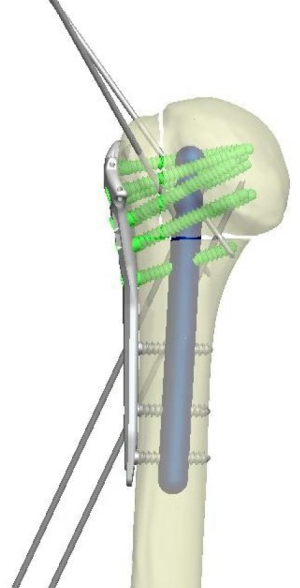
Locking plate fixation for proximal humerus fractures—when do I use a fibular strut?
Introduction
Fractures of the proximal humerus account for 4–8% of all fractures and are the third most common type of injury in patients over 65 years of age (1-3). The incidence of these fractures has increased in the past decade, likely attributable to the aging population and the associated increase in the incidence of osteoporosis (4,5). Despite the relative frequency of these fractures, the majority are amenable to non-operative management with good functional outcomes (6-8).
The treatment of displaced fractures remains controversial. A recent Level I study suggests that at 5 years, displaced surgical neck fractures in the older population managed non-operatively may do as well as those treated surgically (9). Similarly, it is unnecessary to operate on certain low demand patients with 3- and 4-part fractures (10). Based on poor historical outcomes when managed conservatively (4,11-23), treating younger patients with surgery remains a good option.
Due to the deforming forces of the musculature and the suboptimal quality of bone in these cases, operative management presents a challenge. These factors are likely the cause for high failure rates seen with in early attempts at osteosynthesis (11). Suboptimal surgical outcomes have led to many alternative surgical strategies with mixed results. To date, no optimal method of fixation has been identified (4,12-24). In the last two decades, locked plate technology has improved the management of proximal humeral fractures. This can be attributed to the ability to obtain more rigid fixation in compromised bone, thereby eliminating many of the problems associated with standard plating (25-32).
Locked plate fixation was initially expected to dramatically improve patient outcomes; however, this has not been the case. Outcome studies on locking plate technology continue to demonstrate equivocal results with complication rates as high as 20–30% and a reoperation rate of 10% (33,34). Limitations in locking plate technology has led to advances in surgical strategies and techniques resulting in improved outcomes (8). In the case of osteoporotic fractures, surgical management has evolved to include inferiorly placed calcar screws, augmentation with heavy sutures, and the use of a well-positioned intramedullary fibular strut allograft (11,35,36).
Anatomy
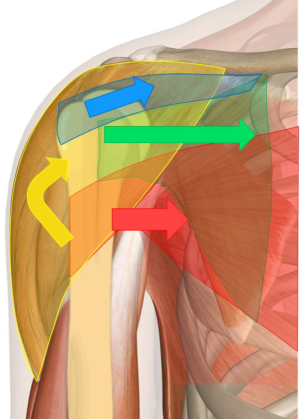
The proximal humerus can be divided into four anatomical parts based on its epiphyseal lines: the head, the greater tuberosity, the lesser tuberosity, and the proximal shaft (37). The average neck-shaft angle is 130° and the average retroversion of the articular surface, relative to the trans-epicondylar axis, is 30° (37,38). For three of the four muscles of the rotator cuff (infraspinatus, supraspinatus, and teres minor), the greater tuberosity serves as the attachment. These muscles serve to abduct and externally rotate the shoulder. The lesser tuberosity serves as the attachment for the subscapularis, which internally rotates the shoulder. The pectoralis attaches on the lateral edge of the bicipital groove and the latissimus dorsi attaches on the medial edge.
With disruption of the bony anatomy after a fracture, these muscles pull the fragments in a predictable manner. The greater tuberosity is displaced superiorly and posteriorly by the pull of its attached muscles. The lesser tuberosity is pulled medially by the subscapularis. The combined forces of the deltoid and pectoralis pull the shaft proximally and medially, respectively, with resultant varus deformity (Figure 1).
