
Free vascularized fibula reconstruction after multilevel total en bloc spondylectomy for primary bone malignancy: a surgical technique
Introduction
Multilevel total en bloc spondylectomy (TES) for primary bone sarcoma is a technically demanding procedure allowing for resection of entire spinal levels in primary bone sarcoma with a negative tumor margin. Durable reconstruction can be accomplished with the addition of a free vascularized fibular grafting (FVFG). We present our preferred technique for management of primary bone malignancy involving multiple spinal levels.
Rationale
Both intralesional and marginal resections demonstrate an unacceptable level of recurrence in primary malignant bone tumors when compared to an en bloc resection. Boriani et al. found that both intralesional and marginal resections had a higher recurrence (hazard ratio of 38.62 and 9.45) when compared to a negative margin in spinal resections (1). In chordoma, when compared to an intralesional resection, only 1 of 21 patients with wide margins recurred at an average of 7.8-year follow-up compared to 22 of 31 marginal or intralesional resections (2). Similarly, in chondrosarcoma patients 12 of 56 wide excisions recurred while 21 of 44 intralesional resections recurred at a median of 3.1-year follow-up (3).
Tomita et al. described the use of pedicular osteotomies to achieve both en bloc corpectomy and en bloc laminectomy, or TES, to minimize tumor spill and provide a wider margin than possible with piecemeal resections (4).
Reconstruction after TES for primary bone sarcomas including chondrosarcoma and chordoma requires a stable and robust fixation for patients that frequently undergo radiation therapy (5,6) and have a prolonged life expectancy when compared to metastatic disease (7). Described techniques have included titanium cages (8,9), auto- and allograft strut (8,10) and vascularized fibular grafts (11) with varying results. The rate of hardware failure has been reported between to be 15–51% (6,9,11,12) and may be due in part to the proportion of primary malignant bone tumors, adjuvant radiation, and levels resected.
From a basic science perspective both allograft and non-vascularized autograft undergo creeping substitution that can manifest with graft resorption during the healing response (13,14) resulting in graft fracture. Vascularized grafts do not carry this risk and have been well studied in spinal deformity correction including included rib, fibula and iliac crest (15-18). Vascularized fibula provides both increased mechanical strength and reliable graft size when compared to both rib and iliac crest (10,11,19-21) making it an ideal graft. However, when the peroneal vascular pedicle is limited, iliac crest may be a reasonable alternative (22). These vascularized grafts provide immediate structural strength, but do incur the additional risk of donor site morbidity. Donor site morbidity associated with FVFG has been reported to be as high as 20%, but mostly consisting of sensory deficits, partial motor weakness, and pain (23,24).
For multilevel primary malignant tumors of the spine, we propose that TES with FVFG provides a wide surgical margin and a high rate of fusion in patients typically undergoing high dose radiation to the surgical field.
Authors preferred technique
Prior to undertaking this complex technique, the most important step is establishing a multidisciplinary team for preoperative planning. At our institution, this includes the orthopaedic spine surgeon, orthopaedic hand surgeon and—depending on the approach—a general surgeon, vascular surgeon, thoracic surgeon, or head/neck surgeon. In other centers the team may comprise a neurosurgeon as well. Preoperative imaging includes relevant staging studies including chest CT, MRI imaging of the entirety of the spine, and angiography of the donor and recipient site.
Patient selection is paramount and we use this modified TES only in primary bone malignancies of the thoracic and lumbar spine. Given the spectrum of disease, we still reserve less technically demanding procedures including isolated anterior or posterior resection when able. In our modified TES, free vascularized fibular grafts are used exclusively for anterior column reconstruction in the posterior column we will use a rotational vascularized rib.
