
Chondrocytes-osteoblast transition in endochondral ossification
Vertebrate skeleton is formed by two kinds of mechanisms: intramembranous ossification (IO) and endochondral ossification (EO). The remarkable difference between them is the formation of cartilage anlage in EO. In the intramembranous ossification, the mesenchymal cells condense and directly differentiate into bone-forming osteoblasts which can secrete bone extracellular matrix and ultimately differentiate into osteocytes. Some craniofacial bones are formed by this way (1). The axial and appendicular skeletons are formed by EO in which a cartilage intermediate is involved. Mesenchymal cells first condense where the future bone will form, and become osteochondroprogenitors. The osteochondroprogenitors in the outer layer of condensation progressively differentiate to osteogenic cells. At the same time, cells in the center of condensation undergo strict and sequential chondrogenic differentiation. At the later stage of chondrocyte development, the immature chondrocytes exit from cell cycle and hypertrophy. When cartilage matrix is mineralized and the vascular invasion occurs, osteoblast precursors differentiate into osteoblasts and deposit bone matrix on the degenerating cartilage scaffolding (Figure 1). However, the ultimate fate of HCs in this process is still controversial.
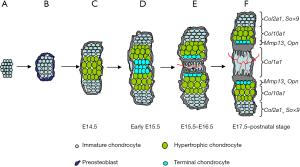
Origin and differentiation program of chondrogenic lineage
After mesenchymal cells migrate from the cranial neural crest, paraxial mesoderm and lateral plate mesoderm, they pack in the presumptive skeleton sites, prefiguring the future skeletal elements (2). Under the control of transcription factor, Sox9, osteochondroprogenitors in the center of condensation begin to express Col2a1 and are committed to early chondrocytes. Chondrocyte is the only cell component that makes up the cartilage tissue. Chondrocyte differentiation process is concomitant with a strong increase in deposition of cartilage matrix. Matrix proteins such as type II collagen, type IX collagen, type XI collagen and aggrecan are highly produced. Besides Sox9, two other Sox members, Sox5 and Sox6, also play critical roles in early chondrocyte differentiation. But whether other unidentified transcription factors are likely to work there together with the Sox trio is still unclear.
Early chondrocytes are small, round, and progressively become flattened and organized into parallel, longitudinal columns, forming columnar chondrocytes. Since these cells proliferate at high rate, they are also called proliferating chondrocytes. Once they exit from cell cycle and undergo irreversible growth arrest, they mature and differentiate into prehypertrophic chondrocytes (preHCs). At gene expression level, columnar chondrocytes distinguish themselves from early chondrocytes by quantitative changes, rather than qualitative changes. No specific molecular markers have been identified for these proliferating chondrocytes. There are some extracellular matrix genes up-regulating progressively concomitantly with the appearance of these cells: e.g., Agc1, and Comp. Many signaling pathways, such as IHH, PTHrP/PPR, FGFs, BMPs and WNT signaling, have been found to interact together to regulate the formation and maintenance of the proliferating chondrocytes.
When proliferating chondrocytes differentiate into prehypertrophic and hypertrophic chondrocytes (HCs), these cells undergo obvious phenotypic switch in some aspects: cell morphology, cell status, gene expression profile and so on. Proliferating chondrocytes exit from cell cycle and progressively increase their cytoplasmic volume up to about 10 times. PreHCs still express high level of Col2a1, Agc1 and most other cartilage matrix genes. However, when chondrocytes are fully hypertrophic, they reduce, even stop, the expression of some cartilage markers and up-regulate Col10a1. It is well known that PTHrP/IHH feedback loop, FGFs, BMPs signaling pathways play essential roles in controlling the rate at which proliferating chondrocytes undergo hypertrophy (3-5).
Terminal differentiation of HCs
It is generally believed that HCs represent the terminal stage of differentiation in chondrogenic cell lineage (6) and these post-mitosis chondrocytes finally undergo apoptosis in the process of EO (6-8).