Classification
Although many classification systems have been proposed, the two most popular systems are the Neer classification and the AO classification (39,40). The Neer classification, which is the most widely used system, categorizes displaced proximal humerus fractures from two to four parts according to its anatomic segments. Displacement is defined as separation of a fragment >1 cm or angulation of a fragment greater than 45°. Fracture lines in nondisplaced segments are not included. The AO classification, a more complex system, is based on the vascular supply of the articular segments. It is divided into three categories (A, B, C) of increasing severity with each category further split into numerical subgroupings (39).
Radiographic analysis
Accurate imaging is crucial to proper diagnosis and treatment. An initial radiographic assessment should include an anteroposterior (AP) view, an AP view in the scapular plane (Grashey view), a scapular Y view, and an axillary lateral view. Obtaining four views is more accurate in denoting fracture displacement in proximal humerus fractures and can help in decision making (41). The axillary view is critical, as it is important in assessing the extent of tuberosity displacement, determining if a posterior dislocation is present, and assessing the integrity of the glenoid. This view can easily be obtained by gently abducting the arm or, if the patient is in too much pain, a Velpeau view is also an option (42).
In patients presenting with completed advanced imaging, however, one may consider forgoing a painful axillary view as one recent study suggested it may not help in decision making (2). Computed tomography (CT) with 2-mm cuts and 3D reconstructions, although not necessary in all cases, may be helpful in more complex articular fractures to better assess the fracture type and the extent of fracture displacement. It is extremely helpful in delineating the locations of the fracture lines in complex fractures. For example, in most 3-part fractures that involve the greater tuberosity, the fracture line is located posterior to the bicipital groove. The bone in the bicipital groove has arguably the greatest density in the humeral head (43,44). Magnetic resonance imaging is not generally helpful but can assist in the diagnosis of avascular necrosis in the more chronic situation.
Non-operative management
Eighty percent of proximal humeral fractures are minimally displaced and amenable to conservative management with good to excellent results (7,40,45). Although debatable in younger patients, Court-Brown & McQueen demonstrated that non-operative management for varus-impacted fractures with angulation >40° is also acceptable with no correlation found between final angulation and overall shoulder pain or function (46). Regardless of fracture pattern, patients with significant comorbidities who are unable to tolerate a surgical procedure should be considered for conservative management.
General non-operative recommendations include a sling and swathe or shoulder immobilizer for comfort with the initiation of gentle pendulum activities within 7–10 days after the injury. Over-aggressive early motion can compromise the fracture position due to the pull of the rotator cuff musculature resulting in a malunion. If early motion is advised, routine imaging at 2-week intervals is recommended to monitor for potential displacement. The authors’ preferred treatment includes a shoulder immobilizer for approximately 4 weeks with rigid immobilization of the shoulder and emphasis on regular elbow and wrist range of motion. Shoulder range of motion is generally initiated at the fourth week with passive and active-assisted motion for the next 6 weeks or until radiographic union is achieved. Rotator cuff strengthening is usually started at 3 months or with radiographic confirmation of union.
Surgical management
Surgical intervention is an option for displaced Neer 2-, 3-, and 4-part fractures in younger active patients (20,40,47). The two main options for surgery are either open reduction and internal fixation (ORIF) or prosthetic replacement. Several techniques of ORIF have been described including percutaneous fixation, various methods of tension band fixation, standard plating with screws and pegs, and intramedullary fixation. The published outcomes of these techniques have been mixed with no preferred treatment identified (48-50). Several of the described techniques are also associated with a relatively high complication rate, including hardware failure, hardware pain, loss of function, and injury to adjacent neurovascular structures (48).
Locking plates with divergent screws are a valuable tool when internal fixation is selected as the preferred technique. Interest has been generated based on its early success in multiple European series and the avoidance of many of the complications associated with the previous devices, such as hardware failure or hardware pain (25,27,29). Biomechanical studies confirm improved fixation with the use of a locked plate, as compared to other fixation techniques (26,30-32). Unlike standard plating, which compresses the screws to the bone, the locked plate construct is more akin to a lever beam. Biomechanical comparative studies have shown that the linear range until failure was extended by 64% in the locked group (30).