The surgical procedure is performed in a staged fashion, with one to seven days between phases. In stage one, the patient is placed in a well-padded prone position for the posterior tumor resection and instrumentation. As described by Shah et al., a midline approach is used, and the paraspinal muscles are elevated (20). Through a combination of blunt finger and instrumented (right-angle clamp) dissection, a pathway is created at the desired level of resection over the anterior vertebral body/disk space. A Penrose drain is then advanced around the anterior vertebral body through which a threadwire saw is subsequently passed to be positioned anterior to the vertebral body, but posterior to the great vessels. This is typically confirmed with intra-operative fluoroscopy. The posterior elements are then resected using a combination of rongeurs and saws. Next, in the thoracic spine, lateral exposure of the vertebral body is obtained by cutting of the ribs, ligation of the segmental nerve roots in the thoracic cavity, and by elevation of the psoas muscle. In the lumbar spine, care must be taken to preserve uninvolved nerve roots while dissecting laterally around the vertebral body and includes elevation of the psoas. After removing the Penrose drain, 1 limb of the threadwire saw is passed through a plane between the thecal sac and the posterior longitudinal ligament. Posterior instrumentation is performed typically 2 levels above and below the resection levels and the threadwire saw is tied to the hardware for later retrieval. A layered closure is performed.
During stage 2, the fibular harvest is performed at the time of the anterior resection with supine positioning for cervical resections and lateral decubitus position for thoracic and lumbar tumors. Approach is dictated by the mass and includes a thoracotomy (Figure 1A), sternotomy, or combined sternotomy and thoracotomy for thoracic tumors, and retroperitoneal approach for the lumbar spine. Careful dissection is performed until the previously placed threadwire saw is reached and is used to complete the vertebral body osteotomy to allow for en bloc resection. If indicated, intraoperative radiation is performed following resection.
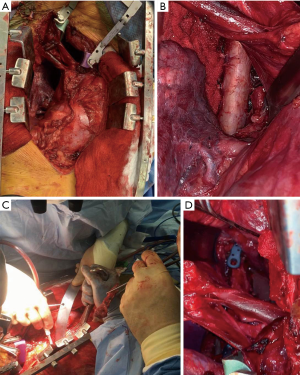
Next, the defect is measured and the fibular reconstruction planned based pre-operative and intraoperative measurements. The defect size should be estimated based on the pre-operative imaging and compared to both the resected specimen and the measured defect in the patient (Figure 1B). The degree of kyphosis or lordosis should be accounted for and its effect on the angulation of the endplates. The overall length of the fibular resection should be slightly longer than the measured defect to allow for wedging of the graft, however overlengthening of a graft may lead to endplate fracture.
The best docking structure for the FVFG (Figure 1C) is a non-irradiated cortical endplate and several factors must be considered. In younger, non-irradiated patients the graft can be placed either against the intact endplate or in cancellous bone with the addition of a “potted” technique to potentially increase graft stability and surface area. When the graft is placed on this non-cortical bone we recommend anterior fixation to prevent graft subsidence. For patients with poor quality or irradiated bone the graft interface should consist of non-irradiated cortical endplates (Figure 1D) (25). This can be accomplished at the resected level, by creating a channel through an osteotomized vertebral, or resecting additional bone to reach an endplate. Additionally, the anatomic accessibility of the endplate is also a consideration for the level of the graft-host interface.
The vascularized fibular graft is harvested through a direct lateral incision under tourniquet (Figure 2A); no skin paddle is taken. The peroneal muscles are elevated (Figure 2B) from the fibula and the interosseous membrane is then incised near the fibular insertion to avoid the peroneal vessels. Prior to bone cuts, the proximal pedicle is found posterolateral to the fibula, distal to the anterior tibial artery, and proximal to the soleus. The peroneal vessel is left intact and an oscillating saw is used to cut the fibula with frequent irrigation to prevent thermal necrosis at the graft ends (Figure 2C). Ten cm of proximal and distal fibula will remain in the wound bed and the vessels are ligated proximally and distally just prior to graft placement.
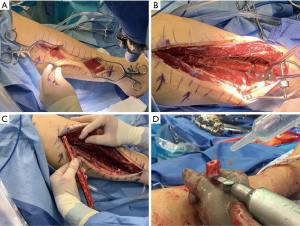
The fibular graft is cut to match the angles of the corresponding vertebral end plates (Figure 2D). The fibula can be grasped with a Kocher clamp (Figure 1C), or other bone grasping instrument, ensuring that the vessels along the side of the fibula are protected during the entirety of the procedure. The fibular graft is fashioned to match the angles of the docking endplates and is then temporarily inset into the defect opening. One end of the graft will typically be colinear to its corresponding endplate and the contralateral will have a more angulated appearance. A surgical elevator, such as a Penfield #1 or 3 or Cobb elevator, is then placed at the more angulated graft interface to act as a shoehorn. A bone tamp is then utilized to gently tap this angled end of the fibula while an assistant gently rotates the surgical elevator out of the field. Once the elevator is removed, both ends of the graft should be colinear with their respective end plates. Alternating taps are then used to advance each graft end until it is firmly set into place. During graft advancement the location of the vessels is continually verified to ensure the bone tamp is not causing injury. The final position of the fibula is confirmed radiographically and, if sized and placed properly, the graft should have inherent stability. If there is any doubt to the stability of the graft, or if additional fixation is desired, vertebral screws (Figure 1D) can be inserted to add slight compression. Additional techniques for compression exist, but are not discussed in this article.