However, some study showed that HC could undergo another phenotypic change and further differentiate into terminal chondrocyte (4). A histological photography of murine humerus stained with alcian blue showed the presence of some smaller cells called terminal chondrocytes. They were found to be located between proximal and distal HCs, adjacent to the bone collar. Furthermore, HCs were shown to only reach its late stage by down-regulating Col10a1 expression and expressing some of osteoblast markers, e.g., alkaline phosphatase (Alp), Mmp13 and Opn (6,9,10).
Owing to its negligible number and the bottom-most location in the postnatal hypertrophic zone, the terminal chondrocytes gain little attention for a long time. Hence, the regulative mechanism of terminal differentiation process and the function of these terminal chondrocyte are still poorly understood.
Besides many regulatory roles of Runx2 in osteogenesis and chondrogenesis, Runx2 was also found to regulate chondrocyte maturation. Runx2 null mice died just after birth, due to a failure to breathe (11-13). These mice showed a lack of hypertrophy and terminal differentiation of chondrocyte in most of the skeleton. Ectopic expression of Runx2 in non-HCs promoted their hypertrophic differentiation and disrupted joint formation (12,14). VEGF, which is normally expressed in hypertrophic chondrocyte, was not expressed in chondrocytes of Runx2 null mutant. Furthermore, VEGF expression was up regulated by Runx2 in fibroblasts in tissue culture (15). These data suggested that Runx2 might be a direct regulator of VEGF expressed by HCs. Moreover, several studies have strongly suggested that Runx2 might directly regulate and activate such genes as Mmp13 and Opn (16-19). Therefore, one hypothesis is that Runx2, which is expressed in HCs, can activate markers of terminal chondrocytes, e.g., Mmp13, Opn.
Sox9 plays a vital and irreplaceable role in the cell fate determination of the chondrogenic lineage. The expression of Sox9 is turned on in osteochondroprogenitor cells prior to condensation. Sox9 maintains its high level of expression in chondrocyte until the beginning of prehypertrophy. The expression of Sox9 is turned off when chondrocytes exit from cell cycle and undergo maturation. Previous gene regulation studies revealed that Sox9 could bind to the enhancer region of some chondrocyte-specific markers such as Col2a1 (20,21), Col11a1 (22) and aggrecan (23), and increase their expression. Consistent with the vital role of Sox9 in chondrocyte differentiation, overexpression of Sox9 in HCs delayed endochondral bone formation with the decrease of VEGFa, Mmp13 and Rankl, indicative of the necessity of “turn-off” of Sox9 in the terminal differentiation of HCs.
Another transcription factor involving in regulating terminal differentiation of HCs is c-Maf, a member of Maf family. This family belongs to the basic leucine zipper (bZIP) superfamily which includes Fos, Jun and the CREB/ATF family. Although c-Maf is believed to act as a developmental regulator, there are few cellular targets of c-Maf identified so far. In situ hybridization (ISH) has shown that c-Maf was expressed specifically in HCs, terminal chondrocytes, primary spongiosa and the perichondrium (24). c-Maf deficient mutant mice displayed normal prehypertrophy and early hypertrophy, determined by normal onset and level of expression of Ppr, Ihh and Col10a1 (24). However, the terminal differentiation of HCs was initially delayed in c-Maf -/- embryos, followed by a subsequent expansion of the hypertrophic chondrocyte domain in the growth plate of fetal and postnatal long bones. These results suggested that c-Maf facilitated the initiation of terminal differentiation of HCs and influenced the disappearance of HCs. The specific decrease of Mmp13 expression in c-Maf -/- embryos at E15.5 suggested it was possible that Mmp13 was a downstream target of c-Maf.
Up to now, our knowledge about terminal differentiation of chondrocytes is at its infancy. Further characterization of the terminally differentiated chondrocytes and investigation of the transcriptional regulation of terminal differentiation will help us understand more about the switch from hypertrophy to terminal differentiation and the ultimate cell fate of chondrocyte in EO.
