
Regeneten bio-inductive collagen scaffold for rotator cuff tears: indications, technique, clinical outcomes, and review of current literature
Introduction
Rotator cuff (RC) tears are one of the most common injuries encountered in an orthopaedic practice. Between 200,000 and 300,000 RC repairs (RCR) are estimated to be performed annually in the United States (1). Patients with symptomatic RC lesions typically experience pain and weakness that can have adverse effects on sleep, work, mobility, and psychosocial functioning (2). Surgical repair of RC tears has been associated with improved patient outcomes and patient satisfaction (3), however, rates of failure of repair have been reported to be as high as 40% in primary repairs (4-6). Despite advances in surgical techniques, re-tear of the RC tendon(s) following primary repair still occurs in nearly 20% of patients (7). Re-tearing of the RC may, in part, be due to a failure to restore biological and mechanical properties of the repaired RC tissue as compared with the native tendon (8).
Various structural augmentations to RCR have been used with minimal success including periosteal patches, extracellular matrix, and even freeze-dried RC tissue (9-11). None of these options have shown improvement in native tendon quality. However, the Regeneten implant (Smith & Nephew, Andover, MA, USA) is a new technology that has been developed with the aim of promoting tendon growth and increasing vascularization at the site of an RCR (12,13).
Restoring the biomechanical environment of the tendon footprint on the greater tuberosity is necessary in achieving a functional and durable RCR. Complete restoration of the normal footprint dimensions along with decreased tissue strain in the healing tendon are both necessary to promote longevity in the repaired RC (14-16). The Regeneten implant was designed with this concept in mind by increasing native tissue at the site of tendon repair and decreasing the strain across a healing and maturing tendon. The use of the Regeneten implant has been shown to induce the formation of well-integrated and mature tendon-like tissue in both partial-thickness and full-thickness RC tears. However, there is limited clinical data for its use at the present time (17-21).
The purpose of this study was to evaluate the indications, clinical outcomes, and complication rates when the Regeneten implant is used for RCR as well as present a review of the available literature. The implant is a new tool available to orthopedic surgeons that may improve healing rates of RCR’s, but long-term and comparative studies are lacking at the current time.
Implant integration
Bioinductive collagen implants have been used for repair of both full- and partial-thickness RC tears because of their unique ability to promote host tissue regeneration and improve the healing environment (17,18). Upon integration of the scaffold, there is a subsequent increase in thickness of the native tendon through the induction and remodeling of tendinous tissue (13). Biopsies from these implants have demonstrated consistent cellular incorporation, tissue maturation, and biocompatibility without signs of foreign body reactions or implant rejection in human studies (12). As early as 5 weeks postoperatively, host cell integration and collagen growth can be expected within the implant, with host incorporation occurring throughout its entirety as early as 8 weeks postoperatively (12). At 3 months, biopsies of the RC tendon-implant complex have shown new collagen formation, maturation of the tendon, and collagen organization on the surface of the incorporated tendon. By 6 months the implant has been shown to be fully incorporated with regularly oriented connective tissue (12).
Radiologically, via MRI, a significant increase in mean tendon thickness compared to published values has been shown by 3 months in human studies, with an average of 2 mm of newly formed tissue over the bursal surface of the supraspinatus tendon (17). At the 2-year follow-up, the new tissue was indistinguishable from the native tendon on MRI, which the study authors have suggested represents complete integration and maturation of the tendon-implant complex (17). In a follow-up study (18), the same authors reported similar results in 13 patients with partial-thickness RC tears. By 3 months, there was a significant increase in implant-induced tissue formation, with an additional 2 mm of tendon thickness at the 2-year follow-up when compared to the tissue thickness at the time of implantation.
Surgical technique/application
The implant is applied into the subacromial space through a lateral portal with a proprietary guide that is provided with the implant. In the setting of partial-thickness tears the implant is applied directly over the site of the tear, and in full-thickness tears the RC is repaired according to surgeon preference and then applied over the site of the repair. The anterior edge of the implant is aligned with the anterior edge of the supraspinatus and placed 5 mm lateral to the most lateral insertion of the tendon. The anterior, medial, and posterior aspects of the implant are secured to the underlying tendon with the included PLLA staples through accessory portals (as needed) created under outside-in localization just off the edge of the acromion. The guide is removed, and the lateral edge is then secured to the bone of the lateral greater tuberosity with the included PEEK bone staples which are inserted similarly through the lateral portal.