Based on preoperative angiography and intraoperative decision making, the recipient vessels are identified. The primary focus of this operation is the removal of the tumor and ligation of adjacent arteries and veins may change the intended anastomosis. If one is able, the intended recipient artery and vessel are protected for the anastomosis however this cannot compromise the safety of the resection. If these vessels are sacrificed then a new recipient vessel must be identified. In the thoracic spine this is often a segmental vessel, however the internal mammary artery has also been utilized in some cases and even a direct anastomosis to the aorta has been undertaken. In the lumbar spine the segmental vessels are the most accessible but they are often ligated during resection of the tumor. This can lead to difficulty in finding appropriate vessels and often the microsurgeon must work closely with the access surgeon to identify appropriate recipient vessels. If no viable recipient vessel is available locally a saphenous vein graft can be utilized for more distant vascular pedicles. Layered closure is then performed over suction drains.
Discussion
Primary malignant bone tumors of the spine require complex resections and reconstructions. These reconstructions often occur in irradiated tumor beds and for malignancies with prolonged survival. Of the options available, we have found TES with FVFG to provide the most reliable surgical margin and construct durability.
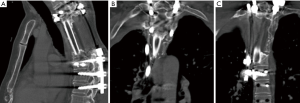
In both chordoma and chondrosarcoma, our institution has increasingly used adjuvant radiotherapy to achieve 5-year local control rates of up to 94% (26). In a prior study looking at 30 structural allografts after multi-level TES in chondrosarcoma and chordoma patients, 28 patients had radiographically intact grafts at an average of 14 months postoperatively. However, of the nine patients that did undergo post-operative CT scan, none demonstrated incorporation of the graft and instead noted limited exterior overgrowth of the graft junction by the host bone (27). As a result of these findings we added FVFG to our reconstructions and in a cohort of 39 patients undergoing multi-level TES and with an average of 68.4 Gy, we achieved successful fusion in 29 patients based on CT scan at an average of 50 months, example in Figure 3A,B,C. An average of 3.7 levels were resected and 28% of patients underwent surgery for prior hardware or graft failure (Schwab JH, 2019, unpublished data). When compared to prior literature, anterior reconstructions with cages and autograft have achieved high rates of success after TES, however the majority of these patients were primary resections without radiation (4,9,12,28,29).
The question is not if FVFG is superior to cage constructs, rather which patients undergoing TES require the additional biology provided by a vascularized graft. In a patient undergoing single level TES without planned radiation, there is ample evidence that resection followed by spinal shortening and autograft packed cages will fuse (4,29). However, in patients undergoing multi-level resections for tumors requiring radiation, vascularized fibular grafting may provide the highest chance of fusion.
In regards to graft choice, biomechanical testing has found compressive failure loads to be as high as 5,070 N for fibular strut graft compared to 1,150 N iliac crest and 452 N for rib grafts (30). Titanium mesh cages vary considerably in yield strength, but are more prone to end plate encroachment than device failure at forces closer to 400–1,300 N (31). In our experience fibular strut graft has been superior in-vivo compared to rib and iliac grafts, however other authors have suggested that iliac crest may be advantageous when the fibular graft has a short pedicle on preoperative angiography and the in thoracic resections can be used as a local rotational graft (22).
The additional time required for fibula harvesting and graft placement has been estimated to be 3–4 hours with minimal blood loss (19,32-34). This corresponds to roughly 40–50% of our average operative time (561 minutes) for the anterior approach (Schwab JH, 2019, unpublished data), potentially increasing the chance of increased blood loss from tumor resection bed and infection.
