Treatment of subchondral insufficiency fracture of the knee by subchondroplasty
Introduction
The notion of subchondral insufficiency fracture is relatively recent; it was derived from the reassessment of the pathophysiology of spontaneous osteonecrosis of the knee (SONK). The main cause of SONK is currently considered to be microfractures of the trabecular bone due to the inability of weakened subchondral bone to tolerate the physiological load to which it is subjected (1). This understanding is based on a study by Yamamoto et al. (2), who detected subchondral bone fractures in the femoral condyle of patients diagnosed with osteonecrosis (1). Since this study, subchondral insufficiency fracture has been increasingly studied, including the identification of risk factors and associated lesions (3).
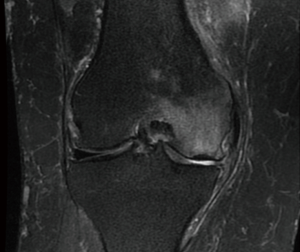
The typical findings of subchondral insufficiency fracture of the knee on magnetic resonance imaging (MRI) include cortical thickening associated with high-intensity signal of the adjacent cancellous bone, which is known as “bone edema”. Additionally, a low signal intensity line (fracture line) might be observed on the subchondral bone (Figure 1). More advanced cases might exhibit flattening or even collapse of the articular surface and consequent degenerative abnormalities (1).
Although widely used in clinical practice, the term “bone edema” is not strictly correct, as histological studies of high signal intensity cancellous bone lesions on MRI have indicated subchondral bone insufficiency with thinner and less dense trabeculae, while local edema was not detected. Therefore, this abnormality is currently called a “bone marrow lesion”.
Subchondral insufficiency fracture usually occurs in association with degenerative joint lesions, including involvement of the meniscus and/or cartilage (2,4-8). Meniscus root or radial tears are frequently associated with subchondral insufficiency fractures because this type of lesion abruptly increases the load on the subchondral bone due to loss of the meniscus’ ability to absorb impact (9-11).
This new understanding raised questions regarding the need for and indication of treatments for subchondral insufficiency fracture. According to various suggestions, conservative treatment is mainly based on restricting the load to the affected limb combined with use of anti-inflammatory and analgesic agents for 3 to 8 months according to the progression of symptoms and radiological abnormalities (4,12). However, some patients do not respond well to nonsurgical treatment, and pain and dysfunction persist over a long period of time, with progression to articular surface depression and degeneration (6,8,13).
Therefore, a new surgical method was developed to afford mechanical stabilization and stimulate the recovery of the local trabecular bone. This technique, called subchondroplasty, consists of injecting a bone substitute paste into the fracture, which once applied undergoes endothermic crystallization, which causes the paste to harden and acquire a consistency adequate to afford mechanical support to the involved area. The bone substitute provides a porous understructure to the blood vessels and newly formed bone and thus exhibits osteoconductive properties. The material is gradually absorbed at a speed that allows for bone regeneration (14,15).
The early clinical results of the first case series in which subchondroplasty was performed for cases of osteoarthritis with bone marrow lesion showed significant improvement of both pain and function (14,15).
Therefore, the aim of the present study was to prospectively assess subchondroplasty for the treatment of subchondral insufficiency fracture in terms of applicability, safety, and results relative to pain and the function of the Knee Injury and Osteoarthritis Outcome Score (KOOS) after at least 6 months of follow-up.
Methods
The present study was conducted at a tertiary university hospital. Approval was granted by the institutional scientific and ethics committees. All participants signed an informed consent form. All patients diagnosed with subchondral insufficiency fracture of the knee from January 2015 through June 2017 were prospectively included, resulting in a total of 11 participants. The diagnosis was based on occurrence of sudden onset pain in the medial or lateral femorotibial compartment, in the absence of trauma, associated with a high signal intensity bone marrow lesion adjacent to low signal intensity cortical thickening on T2-weighted MRI results (Figure 1). For inclusion, patients were required to have undergone conservative treatment, including load restriction for at least 3 months, without response. Only patients with at least 6 months of follow-up were included. The exclusion criteria were osteoarthritis of the knee classified as greater than Kellgren-Lawrence grade 3, articular surface collapse greater than 5 mm, degenerative radiological abnormalities on the patellofemoral joint associated with pain on the anterior side of the knee, and lack of the clinical conditions required for surgical treatment.
For assessment of safety, local or systemic complications associated with the procedure were actively investigated clinically. Patients were assessed based on a pain visual analogue scale (VAS) and the KOOS score (16,17). The epidemiological characteristics of the patients are described in Table 1.