The senior author has also found great success inserting the implant from a posterior portal while viewing from a lateral portal. Due to the rectangular shape of the implant, this technique may provide more coverage of the repaired tendon from anterior to posterior rather than medial to lateral. The long axis of the implant is placed along the lateral borders of the supraspinatus and infraspinatus tendons and secured in the same fashion as if inserted through a lateral portal. In the senior author’s experience, this has been especially helpful in larger tears that consist of two or three tendons.
Indications and contraindications
Indications for the application of the Regeneten patch have varied widely with each published case series (Table 1). The initial series, published by Bokor et al. (17), applied the patch to 8 medium sized (1–3 cm) full-thickness supraspinatus tears and 1 high-grade bursal sided supraspinatus tear which was converted to a full-thickness tear and repaired similar to the other 8 patients (17). Contraindications for its use in their study included previous RCR, recent history of steroid use, insulin-dependent diabetes mellitus (IDDM), genetic collagen disease, chronic inflammatory disease, shoulder instability, grade 3 chondromalacia, and grade 2 or higher goutallier fatty infiltration of the RC muscles. The following year, the same group (18) published a new series where the Regeneten implant was applied to only partial-thickness supraspinatus tears without any rotator cuff repair (RCR) performed. They used the same contraindications as their previous series.

Full table
Schlegel et al. then published a multi-center, prospective trial investigating the application of the Regeneten implant to high-grade partial-thickness tears. Similar to Bokor et al.’s most recent series (18), no RCR was performed in these patients (19). The indication for Regeneten use in their study was chronic, degenerative, partial-thickness supraspinatus tears of 25% or greater that have failed at least 3 months of non-operative treatment. Contraindications were similar to Bokor et al.’s (18) but also included acute traumatic tears, full-thickness tears, oral corticosteroid use, or corticosteroid injection in close proximity to the surgery.
Due to the implant induced tissue growth in single tendon partial- and full-thickness tears, Thon et al. investigated the use of the Regeneten implant in larger full-thickness tears consisting of large (two tendon) or massive (three tendon) tear classifications (20). However, that series did not have exclusion criteria for smokers, patients with IDDM, or patients undergoing revision RCR. In fact, the majority of the patients in that series were undergoing revision RCR with successful results. Similarly, McIntyre et al did not exclude smokers, patients with IDDM, or revision RCR’s (21).
The Regeneten implant has been applied to partial-thickness supraspinatus tears, full-thickness supraspinatus tears, large (two tendon) tears, and massive (three tendon) tears with successful outcomes for both primary and revision repairs (17-21). To date there have been no comparative studies and all published results have been Level IV case series. As such, strict indications and contra-indications have yet to be established for its use, which leaves the decision to apply the Regeneten implant to the treating surgeons’ discretion.
The authors of this report have applied the implant to tears from partial-thickness tears to full-thickness massive RC tears with results that demonstrate acceptable patient outcomes at two years post-operatively (20). Our current views on the most appropriate use for the implant are both in partial thickness tears in high-risk patients where completion and repair has a concerning risk for failure to heal (smokers, IDDM) and for augmentation in full-thickness RC tears that are at risk of failing, which in our practice includes revision repairs and larger full-thickness tears consisting of two or three tendons. While smoking and IDDM are not firm contra-indications, patients undergoing any RC repair should attempt to stop smoking and have their IDDM well-controlled prior to any surgery as it places them at higher risk of failure and complications overall, regardless of whether the Regeneten implant is applied or not (22-24). Table 1 shows a summary of all indications and contraindications for the Regeneten implant.
Literature review
Patient demographics
Five studies have been published reporting on the use of the Regeneten implant in RCR, which included a total of 251 patients across all studies (17-21). One hundred and fifty-one (45.8%) patients had full-thickness tears and 136 (54.2%) patients had partial-thickness tears. The mean patient age at the time of surgery was 54.6 years (range, 24–75 years), and the average follow-up was 14.9 months (range, 10.8–32.0 months). Males made up 58.2% (146/251) of the studied population (Table 2).