Apoptotic fate vs. trans-differentiation fate of terminal HCs
Whether HCs undergo apoptosis or transdifferentiate to bone cells in EO is still unclear. This question is really controversial for more than a century mainly because that the transdifferentiation concept of HCs was difficult to be accepted. Instead, apoptosis detection using many separate techniques have showed strong evidences supporting HCs undergo programmed cell death in EO (8,25-29). And the in-depth investigation of apoptosis showed the specific microenvironment present in the cartilage-bone transition zone linked to the activation of apoptosis. Concomitant with the degeneration of mineralized matrix, the local microenvironment in HZ is changed and it liberates high local concentrations of O2, ions, peptides and glycans, many of which are essential for the activation of apoptosis (30-32).
However, the finding of apoptosis in HCs shown in these studies could not exclude other fate of HCs. Apoptosis occurs frequently in normal tissues and organs. It is not surprising to find them in the process of cartilage-bone conversion. By TUNEL assay, the percentage of apoptotic HCs in all HCs was quantified and varied in different group’s studies, 3.29% or 44%, suggesting that other HCs may have other final than “apoptosis” fate. Serial morphological studies from Crelin and Koch (33) have showed each chondrocyte could generate several small cells and form reticular tissue when undergoing ossification. This conclusion seems more striking, but their observation might give some hints. In 1996, Erenpreisa and Roach described an asymmetrical division of chondrocyte. Chondrocyte gave rise to two daughter cells: one undergoes apoptosis and the other one survives and re-enters cell cycle. This interesting finding provided new insight into the transdifferentiation hypothesis (34-37).
Moreover, the in vitro culture and transplantation experiments also brought more direct evidence to the transdifferentiation hypothesis. Chondrocyte in culture were found to be able to express the markers of osteoblast and the cell morphology also changed to osteoblast-like cells (38-40). And when growth plates from quail were transplanted into the chick chorioallantoic membrane (41), the osteoblasts in the resultant bone were entirely derived from quail, suggestive of transdifferentiation from HCs to osteoblasts.
Even so, some researchers also considered that transdifferentiation was more circumstantial. They believed that hypertrophic chondrocyte was proposed to have the potential to differentiate into osteogenic cells and initially contributed to bone formation. However, only the “borderline chondrocytes”, which were exposed to the appropriate matrix and environment, could differentiate into osteogenic cells (42). The HCs residing in the core of the epiphyseal growth plate was suggested to undergo apoptosis (29). This scenario indeed gives another interesting explanation for the final fate of HCs.
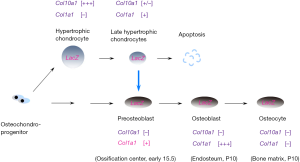
This dispute is still going on until three elegant works published by different groups. The common strategy in these studies is in vivo lineage tracing used to address this challengeable question. The specificity of Cre recombination mouse is the key factor affecting us to draw such an overwhelming conclusion. Cheah’s group first reported chondrocyte-to-osteoblast lineage continuum by using tamoxifen-inducible Col10-Cre knock-in mouse to label HCs temporally (10). Cre fragment is transcriptional expressed by endogenous Col10a1 promoter and Cre expression is strictly limited within the Col10a1-expressing region. This specific Col10-Cre knock-in mouse was adopted to trace the ultimate fate of HCs in vivo by crossing to reporter mice. This study clearly showed HCs, at least partially, survived in cartilage-bone transition and transdifferentiate into osteogenic cells (Figure 2). These HCs-derived osteoblasts (HCOB) are mainly located within the primary spongiosa at embryonic stage. At postnatal stage, these descendant cells are not evenly distributed in metaphyseal and they predominately resided in trabecular bone and endosteum (10). These HCOBs finally differentiated to osteocytes, mostly residing within metaphyseal bone. HCs-derived positive cells were not detected in periosteum and much less positive cells was found in diaphyseal cortical bone. The profound lesson from these discriminative locations of different ontogenies of osteoblasts and osteocytes remained unknown. It might be correlatively connected with some special physiological functions of HCOB. Crombrugghe’s group (43) and Yang’s group (44) also gave consistent conclusion by use of different Cre recombination mouse lines: Col10a1-BAC-Cre, Col10a1int2-Cre and other tamoxifen-induced Agc1-CreERT and Col2a1-CreERT. Besides of osteogenic lineage, Yang’s group also showed some data that HCs exhibited multiple destinies at postnatal stage: vascular endothelial cells, pericytes and adipocytes. These results indicated the in vivo multipotential cell fates of HCs (44). However, we could not jump to the final conclusion of the multi-differentiation potential of HCs descendants and need more solid evidences to support this striking hypothesis. It will overturn current concept of skeletal stem cells if this hypothesis is finally demonstrated in the future.