Despite the improvements in torsional strength with locked plates compared to blade plates, numerous publications reported nebulous initial results, with complication rates generally ranging from 20% to 30% and a reoperation rate of approximately 10% (33,34). With improved understanding of fracture patterns, we have improved our techniques for fracture fixation. It is critical to achieve cortical contact and to augment tuberosity fixation with suture fixation (31). In cases where a comminuted humeral metadiaphyseal segment exists, cortical contact can be attained by using structural fibular allograft or by shortening the humerus by impacting the shaft into the humeral head. In osteoporotic bone where the tuberosities are of poor quality, reliable augmentation can be attained by utilizing heavy sutures passed through the tendon/bone interface of the rotator cuff. These are then secured to the suture islets of the locked plate to balance the forces of the rotator cuff (24).
The clinical results of the use of fibular strut allograft as a supplement to standard locking plate fixation of proximal humerus fractures are promising (49-51). Fibular strut allograft augmentation of repair in ORIF increases overall construct stiffness and maximal failure load (49) while providing medical support and thus decreasing the chance of screw penetration, humeral head collapse, and over-reduction of the greater tuberosity.
The ideal fracture pattern for use of the fibular strut is in a patient with significant metaphyseal comminution that compromises the medial calcar (51). In this situation, the strut acts as a reduction aid to reconstruct the humeral shaft. The humeral head can then be impacted onto the shaft to further provide stability prior to application of the pre-contoured plate. Little et al. justified use of this technique for all 3- and 4-part fractures, and 2-part fractures with angulation greater than 45 degrees and displacement greater than 1 cm (50). Badman et al. described use of the fibular strut to treat nonunions of the surgical neck (51). With this technique, they achieved radiographic union in 17/18 (94%) of patients. Our indications for use of a fibular strut allograft are: posteromedial calcar comminution, metadiaphyseal fracture extension, and fracture nonunion cases. In addition, we have found the strut helpful in patients with osteoporosis to prevent over reduction of the greater tuberosity.
Surgical technique (locked plating)
Anesthesia
An interscalene block is recommended to minimize pain postoperatively. The endotracheal tube should be positioned on the opposite side of the surgical field to prevent inadvertent dislodgement during surgery (52-54).
Positioning
Sufficient fluoroscopic imaging is crucial and appropriate time should be taken to position the patient and the C-arm. The table is rotated 180° so that the patient’s head is sitting at the foot of the table over the radiolucent footplate with which most operating tables are equipped.
Once on the table, the head of the bed is elevated approximately 30° with the head supported with a jelly doughnut and secured in place. The table is then turned 90° relative to the anesthesiologist to allow the C-arm to be positioned at the head of the bed and parallel to the patient. This allows for an unobstructed view of the shoulder and avoids interference with the anesthesiologist. The C-arm can be rotated over the top so that a direct AP view is obtained. Once satisfactory imaging is confirmed, the C-arm can be pulled away from the patient so as not to interfere with the surgical prep. If adequate imaging is not obtained, the patient and C-arm should be repositioned prior to sterile prepping.
Approach
A standard deltopectoral approach is used. A padded Mayo stand is used to help support the arm in the abducted position, both minimizing tension on the neurovascular structures and deltoid and avoiding the need for an extra assistant. The cephalic vein is taken laterally with the deltoid. Gelpi retractors may be placed superficially, allowing for the development of the subdeltoid space. The Browne deltoid retractor [Innomed Inc., Savannah, GA, USA] is placed next and is essential in aiding proper exposure. The clavipectoral fascia is released and the subcoracoid space is developed. We do not recommend placing a self-retaining retractor beneath the conjoined tendon to avoid the risk of musculocutaneous nerve neuropraxia.
The biceps tendon is now identified in the groove superior to the pectoralis major tendon and can easily be palpated as it rolls under the pectoralis insertion on the humeral shaft. Hematoma can often obscure the obvious landmarks so using this as a reference can be helpful. Occasionally, the biceps tendon is interposed within the fracture fragments and may require mobilization.
In cases where the biceps tendon is entrapped within the fracture site, we believe that this tendon will never function normally. Therefore, to avoid a source of postoperative pain, a soft tissue tenodesis to the upper border of the pectoralis major is performed. We do not routinely release the pectoralis tendon, but up to 20% of its upper border can be released to aid in the exposure.
