Parosteal lipoma of humerus with a medical history of 24 years: a case report
Introduction
Parosteal lipoma is an extremely rare benign tumor, being responsible for less than 0.1% of primary bone tumors and only 0.3% of all lipomas (1). This tumor consists mainly of mature adipose tissue and shows contiguity to periosteum of the underlying bone. It is believed to be one of the rarest primary bone tumors, which comprised mostly of mature adipose tissue with a bony component. The most common sites of this tumor are femur followed by proximal radius. This has become great importance of differential diagnosis of malignant tumors, due to bony lesions are found in almost half of patients with this tumor. We hereby describe a case of parosteal lipoma of the humerus in a 70-year-old male patient, which had the longest reported medical history (24 years) in the last 50 years. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aoj-19-152) (2).
Case report
A 70-year-old male patient appeared with a 24-year history of mass on his left upper arm. In 1991, the mass firstly appeared with the size of a bean and asymptomatic. The mass enlarged slowly over the years. In April 2015, the mass displayed the size of a fist and caused night pain. No interventions had been done before. The patient had no history of trauma or other prior similar tumors. There was no drug history or family history.
On clinical examination On April 27, 2015, an approximately 7 cm ×5 cm sized mass was revealed on left upper arm, with an oval shape, elastic, immobile and nontender. Pain was complained while the left shoulder joint moved. There was no sensory deficit in any part of the left arm.
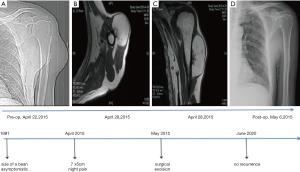
X-ray image showed a radiolucent soft-tissue mass contiguous with left proximal humerus and irregular osseous protuberance of subjacent bone (Figure 1A). On the magnetic resonance imaging (MRI), a large 11 cm ×6 cm ×5 cm well-defined, lobulated, predominantly fat intensity lesion with an underlying area of a multilobulated, and juxtacortical bony excrescence measuring 2.7 cm ×1.9 cm ×0.9 cm showing no contiguity with marrow space of proximal left humerus (Figure 1B,C).
On May 5, 2015, total tumor resection was performed under brachial plexus anesthesia. Tumor was discovered to be situated on the humerus with tight continuity, with minute areas of spiculation palpable on the bone surface.
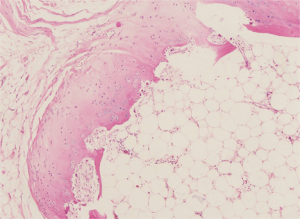
On histopathology (Figure 2), a close relationship has been demonstrated between the lesion composing of mature lipocytes and the periosteum, consistent with parosteal lipoma.
The patient was satisfied and experienced neither movement disorder nor dysesthesia after the surgery. Postoperative X-ray (Figure 1D) of left humerus was normal and presented no bony excrescences. He had no recurrence and no complaints at latest follow-up (June 2020, 5 years after the operation).
All procedures performed in studies involving human participant were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Parosteal lipoma is a rare type of lipoma, “Periosteal lipoma” had been its name once, while it was replaced by “parosteal lipoma” to indicate that the tumor adjacent to the bone but not necessarily originate from periosteal (3). These lesions are typically obtained on the diaphyses of long bones in middle-aged people (4). Followed by the location of proximal radius, the most common sites are femur. The lesion has also been reported originating in scapula, ribs, clavicle, metacarpals, pelvis, metatarsals, mandible and skull (3), perhaps almost all the bones in the body are affected.
According to the degree of chondroid modulation and endochondral ossification, these tumors are classified into 4 subtypes: (I) no ossification; (II) pedunculated exostosis; (III) sessile exostosis; and (IV) patchy chondro-osseous modulation. As revealed in our case, the focal hyperostotic change mimicking malignancy is the most common finding in the underlying bone.
The radiographs clearly illustrated that parosteal lipoma is a well-defined area of lucency located adjacent to a long bone. About 60% of parosteal lipomas may have potential bony alterations, mostly hyperostotic reactive changes (fine linear densities, calcification, cortical thickening or undulation, or frank excrescences of bone), but these lipomas also have cortical bowing (in patients with growing bones), smooth cortical erosions or underlying osteochondroma (5). Bone destruction was absent.
On computed tomography, parosteal lipomas usually present as well-defined fat density mass with lobulated appearance adherent to underlying bone. The presented osseous excrescences can be distinguished from an osteochondroma, since it lacks of contiguity of the marrow space with the adjacent bone. The CT images are helpful in evaluating the relationship of the mass with the adjacent bone, which is important for surgical planning.