Full table
Clinical outcomes
Partial-thickness tears
In the presence of partial-thickness RCT’s the implant is typically applied over the top of the tear site without performing any type of repair. This was first reported by Bokor et al. in 13 patients, in which the primary outcome was tendon thickness as measured on MRI (18). They had comparative data on 10 of 13 patients at final follow-up which showed an average increase of 2.2 mm at 3 months postoperatively. They reported that this value remained stable after 12 months. In 7 of the 10 patients the partial-thickness defect demonstrated complete resolution and the remaining 3 of 10 demonstrated greater than 50% filling in of the defect. All of the 13 patients’ tears were classified as intermediate-grade (3–6 mm) or high-grade (>6 mm) tears and none of the 13 patients had evidence of tear propagation or worsening degeneration. While the final Constant and American Shoulder and Elbow Surgeon score (ASES) were not reported, the authors stated that there were significant improvements in both scores at the final 2-year follow-up. 12 of the 13 patients (92%) were satisfied with their results.
Schlegel et al. reported on 33 patients with partial-thickness tears treated with the Regeneten implant after arthroscopic confirmation (19). Twelve patients had intermediate-grade tears (25–50% of the tendon thickness) and 21 had high-grade tears (>50% of the tendon thickness but less than full thickness). In their series defect tear size was categorized as no tear, low grade (50% of the tendon thickness but less than full thickness), intermediate grade (25%-50% of the tendon thickness), or high grade (>50% of the tendon thickness but less than full thickness). Again, the primary outcome was tendon thickness and tear resolution on MRI. At 12 months, 24% showed complete filling in of the partial-thickness defect and an additional 70% showed a decrease in tear size of at least 1 grade. Only 1 patient (3%) had a tear that remained unchanged. One patient had a propagation of their tear following an acute traumatic injury, but none of the patients who followed the study protocol had any tear worsening or propagation. Mean thickness of the supraspinatus tendon increased by 2.0 mm at final follow-up. Constant scores were significantly improved from 57.1 pre-operatively to 81.4 post-operative. Likewise, ASES scores improved from 57.0 to 89.1 at final follow-up. Patient satisfaction was 94% at final follow-up.
In the largest series to date, McIntyre et al. reported on a subset of patients with partial-thickness tears treated with the Regeneten implant (21). Patient reported outcomes included the single-assessment numeric evaluation (SANE), ASES, and the Western Ontario’s Rotator Cuff (WORC) score. Of the 90 patients reported with partial-thickness tears, SANE scores improved from 42.5 to 86.0, ASES scores from 47.0 to 85.6, and WORC scores from 38.2 to 84.4; all of which were statistically significant increases.
Table 3 shows a summary of all studies reporting on partial-thickness tears.

Full table
Full-thickness tears
The initial series presented by Bokor et al. (17) consisted of 8 full-thickness tears and 1 partial-thickness tear that was converted to and treated as a full-thickness tear. The main outcomes measure was tendon thickness on MRI with Constant and ASES scores as secondary outcomes. At all time points, they showed a significant increase in tendon thickness over published values. Secondarily, Constant and ASES scores also significantly improved from 50.7 to 78.0 and 44.6 to 87.8, respectively.
Thon et al. reported on the use of the Regeneten implant in large and massive full-thickness tears (20). Primary outcome measures were final tendon thickness and healing rates on MRI as well as final ASES scores. Final tendon thickness on MRI was found to be 5.13 mm, tendon healing was 96% (22/23), and final ASES scores were 82.8. Again, in the largest series to date, McIntyre et al. also reported on their results with full-thickness tears in 83 patients reporting SANE, ASES, and WORC scores (21). All three patient reported outcomes showed a statistically significant improvement from pre-operative levels. SANE scores improved from 39.2 to 80.7, ASES scores from 45.5 to 83.8, and WORC scores from 35.0 to 80.1.
Table 4 shows a summary of all studies reporting on full-thickness tears.