Fracture preparation
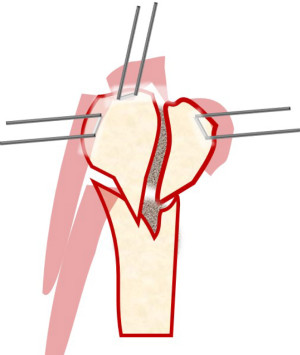
The fracture hematoma is debrided and the tuberosities are mobilized. Heavy sutures are placed at the bone-tendon junction of each tuberosity. If a tuberosity remains attached to the head segment, then a Krackow stitch is placed in the substance of the attached tendon. We routinely place two sutures or tapes in the subscapularis and a minimum of two in the supraspinatus and infraspinatus (Figure 2). These sutures serve as traction sutures to assist with the overall reduction of the fracture and serve to counter the deforming forces of the rotator cuff. The next maneuver requires that the arm is extended to expose the proximal shaft, and this facilitates access to recess behind the greater tuberosity. The clot and hematoma are then removed from within the medullary canal, and the shaft fracture edge is debrided.
The first step to reconstruction requires anatomic reduction of the head segment. A Cobb or Key elevator is utilized to disimpact the head. The head segment can be held in place by inserting a K-wire through the head into the glenoid. In fractures with extensive comminution where a large metaphyseal void is encountered after reduction of the tuberosities, we advise the use of a fibular strut allograft. This can be placed intramedullary within the proximal canal and will serve to prevent subsequent collapse of the head postoperatively and prevent overreduction of the greater tuberosity. The graft is typically impacted into position with 2 cm of bone left exposed. If the humeral intramedullary canal has a large diameter, a unicortical blocking screw placed anteriorly can aid in the positioning of the fibular strut within the canal. Care must be taken to avoid using too long of a strut, as the strut adds significant difficulty to any attempt at conversion to a prosthesis if the patient develops post-traumatic arthritis. A 4 cm strut is ideal in the majority of cases. For small voids, cortico-cancellous bone graft can be used.
The humeral shaft is then reduced to the head and the head is provisionally pinned to the shaft to maintain the overall reduction. The bicipital groove can be utilized to gauge rotation. Placing the pin medial the groove and aiming in the distal to proximal direction avoids a trajectory towards the brachial plexus and axillary artery and allows you to keep the wire in place and out of the way during positioning of the plate lateral to the groove.
Once the head and shaft are anatomically reduced, the tuberosities beneath the head fragment should also be reduced utilizing the traction sutures previously placed in the cuff. If a greater tuberosity fracture is present, it can be pinned by inserting a K-wire from the posterolateral edge of the acromion through the tuberosity and into the shaft (Figure 3). Fluoroscopy is utilized to confirm acceptable reduction.
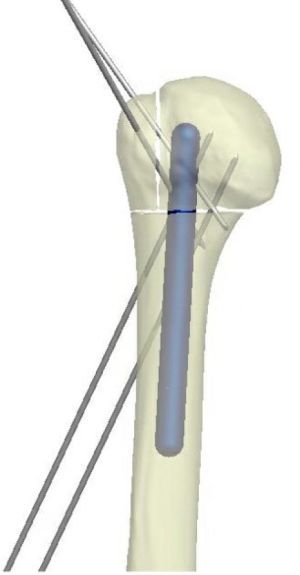
Plate application
The fracture must be reduced anatomically prior to application of the locked plate.
Although many different locking plates are available, the following design characteristics are particularly important:
- low profile to reduce the risk of impingement on the acromion;
- divergent proximal locking screw options to reduce the risk of pullout and improve head fixation;
- the presence of multiple suture eyelets on the plate that facilitate suture augmentation of the rotator cuff to the plate.
General manufacturer recommendations include positioning the plate just lateral to the bicipital groove, typically between 1–3 cm distal to the top of the humeral head based on the plate type. Most plates offer a gliding oblong hole for initial shaft fixation. Placing the screw inferiorly will allow you to later move the plate inferiorly, as the tendency is to initially place the plate too superior. This screw should also be non-locking to allow for proper reduction and compression of the plate to the shaft.