Molecular control of chondrocyte fates during ossification
Apart from the direct observation and study of chondrocyte-osteoblast transdifferentiation, we obtained more about the underlying molecular regulation of this process from analyzing genetically manipulated mice.
Even though the transdifferentiation concept has not been accepted before, there already have some reports showing the terminal differentiation and cell fate were impaired by genetically modifying some key regulators. Osterix (Osx), a Kruppel-like Sp-1 binding factor, is a novel osteoblast-specific transcription factor and belongs to the SP family. Compared to the absence of both of hypertrophic chondrocyte and bone marrow in Runx2-/- mice, Osx deficient mice blocked bone marrow formation only and hypertrophic zone is formed normally (45). This study indicated that Osx function followed downstream of Runx2 and might play more strict role in chondrocyte-osteoblast differentiation. And the unregulated expression of Osx in late HCs of E15.0 also supported its possible function in cartilage-bone transition (10).
WNT proteins are secreted glycoproteins involved in various developmental and Wnt/β-catenin pathway regulates skeleton development at many key levels (46). The indispensable role of canonical Wnt signaling in osteogenic differentiation has been revealed by modifying β-catenin gene expression temporally and spatially. When Wnt/β-catenin pathway was ablated in osteochondroprogenitors, the osteogenic differentiation was arrested at osteochondroprogenitor stage and these cells exhibited chondrocyte characteristics, expressing Sox9 and Col2a1. Further in vivo and in vitro studies suggested that β-catenin functions as a cell fate determinant factor driving osteochondroprogenitors into the osteoblast lineage through preventing chondrogenesis (47-49). Gain of function study of Wnt/β-catenin pathway in immature chondrocytes leads to their dedifferentiation and blockage of further hypertrophic differentiation. Wnt10b and Wnt7b has been considered as a ligand candidate regulating this process because it could induce the mesenchymal cells differentiating to osteogenic cells rather than adipocyte (50,51). After the concept of chondrocyte-to-osteoblast transdifferentiation was accepted, the role of Wnt/β-catenin pathway in this transition got much attention. Although similar work was reporter on 2013 by Klaus von der Mark team (52), Christine Hartmann’s study emphasized the contribution of decreased chondrocyte-derived osteoblast differentiation when Wnt/β-catenin pathway was removed from HCs, other than its indirect regulation on osteoclast differentiation (53). This elegant work provides solid evidence indicating Wnt/β-catenin pathway is the key regulator of chondrocyte-osteoblast transdifferentiation.
Col10a1-13del transgenic mice expressing mutant collagen X as a consequence of a 13-base pair deletion in Col10a1 (13del) elicited endoplasmic reticulum stress (ERS) in HCs because of the misfolded a1(X) chains accumulated within HCs (54). HCs under such situation altered their terminal differentiation and cell fate. They were found to re-enter cell cycle and re-express immature chondrocyte markers, Sox9 and Col2a1. These cells did not show much apoptosis fate; therefore, the most possibility was that they changed their transdifferentiation back to more immature status. Similar finding of ERS-induced cell fate determination was also observed in cartilage oligomeric matrix protein (COMP) deficient mice, indicative of the key role of ER stress and response on cell fate determination of HCs.