Full table
Description of the surgical technique
The MRI findings were mapped in the operating room to define the lesion area and plan the injection at the center of the lesion and the trajectory and entry points of the cannula. The trajectory of the cannula was established by prioritizing an adequate angle of attack for the entry point and large trajectory distances inside the bone to avoid leakage of the bone substitute through the cannula entry hole.
All procedures were performed in the operating room under spinal anesthesia. The patients were placed in supine position on a radiolucent table, with a cushion under the ipsilateral hip for better control of the external rotation of the limb and another cushion under the ipsilateral knee to improve the conditions for lateral fluoroscopy by avoiding overlapping with the image of the contralateral knee.
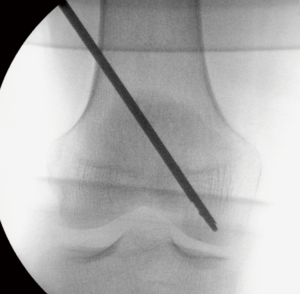
According to the surgical planning and with the aid of fluoroscopy in the frontal and lateral views, the entry point of the cannula was established, and the cannula was placed on the skin. A 5-mm incision was made at the entry point on the skin. The cannula was introduced under fluoroscopic guidance toward the preset center of the lesion (Figure 2) and placed manually or using a mallet in the case of considerable resistance.
Fluoroscopy was performed during the insertion of the cannula in oblique views to avoid perforating the opposite cortical bone. Particular care was taken during the insertion of the cannula when it came in close proximity to the opposite cortical bone, and eventually, a blunt cannula was used to reduce the chance of perforation.
The bone substitute was then prepared. The solid part was mixed with the fluid part until a fluid/pasty consistency was obtained, and the mixture was transferred to 1 mL syringes. The syringes were connected to the cannula previously placed at the site selected for filling. The bone substitute was injected in alternation with a passage of a metallic piston through the cannula between injections. Fluoroscopy allowed visualization of the distribution of the compound across the bone marrow, thus ensuring that the application matched the lesion mapping and avoided possible leakage. The amount of bone substitute injected was determined based on the degree of filling as assessed by fluoroscopy. The cannula was removed 5 minutes after the end of injection. The Graftys® HBS (Graftys, Aix-en-Provence, France) bone substitute was used.
The patients remained in the hospital until the following day. Full weight bearing was allowed as tolerated with free range of motion; analgesics were administered as needed.
Assessment of MRI results
All participants underwent at least one 1.5 T MRI scan before the procedure. T2-weighted images were assessed for lesion size and cortical thickness in the coronal and sagittal planes (18).
Statistical analysis
Statistical analysis of the KOOS and VAS scores before and after surgery was performed using the Wilcoxon signed rank test with Stata 14.2 software (StataCorp, Texas, USA).
Results
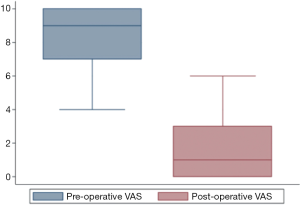
The KOOS score increased from 43.0 (35.1–48.2) before surgery to 89.9 (89.3–94.0) [median (interquartile range), P=0.0033] after surgery, and the VAS score decreased from 9 [7–10] to 1 [0–3] [median (interquartile range), P=0.0032], as shown in Figures 3,4.

The lesions were located on the medial femoral condyle in 8 (72.7%) cases and on the medial tibial plateau in 3 cases (27.3%). Two (18.18%) participants were classified as Kellgren-Lawrence grade 1, 6 (54.55%) were classified as grade 2, and 3 (27.3%) were classified as grade 3. All the participants reported satisfaction with the results of the procedure.
The average size of the lesions was 14.9±5 mm in the coronal plane and 22.0±4.5 mm in the sagittal plane. The mean cortical thickness was 4.0±1.5 mm.
All participants could walk without additional support on the day after the procedure, which was the day that they were discharged.
Regarding outcome safety, no participant required additional procedures during follow-up or developed postoperative complications or local or systemic adverse reactions to the bone substitute.
The follow-up period lasted 21.2±11.4 months, on average, varying from 6.2 to 36.6 months.
Discussion
Subchondroplasty represents an interesting therapeutic option for treatment of subchondral bone lesions that allows correction of local mechanical and biological insufficiency, is minimally invasive, and is associated with low morbidity (14,15,19). In the present study, the application of this technique was successful. To the best of our knowledge, this was the first study in which subchondroplasty was performed on a group of patients diagnosed with subchondral insufficiency fracture, without the generalization of the term “bone marrow edema” or “bone marrow lesion”, but with a more specific diagnosis.