Once plate height is acceptable, most plates allow for a K-wire to be placed through the plate and into the head. Proximal locking screws are then sequentially placed. Our preference is to only drill the outer cortex and then place the depth gauge under fluoroscopy, as this is felt to decrease the chance of penetrating the articular surface. For younger patients or those with better bone stock or if a fibular strut is used, a drill may be necessary.
Superior screws are intentionally left shorter, as these screws are at higher risk for late perforation and are less important in terms of fixation than the more inferior calcar screws (55,56). Anatomic studies show that the strongest bone is located in the inferior posteromedial portion of the humeral head (57). Once all locking screws are inserted, the remaining shaft screws are placed with a minimum of three shaft screws (Figures 4,5).
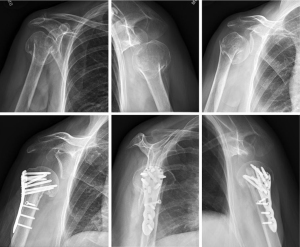
Tuberosity fixation
An important tenet involves fixation of the tuberosities to the plate. Much like the principles involved with arthroplasty for fracture, the deforming forces of the rotator cuff need to be neutralized in order to avoid subsequent displacement and failure. The deforming forces of the rotator cuff are counterbalanced by using heavy sutures in the rotator cuff and tying them to the plate via suture eyelets at the plate’s proximal end. Some plates which require that the sutures be passed prior to application are less desirable, as this can be time consuming. Instead, some eyelets or “cleats” have been incorporated as design options that allow suture passage after the plate is secured. Typically, two sutures are placed through the subscapularis and 2–3 sutures are passed into the substance of the supraspinatus and infraspinatus for a total of at least four sutures tied to the plate. Once fixation is secure and the sutures are tied to the plate, the arm is rotated to assure fracture stability and final fluoroscopic imaging is obtained.
The rotator interval is closed with a FiberWire or No. 2 Ethibond and the wound is closed in a standard fashion.
Post-operative management
Patients are placed in an abduction shoulder immobilizer and typically admitted post-operatively for a period of 24 hours for intravenous antibiotics and 1 to 2 days for pain control. For stable 2-part fractures in younger patients, the immobilizer is discontinued at 2 weeks, allowing gentle pendulum and passive and active assisted range of motion, focusing on forward flexion. Three- and four-part fractures are usually immobilized for 4–6 weeks with an emphasis on elbow and wrist range of motion only. Generally, formal physical therapy is not initiated until at least 4 weeks for all fracture types. Active range of motion usually begins at 8–12 weeks or with the first radiograph showing evidence of callous formation. Strengthening is started in the last phase of formal physical therapy, typically at 12 weeks.
Follow-up radiographs are obtained at the initial post-op visit and then at 6 weeks, 3 months, 6 months, and annually thereafter for a period of at least 2 years. Close monitoring is important to identify hardware complications as well as the late development of avascular necrosis.