Besides of autonomous regulation by transcription factors and signaling pathways, the in vivo cell fate of HCs was also influenced by surrounding matrix environment. Matrix metalloproteases, such as MMP13 and MMP14 (also known as MT1-MMP), were synthesized by HCs and degraded cartilage matrix in cartilage-bone transition. Loss of Mmp13 or Mmp14 induced an expansion of hypertrophic zone due to less vascular invasion and bone resorption defects (55,56). Undoubtedly, the cartilage-bone transition process was interrupted by bone resorption defects. And the terminal differentiation and final fate of HCs could be predicted to have changed a lot because of an expanded hypertrophic zone. However because of the absence of detecting more molecular markers, the status and the ending of HCs are hardly determined in these two mutant mice. It will be more convincing if the fate of mutant HCs was explored and followed by further lineage tracing experiments.
Most of specific transcription factors and signaling pathways of osteoblast differentiation, such as Osx and Wnt/β-catenin pathway, are involved in the chondrocyte-osteoblast transdifferentiation. But whether there is any special regulator responsible for this lineage-crossing differentiation remains unclear. The finding of such a key lineage-crossing factor might give us more insight for many lineage studies in other organs.
Perspectives on physiological function of chondrocyte-derived osteogenitors
Up to now, the concept of chondrocyte-osteoblast transdifferentiation has been well known as an alternative fate of HCs besides of undergoing apoptosis. Therefore, there are two sources of osteogenitors in endochondral bone formation: perichondral cells and chondrocytes. Their locations in long bone are obviously different, with chondrocyte-derived osteoblasts in endosteum and chondrocyte-derived osteocyte mainly in metaphyseal bone, indicating they might have variant physiological functions in maintaining the homeostasis of bone marrow. And whether they exhibit differently in disease cases are also unknown. Upon aging and osteoporosis, these perichondrium-derived and chondrocyte-derived osteoblasts and osteocytes loss their biological function equally or not. All these answers will help us understand more about skeleton homeostasis and develop more suitable strategy to maintain bone mass.
Of articular cartilage, chondrocytes in calcified zone are positive for Col10a1, and these cells were also labelled in lineage tracing experiment by using of Col10a1-Cre mice. Unlike of the formation of primary ossification center in which the perichondrium-derived osteoprogenitors are much involved, the secondary ossification center is much clear and the involved of another origin of subchondral osteoblasts was suspected except for articular cartilage-derived. The ultimate fate of these articular chondrocytes is still absent and it need to be addressed urgently by solid in vivo data. Notably in osteoarthritis (OA), articular cartilage undergoes degeneration and calcified zone was expanded in some cases. Whether these degenerated cartilages produce more chondrocyte-derived osteoblasts for subchondral bone was still an open question. The answer will extend our knowledge on joint homeostasis and disease, helping us develop more effective drugs or interventions for future.
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (81472043 and 81572192).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamashiro T, Wang XP, Li Z, et al. Possible roles of Runx1 and Sox9 in incipient intramembranous ossification. J Bone Miner Res 2004;19:1671-7. [Crossref] [PubMed]
- Aubin JE, Liu F, Malaval L, et al. Osteoblast and chondroblast differentiation. Bone 1995;17:77S-83S. [Crossref] [PubMed]
- Karaplis AC. PTHrP: novel roles in skeletal biology. Curr Pharm Des 2001;7:655-70. [Crossref] [PubMed]
- Lefebvre V, Smits P. Transcriptional control of chondrocyte fate and differentiation. Birth Defects Res C Embryo Today 2005;75:200-12. [Crossref] [PubMed]
- Day TF, Yang Y. Wnt and hedgehog signaling pathways in bone development. J Bone Joint Surg Am 2008;90:19-24. [Crossref] [PubMed]