The results indicated improvement of pain and function scores among patients with subchondral insufficiency fracture of the knee who had previously undergone unsuccessful conservative treatment. These lesions cause substantial impairment of the functioning and quality of life of patients. The magnitude of improvement following treatment was considerable.
While previous studies have reported favorable outcomes following subchondroplasty, the participants did not have a specific diagnosis of high signal intensity lesions on T2-weighted images but were systematically described as having bone marrow edema. In 2015, Chatterjee et al. (20) performed a retrospective study of 22 patients subjected to subchondroplasty and followed up for at least 6 months. The mean KOOS score was 39.5 (±21.8) before surgery and 71.3 (±23) (P<0.01) after surgery. The Tegner-Lysholm scale scores were 48 (±15.1) before surgery and 77.5 (±20.6) (P<0.01) after surgery. Those authors also found an inverse correlation between the degree of osteoarthritis, assessed using the Kellgren-Lawrence scale, and the Tegner-Lysholm scale score after surgery. These findings indicate that patients with more advanced joint degeneration have a poorer final functional outcome. Chatterjee et al. further reported that based on the Tegner-Lysholm scale results, the outcome was poor for 7 of the 22 patients, moderate for 3 patients, good for 5 patients, and excellent for 7 patients. Given that the success rate was only 55%, they concluded that subchondroplasty is inefficient for the treatment of bone edema lesions due to osteoarthritis. However, they observed that 20 of the 22 participants (91%) exhibited some improvement compared to their functional state before surgery.
In 2015, Cohen et al. (15) published the results of a retrospective cohort of 66 patients subjected to subchondroplasty. The participants were patients indicated for knee arthroplasty and exhibited bone marrow edema on MRI and local pain in the lesion topography. Patients presented symptoms for 22.4 months, on average, an osteoarthritis Kellgren-Lawrence grade of 3, and failure of conservative treatment. The VAS pain score exhibited significant improvement of 4.3 and 4.5 points, on average, after 6 weeks and 2 years of follow-up, respectively. Additionally, the Internal Knee Documentation Center (IKDC) Subjective Knee Evaluation Form score exhibited improvement of 17.2 and 17.8 at 6 months and 2 years of follow-up, respectively. Kaplan-Meier analysis showed a 2-year joint preservation survivorship (non-performance of arthroplasty) rate of 70%.
A series of 5 cases published in 2017 described an early experience with injection of a calcium phosphate bone substitute for treatment of bone edema lesions in osteoarthritic knees. The short-term results indicated improvement of pain and function scores (21).
However, it is worth noting that the studies that have assessed the results of subchondroplasty up to the present time did not seek to distinguish the specific cause of the abnormal bone marrow lesion signals on MRI. Kon et al. (4) described several conditions and causes associated with subchondral bone abnormalities with different pathophysiology and therefore different treatment. We do not believe that subchondroplasty is indicated for all subchondral bone abnormalities. Therefore, studies assessing this technique should seek to establish the cause of bone marrow edema.
We suggest that subchondroplasty is best indicated for subchondral insufficiency, as it is able to efficiently treat the main cause of this condition by immediately increasing the ability to tolerate load, in association with biological aid for bone repair through the use of an osteoconductive material. As the results of the present study show, these characteristics lead to rapid improvement of symptoms, which persisted during the follow-up period.
One limitation of the present study was that the sample size was small; however, the sample was significant given the low prevalence of lesions. A second limitation is the lack of a control group for assessment of the efficacy of subchondroplasty. In addition, the follow-up period was too short for a comprehensive assessment of the effect of treatment on the progression of osteoarthritis. Nevertheless, these limitations do not diminish the relevance of the conclusions.