Conclusions
Our indications for use of a fibular strut allograft in proximal humerus fractures are: posteromedial calcar comminution, metadiaphyseal fracture extension, and fracture nonunion cases. Additionally, the fibular strut is useful in patients with osteoporosis to prevent over reduction of the greater tuberosity. With these indications, we feel that the use of a fibular strut will lead to improved construct stiffness, decreased chance of humeral head screw penetration, and thus improved clinical outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Adam Seidl) for the series “Management of Fractures Around the Shoulder” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-42). The series “Management of Fractures Around the Shoulder” was commissioned by the editorial office without any funding or sponsorship. GPS reports personal fees from DJO Global, personal fees from Smith and Nephew, outside the submitted work. MAM reports personal fees from DJO Surgical, personal fees from Stryker, personal fees from DePuy Mitek, outside the submitted work. KNC reports personal fees from DJO Surgical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Badman BL, Mighell M, Drake GN. Proximal humeral nonunions: surgical technique with fibular strut allograft and fixed-angle locked plating. Tech Shoulder Elb Surg 2006;7:95-101. [Crossref]
- Berkes MB, Dines JS, Birnbaum JF, et al. The axillary view typically does not contribute to decision making in care for proximal humeral fractures. HSS J 2015;11:192-7. [Crossref] [PubMed]
- Koukakis A, Apostolou CD, Taneja T, et al. Fixation of proximal humerus fractures using the PHILOS plate: early experience. Clin Orthop Relat Res 2006;115-20. [Crossref] [PubMed]
- Hawkins RJ, Bell R, Gurr K. The three-part fracture of the proximal part of the humerus. Operative treatment. J Bone Joint Surg Am 1986;68:1410-4. [Crossref] [PubMed]
- Kannus P, Palvanen M, Niemi S, et al. Osteoporotic fractures of the proximal humerus in elderly Finnish persons: sharp increase in 1970-1998 and alarming projections for the new millennium. Acta Orthopaedica Scandinavica 2000;71:465-70. [Crossref] [PubMed]
- Gerber C, Werner C, Vienne P. Internal fixation of complex fractures of the proximal humerus. J Bone Joint Surg Br 2004;86:848-55. [Crossref] [PubMed]
- Iannotti JP, Ramsey ML, Williams GR, et al. Nonprosthetic management of proximal humeral fractures. JBJS 2003;85:1578-93. [Crossref]
- Jawa A, Burnikel D. Treatment of proximal humeral fractures: a critical analysis review. JBJS Rev 2016; [Crossref] [PubMed]
- Handoll HH, Keding A, Corbacho B, et al. Five-year follow-up results of the PROFHER trial comparing operative and non-operative treatment of adults with a displaced fracture of the proximal humerus. Bone Joint J 2017;99-B:383-92. [Crossref] [PubMed]
- Ring D, McKee MD, Perey BH, et al. The use of a blade plate and autogenous cancellous bone graft in the treatment of ununited fractures of the proximal humerus. J Shoulder Elbow Surg 2001;10:501-7. [Crossref] [PubMed]
- Badman BL, Mighell M. Fixed-angle locked plating of two-, three-, and four-part proximal humerus fractures. J Am Acad Orthop Surg 2008;16:294-302. [Crossref] [PubMed]
- Darder A, Darder JA, Sanchis V, et al. Four-part displaced proximal humeral fractures: operative treatment using Kirschner wires and a tension band. J Orthop Trauma 1993;7:497-505. [Crossref] [PubMed]
- Esser RD. Treatment of three-and four-part fractures of the proximal humerus with a modified cloverleaf plate. J Orthop Trauma 1994;8:15-22. [Crossref] [PubMed]
- Cuomo F, Flatow EL, Maday MG, et al. Open reduction and internal fixation of two-and three-part displaced surgical neck fractures of the proximal humerus. J Shoulder Elbow Surg 1992;1:287-95. [Crossref] [PubMed]
- Jaberg H, Warner J, Jakob R. Percutaneous stabilization of unstable fractures of the humerus. J Bone Joint Surg Am 1992;74:508-15. [Crossref] [PubMed]
- Jakob RP, Miniaci A, Anson PS, et al. Four-part valgus impacted fractures of the proximal humerus. J Bone Joint Surg Br 1991;73:295-8. [Crossref] [PubMed]