Full table
Re-operation rates and failures
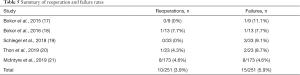
Overall, there have been 251 patients reported in the literature with the majority recently reported on by McIntyre et al. (17-21). In this initial combined cohort, there has been only a 3.9% (10/251 patients) re-operation rate. One patient in Bokor et al.’s partial-thickness series (18) developed severe bursitis 12 months after the index procedure and was taken for arthroscopic debridement. At that time, arthroscopic tissue cultures and tissue biopsies obtained showed no evidence of infection nor any discernible reaction to the implant. The authors postulated that the bursitis could have been caused by the addition of the implant, but no conclusive histological evidence was found to support that hypothesis. One patient in Thon et al.’s case series underwent conversion of a previous RCR with Regeneten implant to a reverse shoulder arthroplasty due to worsening glenohumeral arthritis and atrophy (20). They also had a second patient who would have been eligible for an additional operation due to recurrent tearing, but the patient declined further surgery.
Eight patients in McIntyre et al.’s study underwent re-operation (21). One patient developed an acute infection requiring irrigation and debridement at which time the implant was removed. That patient underwent repeat RCR once the infection had resolved. A second patient developed a deep-vein thrombosis (DVT) as well as adhesive capsulitis of the operated shoulder and was treated with eventual lysis of adhesions and manipulation with satisfactory results. A third patient from their series developed post-operative stiffness and was later taken for an additional arthroscopy 4 months following the initial procedure. During the arthroscopic evaluation it was found that part of the graft had torn and was loose in the bursa. This piece was removed, and a thorough debridement was performed which resolved the issue. One patient developed recurrent effusion which also eventually required repeat arthroscopy. At that time, a synovectomy for bursitis was performed. The last four patients requiring re-operation were due to failure of the RC to heal with recurrent tearing, including one partial tear and three full-thickness tears. The patient with a partial tear was later taken for tear completion and revision repair, while two of the full-thickness patients underwent revision RCR, and the last underwent reverse total shoulder arthroplasty.
“Failure” of the operation was determined differently across each of the studies, making direct comparisons challenging. However, if failure is combined to mean lack of patient satisfaction, lack of tendon healing, or need for re-operation, the failure rate was 5.9% (15/251 patients) across all studies utilizing the Regeneten implant (17-21). Bokor et al.’s (17) initial series of full-thickness tears had one unsatisfied patient due to continued pain. They noted that this patient failed to attend their follow-up appointments and was “intending to file a worker’s compensation claim”. Their second series of partial-thickness tears (18) similarly had one unsatisfied patient due to significant pain. Three patients from Schlegel et al.’s series can be considered failures by these criteria (19). One patient did not follow their post-operative protocol and had an acute injury which caused tear progression from partial-thickness to full-thickness and two patients did not consider the results of their surgery to be satisfactory. Thon et al.’s case series had two clinical failures (20). One was a smoker who failed to heal his supraspinatus but declined any further surgery. The other developed worsening glenohumeral arthritis with subsequent atrophy of the RC, and eventually went on to reverse total shoulder arthroplasty. The remaining 8 patients who underwent re-operation in McIntyre et al.’s series were described above (21).
Table 5 shows a summary of all studies reporting on reoperation and failure rates.
Full table
Complications
To date, complications with the use of the Regeneten implant have been reported in the literature at a rate of 9.9% (25/251) overall. Bokor et al.’s full-thickness series (17) reported one patient with pre-operative adhesive capsulitis which worsened in the post-operative period. It eventually improved over the 24-month study period and there was no mention of any repeat operation. In Bokor et al.’s partial-thickness series they had one patient develop severe swelling requiring conversion to an open procedure at the time of operation, 1 patient developed adhesive capsulitis which resolved by final follow-up, 1 patient with a spontaneous rupture of the long head of the biceps tendon, and 1 patient with significant pain due to bursitis requiring re-operation that was described above (18).
Schlegel et al. reported 4 complications (19). One patient with significant, but asymptomatic, subacromial bursal fluid which was drained under ultrasound guidance. The fluid was found to be “normal physiologic fluid” and “considered nonreactive to the implant” by an independent pathologist. One patient reported increased pain after a specific movement during an electrical fire. Following MRI to confirm the tendon and implant complex were intact, a subacromial injection was given and the pain resolved. The remaining 2 complications were superficial skin issues; 1 for an allergic reaction to the skin preparation and 1 for wound drainage due to a stitch abscess.
