preliminary retrospective evaluation of 14 dogs with the disease. Vet Comp Oncol 2005;3:1-7. [Crossref] [PubMed]
- Pagano C, Boudreaux B, Shiomitsu K. Safety and toxicity of an accelerated coarsely fractionated raditiaon protocol for treatment of appendicular osteosarcoma in 14 dogs: 10 Gy x 2 fractions. Vet Radiol Ultrasound 2016;57:551-6. [Crossref] [PubMed]
- Boston SE, Vinayak A, Lu X, et al. Outcome and complications in dogs with appendicular primary bone tumors treated with stereotactic radiotherapy and concurrent surgical stabilization. Vet Surg 2017;46:829-37. [Crossref] [PubMed]
- Covey JL, Farese JP, Bacon NJ, et al. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet Surg 2014;43:174-81. [Crossref] [PubMed]
- Farese JP, Milner R, Thompson MS, et al. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc 2004;225:1567-1572. [Crossref] [PubMed]
- Kubicek L, Vanderhart D, Wirth K, et al. Association between computed tomographic characteristics and fractures following stereotactic radiosurgery in dogs with appendicular osteosarcoma. Vet Radiol Ultrasound 2016;57:321-30. [Crossref] [PubMed]
- Swift KE, LaRue SM. Outcome of 9 dogs treated with stereotactic radiation therapy for primary or metastatic vertebral osteosarcoma. Vet Comp Oncol 2018;16:E152-8. [Crossref] [PubMed]
- Heidner GL, Page RL, McEntee MC, et al. Treatment of canine appendicular osteosarcoma using cobalt 60 radiation and intraarterial cisplatin. J Vet Intern Med 1991;5:313-6. [Crossref] [PubMed]
- Powers BE, Withrow SJ, Thrall DE, et al. Percent tumor necrosis as a predictor of treatment response in canine osteosarcoma. Cancer 1991;67:126-34. [Crossref] [PubMed]
- Withrow SJ, Thrall DE, Straw RC, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer 1993;71:2484-90. [Crossref] [PubMed]
Cite this article as: Culp WTN, Rebhun RB, Alvarez EM, Malogolowkin MM, Randall RL, Thorpe SW. Canine osteosarcoma: where human and canine research intersect. Ann Joint 2020;5:30.