- Naranja RJ, Iannotti JP. Displaced three-and four-part proximal humerus fractures: evaluation and management. J Am Acad Orthop Surg 2000;8:373-82. [Crossref] [PubMed]
- Paavolainen P, Björkenheim JM, Slätis P, et al. Operative treatment of severe proximal humeral fractures. Acta Orthopaedica Scandinavica 1983;54:374-9. [Crossref] [PubMed]
- Savoie FH, Geissler W, Vander Griend R. Open reduction and internal fixation of three-part fractures of the proximal humerus. Orthopedics 1989;12:65-70. [PubMed]
- Stableforth PG. Four-part fractures of the neck of the humerus. J Bone Joint Surg Br 1984;66:104-8. [Crossref] [PubMed]
- Sturzenegger M, Fornaro E, Jakob R. Results of surgical treatment of multifragmented fractures of the humeral head. Arch Orthop Trauma Surg 1982;100:249-59. [Crossref] [PubMed]
- Szyszkowitz R, Seggl W, Schleifer P, et al. Proximal humeral fractures. Management techniques and expected results. Clin Orthop Relat Res 1993;13-25. [Crossref] [PubMed]
- Weseley MS, Barenfeld PA, Eisenstein AL. Rush pin intramedullary fixation for fractures of the proximal humerus. J Trauma 1977;17:29-37. [Crossref] [PubMed]
- Wijgman AJ, Roolker W, Patt T, et al. Open reduction and internal fixation of three and four-part fractures of the proximal part of the humerus. J Bone Joint Surg Am 2002;84:1919-25. [Crossref] [PubMed]
- Björkenheim JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral fractures with a locking compression plate A retrospective evaluation of 72 patients followed for a minimum of 1 year. Acta Orthopaedica Scandinavica 2004;75:741-5. [Crossref] [PubMed]
- Edwards SL, Wilson NA, Zhang LQ, et al. Two-part surgical neck fractures of the proximal part of the humerus: a biomechanical evaluation of two fixation techniques. J Bone Joint Surg Am 2006;88:2258-64. [PubMed]
- Fankhauser F, Boldin C, Schippinger G, et al. A new locking plate for unstable fractures of the proximal humerus. Clin Orthop Relat Res (1976-2007) 2005;430:176-81.
- Fulkerson E, Egol KA, Kubiak EN, et al. Fixation of diaphyseal fractures with a segmental defect: a biomechanical comparison of locked and conventional plating techniques. J Trauma 2006;60:830-5. [Crossref] [PubMed]
- Rose PS, Adams CR, Torchia ME, et al. Locking plate fixation for proximal humeral fractures: initial results with a new implant. J Shoulder Elbow Surg 2007;16:202-7. [Crossref] [PubMed]
- Seide K, Triebe J, Faschingbauer M, et al. Locked vs. unlocked plate osteosynthesis of the proximal humerus–a biomechanical study. Clin Biomech (Bristol, Avon) 2007;22:176-82. [Crossref] [PubMed]
- Weinstein DM, Bratton DR, Ciccone WJ II, et al. Locking plates improve torsional resistance in the stabilization of three-part proximal humeral fractures. J Shoulder Elbow Surg 2006;15:239-43. [Crossref] [PubMed]
- Wheeler DL, Colville MR. Biomechanical comparison of intramedullary and percutaneous pin fixation for proximal humeral fracture fixation. J Orthop Trauma 1997;11:363-7. [Crossref] [PubMed]
- Südkamp N, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate: results of a prospective, multicenter, observational study. J Bone Joint Surg Am 2009;91:1320-8. [Crossref] [PubMed]
- Thanasas C, Kontakis G, Angoules A, et al. Treatment of proximal humerus fractures with locking plates: a systematic review. J Shoulder Elbow Surg 2009;18:837-44. [Crossref] [PubMed]
- Walch G, Badet R, Nove-Josserand L, et al. Nonunions of the surgical neck of the humerus: surgical treatment with an intramedullary bone peg, internal fixation, and cancellous bone grafting. J Shoulder Elbow Surg 1996;5:161-8. [Crossref] [PubMed]
- Ring D. Current concepts in plate and screw fixation of osteoporotic proximal humerus fractures. Injury 2007;38:S59-68. [Crossref] [PubMed]
- Codman E. The Shoulder Boston. Thomas Todd 1934:123-77.
- Keene JS, Huizenga R, Engber W, et al. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics 1983;6:173-8. [Crossref] [PubMed]
- Jakob RP, Kristiansen T, Mayo K, et al. Classification and aspects of treatment of fractures of the proximal humerus. In: Bateman JE, Welsh RP. editor. Surgery of the shoulder. Philadelphia: BC Decker Inc, 1984:330-43.