Complications from the FVFG harvest can include weakness in ankle dorsiflexion, pain, loss of sensation (10,23,24,32), and progressive ankle valgus deformity in children (35). However, given the overall morbidity associated with multi-level total en bloc spondylectomy for a primary bone sarcoma, this represents an incremental risk when compared to the greater risk of construct failure.
Conclusions
Total en bloc spondylectomy is a radical procedure designed to provide disease-free margins in primary bone malignancies of the spine. Reconstruction in these patients can be challenging and with increasing spinal levels and radiation dosing free fibular grafts may provide the required structural and vascular support for a long-lasting fusion.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Wei Guo, Tao Ji, Paul Jutte and Eric Henderson) for the series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.02.06). The series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participant were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from all patients and institutional IRB approval was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boriani S, Gasbarrini A, Bandiera S, et al. En bloc resections in the spine: the experience of 220 patients during 25 years. World Neurosurg 2017;98:217-29. [Crossref] [PubMed]
- Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am 2005;87:2211-6. [PubMed]
- Fisher CG, Versteeg AL, Dea N, et al. Surgical Management of Spinal Chondrosarcomas. Spine (Phila Pa 1976) 2016;41:678-85. [Crossref] [PubMed]
- Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy: a new surgical technique for primary malignant vertebral tumors. Spine (Phila Pa 1976) 1997;22:324-33. [Crossref] [PubMed]
- Yanamadala V, Rozman PA, Kumar JI, et al. Vascularized Fibular Strut Autografts in Spinal Reconstruction after Resection of Vertebral Chordoma or Chondrosarcoma: A Retrospective Series. Neurosurgery 2017;81:156-64. [Crossref] [PubMed]
- Wagner TD, Kobayashi W, Dean S, et al. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys 2009;73:259-66. [Crossref] [PubMed]
- Cloyd JM, Acosta FL, Polley MY, et al. En bloc resection for primary and metastatic tumors of the spine: a systematic review of the literature. Neurosurgery 2010;67:435-44; discussion 444-5. [Crossref] [PubMed]
- Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine (Phila Pa 1976) 1996;21:1569-77. [Crossref] [PubMed]
- Acosta FL Jr, Aryan HE, Ames CP. Successful outcome of six-level cervicothoracic corpectomy and circumferential reconstruction: case report and review of literature on multilevel cervicothoracic corpectomy. Eur Spine J 2006;15:670-4. [Crossref] [PubMed]
- Wright NM, Kaufman BA, Haughey BH, et al. Complex cervical spine neoplastic disease: reconstruction after surgery by using a vascularized fibular strut graft. Case report. J Neurosurg 1999;90:133-7. [PubMed]
- Vrints I, Depreitere B, Vranckx JJ. Multilevel cervical reconstruction with no remaining hardware: The potential of a vascularised fibular strut graft. J Plast Reconstr Aesthet Surg 2012;65:e344-7. [Crossref] [PubMed]
- Houdek MT, Rose PS, Bakri K, et al. Outcomes and complications of reconstruction with use of free vascularized fibular graft for spinal and pelvic defects following resection of a malignant tumor. J Bone Joint Surg Am 2017;99:e69 [Crossref] [PubMed]
- Goldberg VM, Shaffer JW, Field G, et al. Biology of vascularized bone grafts. Orthop Clin North Am 1987;18:197-205. [PubMed]
- Shaffer JW, Field GA, Goldberg VM, et al. Fate of vascularized and nonvascularized autografts. Clin Orthop Relat Res 1985;32-43. [PubMed]
- Bradford DS. Juvenile kyphosis. Clin Orthop Relat Res 1977;45-55. [PubMed]
- Bradford DS. Anterior vascular pedicle bone grafting for the treatment of kyphosis. Spine (Phila Pa 1976) 1980;5:318-23. [Crossref] [PubMed]