Conclusions
Subchondroplasty was shown to be safe, applicable, and efficacious for improvement of pain and the KOOS score for subchondral insufficiency fractures of the knee in the short- and medium-term.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.10.02). MBB and MKD report other from LAS BRASIL, from LAS BRASIL, during the conduct of the study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was conducted at a tertiary university hospital. Approval was granted by the institutional scientific and ethics committees (No. 37392614.9.0000.0068). All participants signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jose J, Pasquotti G, Smith MK, et al. Subchondral insufficiency fractures of the knee: review of imaging findings. Acta Radiol 2015;56:714-9. [Crossref] [PubMed]
- Yamamoto T, Bullough PG. Spontaneous Osteonecrosis of the Knee: The Result of Subchondral Insufficiency Fracture. J Bone Joint Surg Am 2000;82:858-66. [Crossref] [PubMed]
- Hussain ZB, Chahla J, Mandelbaum BR, et al. The Role of Meniscal Tears in Spontaneous Osteonecrosis of the Knee: A Systematic Review of Suspected Etiology and a Call to Revisit Nomenclature. Am J Sports Med 2019;47:501-7. [Crossref] [PubMed]
- Kon E, Ronga M, Filardo G, et al. Bone marrow lesions and subchondral bone pathology of the knee. Knee Surg Sports Traumatol Arthrosc 2016;24:1797-814. [Crossref] [PubMed]
- Camanho GL. Dor aguda no joelho do paciente idoso. Revista Brasileira de Ortopedia 2008;43:361-6. [Crossref]
- Plett SK, Hackney LA, Heilmeier U, et al. Femoral condyle insufficiency fractures: associated clinical and morphological findings and impact on outcome. Skeletal Radiol 2015;44:1785-94. [Crossref] [PubMed]
- Taljanovic MS, Graham AR, Benjamin JB, et al. Bone marrow edema pattern in advanced hip osteoarthritis: quantitative assessment with magnetic resonance imaging and correlation with clinical examination, radiographic findings, and histopathology. Skeletal Radiol 2008;37:423-31. [Crossref] [PubMed]
- Gourlay ML, Renner JB, Spang JT, et al. Subchondral insufficiency fracture of the knee: a non-traumatic injury with prolonged recovery time. BMJ Case Rep 2015; [Crossref] [PubMed]
- Camanho GL, Hernandez AJ, Bitar AC, et al. Results of meniscectomy for treatment of isolated meniscal injuries: correlation between results and etiology of injury. Clinics (Sao Paulo) 2006;61:133-8. [Crossref] [PubMed]
- Demange MK, Gobbi RG, Camanho GL. “Fatigue meniscal tears”: a description of the lesion and the results of arthroscopic partial meniscectomy. Int Orthop 2016;40:399-405. [Crossref] [PubMed]
- Yao L, Stanczak J, Boutin RD. Presumptive subarticular stress reactions of the knee: MRI detection and association with meniscal tear patterns. Skeletal Radiol 2004;33:260-4. [Crossref] [PubMed]
- Yates PJ, Calder JD, Stranks GJ, et al. Early MRI diagnosis and non-surgical management of spontaneous osteonecrosis of the knee. Knee 2007;14:112-6. [Crossref] [PubMed]
- Karim AR, Cherian JJ, Jauregui JJ, et al. Osteonecrosis of the knee Ann Transl Med 2015;3:6. review. [PubMed]
- Sharkey PF, Cohen SB, Leinberry CF, et al. Subchondral bone marrow lesions associated with knee osteoarthritis. Am J Orthop (Belle Mead NJ) 2012;41:413-7. [PubMed]
- Cohen SB, Sharkey PF. Subchondroplasty for Treating Bone Marrow Lesions. J Knee Surg 2016;29:555-63. [PubMed]
- Bekkers JE, de Windt TS, Raijmakers NJ, et al. Validation of the Knee Injury and Osteoarthritis Outcome Score (KOOS) for the treatment of focal cartilage lesions. Osteoarthritis Cartilage 2009;17:1434-9. [Crossref] [PubMed]
- Gonçalves RS, Cabri J, Pinheiro JP, et al. Cross-cultural adaptation and validation of the Portuguese version of the Knee injury and Osteoarthritis Outcome Score (KOOS). Osteoarthritis Cartilage 2009;17:1156-62. [Crossref] [PubMed]
- Lecouvet FE, van de Berg BC, Maldague BE, et al. Early irreversible osteonecrosis versus transient lesions of the femoral condyles: prognostic value of subchondral bone and marrow changes on MR imaging. AJR Am J Roentgenol 1998;170:71-7. [Crossref] [PubMed]
- Farr J, Cohen SB. Expanding Applications of the Subchondroplasty Procedure for the Treatment of Bone Marrow Lesions Observed on Magnetic Resonance Imaging. Oper Tech Sports Med 2013;21:138-43. [Crossref]
- Chatterjee D, McGee A, Strauss E, et al. Subchondral Calcium Phosphate is Ineffective for Bone Marrow Edema Lesions in Adults With Advanced Osteoarthritis. Clin Orthop Relat Res 2015;473:2334-42. [Crossref] [PubMed]
- Bonadio MB, Giglio PN, Helito CP, et al. Subcondroplastia no tratamento de lesões medulares ósseas no joelho – Experiência inicial. Revista Brasileira de Ortopedia 2017;52:325-30. [Crossref] [PubMed]
Cite this article as: Bonadio MB, Giglio PN, Helito CP, da Silva HP, Gobbi RG, Camanho GL, Demange MK. Treatment of subchondral insufficiency fracture of the knee by subchondroplasty. Ann Joint 2020;5:37.