MRI is considered most valuable for evaluation of parosteal lipoma. On MRI, despite of pulse sequence, the tumor is recognized as a juxtacortical mass with same signal intensity to that of subcutaneous fat. Low-signal-intensity strands on T1-weighted images in the lesion, corresponding to fibrovascular strands, can be differentiated from those of well-differentiated liposarcoma, as these are thin and lack postcontrast enhancement (6). Adjacent muscle atrophy, caused by associated nerve entrapment, is identified on MRI as general pattern of increased striations of fat in the affected muscle. This finding is best valued on T2-weighted images because of the reduced signal intensity of normal muscle relative to fat. Cartilaginous components in parosteal lipoma showed intermediate signal intensity on T1-weighted images but high signal intensity on T2-weighted images (7). From the above, MRI is the best approach to exhibit the relationship between the tumor and the underlying bone, which is crucial for surgical planning.
Pathologically, a parosteal lipoma is usually a multi-lobulated mass circumscribed by a thin, fibrous capsule, and it is well encapsulated with a broad-based attachment to the underlying bone (8). Under the microscope, the adipocytes of a parosteal lipoma occur identical to fat cells found in the subcutaneous tissues. There are no reports that this tumor undergoes malignant transformation. The differential diagnosis with low-grade liposarcoma may be difficult, despite there are no previous reports of either primary parosteal liposarcoma or degenerated benign parosteal liposarcoma with these parosteal lipomas of patients (9).
The treatment of parosteal lipoma is intact surgical excision with further subperiosteal dissection, osteotomy, or segmental resection of the bone in cases with hyperostosis (10,11). The prognosis is good and local recurrence is unusual. Due to its negligible malignant potential, parosteal lipoma can be followed conservatively. In our case, it would be better if an X-ray or MRI was performed at the time of 2-year follow-up.
Conclusions
Parosteal lipomas are benign with an excellent prognosis. Surgery, which is ideal treatment, requires particular attention to ensure that any periosteal involvement is removed completely. For purpose of providing suitable treatment, parosteal lipoma should be incorporated in the differential diagnosis of soft tissue tumors.
Acknowledgments
Funding: This study was supported by National Nature Science Foundation of China (NSFC, 81472508).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aoj-19-152
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-19-152). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participant were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aoki S, Kiyosawa T, Nakayama E, et al. Large Parosteal Lipoma without Periosteal Changes. Plast Reconstr Surg Glob Open 2015;3:e287 [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Greco M, Mazzocchi M, Ribuffo D, et al. Parosteal lipoma. Report of 15 new cases and a review of the literature. Ann Ital Chir 2013;84:229-35. [PubMed]
- Miller MD, Ragsdale BD, Sweet DE. Parosteal lipomas: a new perspective. Pathology 1992;24:132-9. [Crossref] [PubMed]
- Fleming RJ, Alpert M, Garcia A. Parosteal lipoma. Am J Roentgenol 1962;87:1075-84.
- Balani A, Sankhe A, Dedhia T, et al. Lump on back: a rare case of parosteal lipoma of scapula. Case Rep Radiol 2014;2014:169157 [Crossref] [PubMed]
- Murphey MD, Johnson DL, Bhatia PS, et al. Parosteal lipoma: MR imaging characteristics. AJR Am J Roentgenol 1994;162:105-10. [Crossref] [PubMed]
- Yu JS, Weis L, Becker W. MR imaging of a parosteal lipoma. Clin Imaging 2000;24:15-8. [Crossref] [PubMed]
- Fernández-Sueiro JL, Pinto JA, Blanco FJ, et al. Multiple parosteal lipoma associated to polyarthritis. Joint Bone Spine 2006;73:202-4. [Crossref] [PubMed]
- Bispo Junior RZ, Guedes AV. Parosteal lipoma of the femur with hyperostosis: case report and literature review. Clinics (Sao Paulo) 2007;62:647-52. [Crossref] [PubMed]
- Kapukaya A, Subasi M, Dabak N, et al. Osseous lipoma: eleven new cases and review of the literature. Acta Orthop Belg 2006;72:603-14. [PubMed]
Cite this article as: Lu J, Fan G, Zhou G. Parosteal lipoma of humerus with a medical history of 24 years: a case report. Ann Joint 2020;5:45.