- Pacifici M, Golden EB, Oshima O, et al. Hypertrophic chondrocytes. The terminal stage of differentiation in the chondrogenic cell lineage? Ann N Y Acad Sci 1990;599:45-57. [Crossref] [PubMed]
- Cheung JO, Grant ME, Jones CJ, et al. Apoptosis of terminal hypertrophic chondrocytes in an in vitro model of endochondral ossification. J Pathol 2003;201:496-503. [Crossref] [PubMed]
- Gibson G, Lin DL, Roque M. Apoptosis of terminally differentiated chondrocytes in culture. Exp Cell Res 1997;233:372-82. [Crossref] [PubMed]
- Bi W, Deng JM, Zhang Z, et al. Sox9 is required for cartilage formation. Nat Genet 1999;22:85-9. [Crossref] [PubMed]
- Yang L, Tsang KY, Tang HC, et al. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA 2014;111:12097-102. [Crossref] [PubMed]
- Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89:755-64. [Crossref] [PubMed]
- Kim IS, Otto F, Zabel B, et al. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev 1999;80:159-70. [Crossref] [PubMed]
- Otto F, Thornell AP, Crompton T, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997;89:765-71. [Crossref] [PubMed]
- Takeda S, Bonnamy JP, Owen MJ, et al. Continuous expression of Cbfa1 in nonhypertrophic chondrocytes uncovers its ability to induce hypertrophic chondrocyte differentiation and partially rescues Cbfa1-deficient mice. Genes Dev 2001;15:467-81. [Crossref] [PubMed]
- Zelzer E, Glotzer DJ, Hartmann C, et al. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech Dev 2001;106:97-106. [Crossref] [PubMed]
- Ducy P, Zhang R, Geoffroy V, et al. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 1997;89:747-54. [Crossref] [PubMed]
- Frendo JL, Xiao G, Fuchs S, et al. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J Biol Chem 1998;273:30509-16. [Crossref] [PubMed]
- Kern B, Shen J, Starbuck M, et al. Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J Biol Chem 2001;276:7101-7. [Crossref] [PubMed]
- Hess J, Porte D, Munz C, et al. AP-1 and Cbfa/runt physically interact and regulate parathyroid hormone-dependent MMP13 expression in osteoblasts through a new osteoblast-specific element 2/AP-1 composite element. J Biol Chem 2001;276:20029-38. [Crossref] [PubMed]
- Bell DM, Leung KK, Wheatley SC, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet 1997;16:174-8. [Crossref] [PubMed]
- Lefebvre V, Huang W, Harley VR, et al. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol 1997;17:2336-46. [Crossref] [PubMed]
- Bridgewater LC, Lefebvre V, de Crombrugghe B. Chondrocyte-specific enhancer elements in the Col11a2 gene resemble the Col2a1 tissue-specific enhancer. J Biol Chem 1998;273:14998-5006. [Crossref] [PubMed]
- Sekiya I, Tsuji K, Koopman P, et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem 2000;275:10738-44. [Crossref] [PubMed]
- MacLean HE, Kim JI, Glimcher MJ, et al. Absence of transcription factor c-maf causes abnormal terminal differentiation of hypertrophic chondrocytes during endochondral bone development. Dev Biol 2003;262:51-63. [Crossref] [PubMed]
- Farnum CE, Wilsman NJ. Condensation of hypertrophic chondrocytes at the chondro-osseous junction of growth plate cartilage in Yucatan swine: relationship to long bone growth. Am J Anat 1989;186:346-58. [Crossref] [PubMed]
- Bronckers AL, Goei W, Luo G, et al. DNA fragmentation during bone formation in neonatal rodents assessed by transferase-mediated end labeling. J Bone Miner Res 1996;11:1281-91. [Crossref] [PubMed]
- Ohyama K, Farquharson C, Whitehead CC, et al. Further observations on programmed cell death in the epiphyseal growth plate: comparison of normal and dyschondroplastic epiphyses. J Bone Miner Res 1997;12:1647-56. [Crossref] [PubMed]
- Suda N, Shibata S, Yamazaki K, et al. Parathyroid hormone-related protein regulates proliferation of condylar hypertrophic chondrocytes. J Bone Miner Res 1999;14:1838-47. [Crossref] [PubMed]
- Shapiro IM, Adams CS, Freeman T, et al. Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today 2005;75:330-9. [Crossref] [PubMed]