In addition to the previous two failures described above, Thon et al. reported 8 additional patients who required prolonged physical therapy of greater than 6 months for scapular dyskinesia (20). The authors believed that the prolonged therapy was likely necessary due to the larger pre-operative tear size and chronicity of the tears. They otherwise reported no adverse events due to the addition of the implant.
McIntrye et al. reported on 8 patients that required re-operation which were described above (21). Of those 8, 1 had a post-operative infection, 1 had a separate loose piece of implant causing stiffness and bursitis, 1 developed deep vein thrombosis and adhesive capsulitis of the glenohumeral joint, 1 had recurrent effusions and bursitis requiring debridement, and 4 patients had recurrent tearing or failures to heal.
Discussion
The use of the Regeneten Implant has been shown to be effective and have improved patient reported outcomes in RCT’s (17-21). Successful outcomes have been achieved in tear sizes from partial-thickness tears, to full-thickness tears, to massive 3-tendon tears (17-21). While the technology is new, it shows promise in its broad application across different tear types and repair settings. However, the use of the implant should be considered in the context that there are no long-term follow-up studies associated with its use. The longest studies in the published literature, while showing promising short-term results, had only 2 years of follow-up. It has yet to be determined if the use of the implant has lasting results that are durable as time progresses.
While complication rates have been limited, it cannot be determined with complete certainty how much the use of the implant has contributed to specific complications and to complication rates. Large, comparative studies are needed to determine the implants specific indications, contra-indications, and pitfalls with its use. Comparative studies would also help determine which patients would benefit from addition of a Regeneten Implant during a RCR. Another reason that this cannot be ignored is the ever-increasing emphasis of “cost” and “value” in today’s current medical climate. The Regeneten Implant is a new technology, and with that, has an increased cost associated with its use compared to a standard RCR. The value of the implant will need to be determined in different tear types and tear sizes to determine its most appropriate use.
The biological nature of reconstituted collagen implants for repair of either full- or partial-thickness RC tears has been repeatedly demonstrated to induce formation of well-integrated and mature tendon-like tissue. While this promotes and possibly accelerates healing of the initial tear, it is also possible that the new tissue prevents future tear propagation and decreases the risk of degenerative changes within the tendon (18). Its use appears to be safe with an overall low complication rate (9.9%), with most complications being transient and fully resolving at final follow-up. Again, more work is needed to determine if the implant is a direct cause of some of these complications, even if they are less frequent overall. This will be necessary to provide counseling to future patients who may undergo a procedure with the implant.
Limitations
This review is limited by the available literature on the use of the Regeneten Implant. All published series to date are only Level IV studies with limited follow-up of 2 years or less (17-21). Lack of control groups across studies makes comparisons difficult and unavailable at the current time. In addition, repair techniques differed greatly and were decided individually by the treating surgeons in each study. This may introduce some bias into the patient selection, repair techniques, and to the reported complications.
Conclusions
The use of the Regeneten implant has shown improved patient reported outcomes and success when used during RCR compared to isolated RCR without Regeneten augmentation. At the current time, clear indications and contra-indications for its use have yet to be defined. More work is needed to determine which patients it is most appropriate for and if the results stand the test of time with long-term follow-up. Because of these factors, treating physicians should approach its use with caution, but also optimism. Early results with 2-year follow-up are especially encouraging and point to broad applicability for the implants use.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Adnan Saithna) for the series “Current and Emerging Concepts in the Management of Rotator Cuff Tears” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.03.04). The series “Current and Emerging Concepts in the Management of Rotator Cuff Tears” was commissioned by the editorial office without any funding or sponsorship. JTB reports other from DJO, other from Smith & Nephew, other from Shukla Medical, other from Biomet, other from Stryker, other from Mitek, other from AOSSM, other from null, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am 2012;94:227-33. [Crossref] [PubMed]