- Neer C. Displaced proximal humerus fractures, Part 1. J Bone Joint Surg A 1970;52:1077-89. [Crossref]
- Parsons BO, Klepps SJ, Miller S, et al. Reliability and reproducibility of radiographs of greater tuberosity displacement: a cadaveric study. J Bone Joint Surg Am 2005;87:58-65. [Crossref] [PubMed]
- Sidor ML, Zuckerman JD, Lyon T, et al. Classification of proximal humerus fractures: The contribution of the scapular lateral and axillary radiographs. J Shoulder Elbow Surg 1994;3:24-7. [Crossref] [PubMed]
- Hasan AP, Phadnis J, Jaarsma RL, et al. Fracture line morphology of complex proximal humeral fractures. J Shoulder Elbow Surg 2017;26:e300-8. [Crossref] [PubMed]
- Sukthankar AV, Leonello DT, Hertel RW, et al. A comprehensive classification of proximal humeral fractures: HGLS system. J Shoulder Elbow Surg 2013;22:e1-6. [Crossref] [PubMed]
- Gerber C, Schneeberger A, Vinh T. The arterial vascularization of the humeral head. An anatomical study. J Bone Joint Surg Am 1990;72:1486-94. [Crossref] [PubMed]
- Court-Brown CM, McQueen M. The impacted varus (A2. 2) proximal humeral fracture Prediction of outcome and results of nonoperative treatment in 99 patients. Acta Orthopaedica Scandinavica 2004;75:736-40. [Crossref] [PubMed]
- Clifford PC. Fractures of the neck of the humerus: a review of the late results. Injury 1980;12:91-5. [Crossref] [PubMed]
- Sanders RJ, Thissen LG, Teepen JC, et al. Locking plate versus nonsurgical treatment for proximal humeral fractures: better midterm outcome with nonsurgical treatment. J Shoulder Elbow Surg 2011;20:1118-24. [Crossref] [PubMed]
- Bae JH, Oh JK, Chon CS, et al. The biomechanical performance of locking plate fixation with intramedullary fibular strut graft augmentation in the treatment of unstable fractures of the proximal humerus. J Bone Joint Surg Br 2011;93:937-41. [Crossref] [PubMed]
- Little MT, Berkes MB, Schottel PC, et al. The impact of preoperative coronal plane deformity on proximal humerus fixation with endosteal augmentation. J Orthop Trauma 2014;28:338-47. [Crossref] [PubMed]
- Badman BL, Mighell M, Kalandiak SP, et al. Proximal humeral nonunions treated with fixed-angle locked plating and an intramedullary strut allograft. J Orthop Trauma 2009;23:173-9. [Crossref] [PubMed]
- Abildgaard JT, Lonergan KT, Tolan SJ, et al. Liposomal bupivacaine versus indwelling interscalene nerve block for postoperative pain control in shoulder arthroplasty: a prospective randomized controlled trial. J Shoulder Elbow Surg 2017;26:1175-81. [Crossref] [PubMed]
- Sabesan VJ, Shahriar R, Petersen-Fitts GR, et al. A prospective randomized controlled trial to identify the optimal postoperative pain management in shoulder arthroplasty: liposomal bupivacaine versus continuous interscalene catheter. J Shoulder Elbow Surg 2017;26:1810-7. [Crossref] [PubMed]
- Namdari S, Nicholson T, Abboud J, et al. Randomized controlled trial of interscalene block compared with injectable liposomal bupivacaine in shoulder arthroplasty. J Bone Joint Surg Am 2017;99:550-6. [Crossref] [PubMed]
- Ockert B, Braunstein V, Kirchhoff C, et al. Monoaxial versus polyaxial screw insertion in angular stable plate fixation of proximal humeral fractures: radiographic analysis of a prospective randomized study. J Trauma 2010;69:1545-51. [Crossref] [PubMed]
- Padegimas EM, Zmistowski B, Lawrence C, et al. Defining optimal calcar screw positioning in proximal humerus fracture fixation. J Shoulder Elbow Surg 2017;26:1931-7. [Crossref] [PubMed]
- Hepp P, Lill H, Bail H, et al. Where should implants be anchored in the humeral head? Clin Orthop Relat Res 2003;139-47. [Crossref] [PubMed]
Cite this article as: Stone GP, Christmas KN, Mighell MA. Locking plate fixation for proximal humerus fractures—when do I use a fibular strut? Ann Joint 2020;5:44.