- Magne D, Bluteau G, Faucheux C, et al. Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res 2003;18:1430-42. [Crossref] [PubMed]
- Mansfield K, Teixeira CC, Adams CS, et al. Phosphate ions mediate chondrocyte apoptosis through a plasma membrane transporter mechanism. Bone 2001;28:1-8. [Crossref] [PubMed]
- Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci U S A 2005;102:9637-42. [Crossref] [PubMed]
- Crelin ES, Koch WE. An autoradiographic study of chondrocyte transformation into chondroclasts and osteocytes during bone formation in vitro. Anat Rec 1967;158:473-83. [Crossref] [PubMed]
- Roach HI, Erenpreisa J. The phenotypic switch from chondrocytes to bone-forming cells involves asymmetric cell division and apoptosis. Connect Tissue Res 1996;35:85-91. [Crossref] [PubMed]
- Erenpreisa J, Roach HI. Epigenetic selection as a possible component of transdifferentiation. Further study of the commitment of hypertrophic chondrocytes to become osteocytes. Mech Ageing Dev 1996;87:165-82. [Crossref] [PubMed]
- Roach HI. Trans-differentiation of hypertrophic chondrocytes into cells capable of producing a mineralized bone matrix. Bone Miner 1992;19:1-20. [Crossref] [PubMed]
- Roach HI. New aspects of endochondral ossification in the chick: chondrocyte apoptosis, bone formation by former chondrocytes, and acid phosphatase activity in the endochondral bone matrix. J Bone Miner Res 1997;12:795-805. [Crossref] [PubMed]
- Galotto M, Campanile G, Robino G, et al. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res 1994;9:1239-49. [Crossref] [PubMed]
- Descalzi Cancedda F, Gentili C, Manduca P, et al. Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol 1992;117:427-35. [Crossref] [PubMed]
- Holtrop ME. The potencies of the epiphyseal cartilage in endochondral ossification. Proc K Ned Akad Wet C 1967;70:21-8. [PubMed]
- Kahn AJ, Simmons DJ. Chondrocyte-to-osteocyte transformation in grafts of perichondrium-free epiphyseal cartilage. Clin Orthop Relat Res 1977;299-304. [Crossref] [PubMed]
- Bianco P, Cancedda FD, Riminucci M, et al. Bone formation via cartilage models: the "borderline" chondrocyte. Matrix Biol 1998;17:185-92. [Crossref] [PubMed]
- Zhou X, von der Mark K, Henry S, et al. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet 2014;10:e1004820 [Crossref] [PubMed]
- Yang G, Zhu L, Hou N, et al. Osteogenic fate of hypertrophic chondrocytes. Cell Res 2014;24:1266-9. [Crossref] [PubMed]
- Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcriptional factor osterix is required for osteoblast differentiation and bone formation. Cell 2002;108:17-29. [Crossref] [PubMed]
- Kühl M, Sheldahl LC, Park M, et al. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000;16:279-83. [PubMed]
- Day TF, Guo X, Garrett-Beal L, et al. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005;8:739-50. [Crossref] [PubMed]
- Hill TP, Spater D, Taketo MM, et al. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 2005;8:727-38. [Crossref] [PubMed]
- Hu H, Hilton MJ, Tu X, et al. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005;132:49-60. [Crossref] [PubMed]
- Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A 2005;102:3324-9. [Crossref] [PubMed]
- Chen J, Tu X, Esen E, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet 2014;10:e1004145 [Crossref] [PubMed]
- Golovchenko S, Hattori T, Hartmann C, et al. Deletion of beta catenin in hypertrophic growth palte chondrocytes impairs trabecular bone formation. Bone 2013;55:102-12. [Crossref] [PubMed]
- Houben A, Kostanova-Poliakova D, Weissenböck M, et al. β-catenin activity in late hypertrophic chondrocytes locally orchestrates osteoblastogenesis and osteoclastogenesis. Development 2016;143:3826-38. [Crossref] [PubMed]
- Tsang KY, Chan D, Cheslett D, et al. Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol 2007;5:e44 [Crossref] [PubMed]
- Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A 2004;101:17192-7. [Crossref] [PubMed]
- Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004;131:5883-95. [Crossref] [PubMed]
Cite this article as: Wang L, Jie Q, Yang L. Chondrocytes-osteoblast transition in endochondral ossification. Ann Joint 2017;2:4.