- Minns Lowe CJ, Moser J, Barker K. Living with a symptomatic cuff tear ‘bad days, bad nights’: a qualitative study. BMC Musculoskelet Disord 2014;15:228. [Crossref] [PubMed]
- Simank HG, Dauer G, Schneider S, et al. Incidence of rotator cuff tears in shoulder dislocations and results of therapy in older patients. Arch Orthop Trauma Surg 2006;126:235-40. [Crossref] [PubMed]
- Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am 2005;87:1229-40. [PubMed]
- Cole BJ, McCarty LP 3rd, Kang RW, et al. Arthroscopic rotator cuff repair: prospective functional outcome and repair integrity at minimum 2-year follow-up. J Shoulder Elbow Surg 2007;16:579-85. [Crossref] [PubMed]
- DeFranco MJ, Bershadsky B, Ciccone J, et al. Functional outcome of arthroscopic rotator cuff repairs: a correlation of anatomic and clinical results. J Shoulder Elbow Surg 2007;16:759-65. [Crossref] [PubMed]
- Le BT, Wu XL, Lam PH, et al. Factors predicting rotator cuff retears: an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med 2014;42:1134-42. [Crossref] [PubMed]
- Longo UG, Franceschi F, Ruzzini L, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med 2008;36:533-8. [PubMed]
- Iannotti JP, Codsi MJ, Kwon YW, et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am 2006;88:1238-44. [Crossref] [PubMed]
- Neviaser JS, Neviaser RJ, Neviaser TJ. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am 1978;60:681-4. [Crossref] [PubMed]
- Walton JR, Bowman NK, Khatib Y, et al. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am 2007;89:786-91. [PubMed]
- Arnoczky SP, Bishai SK, Schofield B, et al. Histologic evaluation of biopsy specimens obtained after rotator cuff repair augmented with a highly porous collagen implant. Arthroscopy 2017;33:278-83. [Crossref] [PubMed]
- Van Kampen C, Arnoczky S, Parks P, et al. Tissue-engineered augmentation of a rotator cuff tendon using a reconstituted collagen scaffold: a histological evaluation in sheep. Muscles Ligaments Tendons J 2013;3:229-35. [Crossref] [PubMed]
- Ahmad S, Haber M, Bokor DJ. The influence of intraoperative factors and postoperative rehabilitation compliance on the integrity of the rotator cuff after arthroscopic repair. J Shoulder Elbow Surg 2015;24:229-35. [Crossref] [PubMed]
- Lee TQ. Current biomechanical concepts for rotator cuff repair. Clin Orthop Surg 2013;5:89-97. [Crossref] [PubMed]
- Park MC, Tibone JE, ElAttrache NS, et al. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg 2007;16:469-76. [Crossref] [PubMed]
- Bokor DJ, Sonnabend D, Deady L, et al. Preliminary investigation of a biological augmentation of rotator cuff repairs using a collagen implant: a 2-year MRI follow-up. Muscles Ligaments Tendons J 2015;5:144-50. [Crossref] [PubMed]
- Bokor DJ, Sonnabend D, Deady L, et al. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: a 2-year MRI follow-up. Muscles Ligaments Tendons J 2016;6:16-25. [Crossref] [PubMed]
- Schlegel TF, Abrams JS, Bushnell BD, et al. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: a prospective multicenter study. J Shoulder Elbow Surg 2018;27:242-51. [Crossref] [PubMed]
- Thon SG, O’Malley L 2nd, O’brien MJ, et al. Evaluation of healing rates and safety with a bioinductive collagen patch for large and massive rotator cuff tears: 2-year safety and clinical outcomes. Am J Sports Med 2019;47:1901-8. [Crossref] [PubMed]
- McIntyre LF, Bishai SK, Brown PB 3rd, et al. Patient-reported outcomes after use of a bioabsorbable collagen implant to treat partial and full-thickness rotator cuff tears. Arthroscopy 2019;35:2262-71. [Crossref] [PubMed]
- Park JH, Oh KS, Kim TM, et al. Effect of smoking on healing failure after rotator cuff repair. Am J Sports Med 2018;46:2960-8. [Crossref] [PubMed]
- Naimark M, Robbins CB, Gagnier JJ, et al. Impact of smoking on patient outcomes after arthroscopic rotator cuff repair. BMJ Open Sport Exerc Med 2018;4:e000416 [Crossref] [PubMed]
- Bedi A, Fox AJ, Harris PE, et al. Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg 2010;19:978-88. [Crossref] [PubMed]
Cite this article as: Thon SG, Belk JW, Bravman JT, McCarty EC, Savoie FH 3rd. Regeneten bio-inductive collagen scaffold for rotator cuff tears: indications, technique, clinical outcomes, and review of current literature. Ann Joint 2020;5:41.