- Bradford DS, Ganjavian S, Antonious D, et al. Anterior strut-grafting for the treatment of kyphosis. Review of experience with forty-eight patients. J Bone Joint Surg Am 1982;64:680-90. [Crossref] [PubMed]
- Hsu RW, Wood MB, Sim FH, et al. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br 1997;79:36-42. [Crossref] [PubMed]
- Ackerman DB, Rose PS, Moran SL, et al. The results of vascularized-free fibular grafts in complex spinal reconstruction. J Spinal Disord Tech 2011;24:170-6. [Crossref] [PubMed]
- Shah AA, Paulino Pereira NR, Pedlow FX, et al. Modified en bloc spondylectomy for tumors of the thoracic and lumbar spine: surgical technique and outcomes. J Bone Joint Surg Am 2017;99:1476-84. [Crossref] [PubMed]
- Winters HA, Kraak J, Oosterhuis JW, et al. Spinal reconstruction with free vascularised bone grafts; approaches and selection of acceptor vessels. Scand J Surg 2013;102:42-8. [Crossref] [PubMed]
- Wuisman PI, Jiya TU, Van Dijk M, et al. Free vascularized bone graft in spinal surgery: indications and outcome in eight cases. Eur Spine J 1999;8:296-303. [Crossref] [PubMed]
- Babovic S, Johnson CH, Finical SJ. Free fibula donor-site morbidity: the Mayo experience with 100 consecutive harvests. J Reconstr Microsurg 2000;16:107-10. [Crossref] [PubMed]
- Vail TP, Urbaniak JR. Donor-site morbidity with use of vascularized autogenous fibular grafts. J Bone Joint Surg Am 1996;78:204-11. [Crossref] [PubMed]
- van Wulfften Palthe O, Jee KW, Bramer JAM, et al. What Is the Effect of High-dose Radiation on Bone in Patients With Sacral Chordoma? A CT Study. Clin Orthop Relat Res 2018;476:520-8. [Crossref] [PubMed]
- DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol 2014;110:115-22. [Crossref] [PubMed]
- Lewandrowski KU, Hecht AC, DeLaney TF, et al. Anterior spinal arthrodesis with structural cortical allografts and instrumentation for spine tumor surgery. Spine (Phila Pa 1976) 2004;29:1150-8; discussion 1159. [Crossref] [PubMed]
- Yoshioka K, Murakami H, Demura S, et al. Clinical outcomes of spinal reconstruction after total en bloc spondylectomy at 3 or more levels. Spine (Phila Pa 1976) 2013;38:E1511-6. [Crossref] [PubMed]
- Yoshioka K, Murakami H, Demura S, et al. Risk factors of instrumentation failure after multilevel total en bloc spondylectomy. Spine Surg Relat Res 2017;1:31-9. [Crossref] [PubMed]
- Wittenberg RH, Moeller J, Shea M, et al. Compressive strength of autologous and allogenous bone grafts for thoracolumbar and cervical spine fusion. Spine (Phila Pa 1976) 1990;15:1073-8. [Crossref] [PubMed]
- Hasegawa K, Abe M, Washio T, et al. An experimental study on the interface strength between titanium mesh cageand vertebra in reference to vertebral bone mineral density. Spine (Phila Pa 1976) 2001;26:957-63. [Crossref] [PubMed]
- Ling XF, Peng X. What is the price to pay for a free fibula flap? A systematic review of donor-site morbidity following free fibula flap surgery. Plast Reconstr Surg 2012;129:657-74. [Crossref] [PubMed]
- Shaker AS, Addosooki AI, El-Deen MA. Anterior Cervical Corpectomy with free vascularized fibular graft versus multilevel discectomy and grafting for Cervical Spondylotic Myelopathy. Int J Spine Surg. 2015;9:60. [Crossref] [PubMed]
- Pedreira R, Siotos C, Cho BH, et al. Vascularized Bone Grafting for Reconstruction of Oncologic Defects in the Spine: A Systematic Review and Pooled Analysis of the Literature. J Reconstr Microsurg 2018;34:708-18. [Crossref] [PubMed]
- Hsu LC, Yau AC, O’Brien JP, et al. Valgus deformity of the ankle resulting from fibular resection for a graft in subtalar fusion in children. J Bone Joint Surg Am 1972;54:585-94. [Crossref] [PubMed]
Cite this article as: Kelly SP, Ramkumar D, Bongers M, Schwab JH. Free vascularized fibula reconstruction after multilevel total en bloc spondylectomy for primary bone malignancy: a surgical technique. Ann Joint 2020;5:46.


