
Streptococcus viridans periprosthetic joint infections
Introduction
The incidence of total knee arthroplasty (TKA) and total hip arthroplasty (THA) operations is increasing in the United States, along with the number of complications including periprosthetic joint infection (PJI) (1). Although the rate of PJI after total joint arthroplasty (TJA) is low, with a reported rate of 0.3–1.7% for TKA and 0.8–1.9% for THA, the rate is slowly increasing despite interventions (2).
Organisms responsible for PJI are often bacteria, which may spread from other parts of the body or may originate from the surgical wound itself. For example, organisms from the urogenital system, skin, gastrointestinal tract, and oral mucosa have all been found to cause PJI (3). The varieties of sources mirror the diversity of organisms that have the potential to cause PJI. Current literature identifies Staphylococcus aureus as the most common bacterial source of infection, followed by coagulase-negative Staphylococcus, beta-hemolytic Streptococcus, Enterococci and Streptococcus viridans (S. viridans) (4). However, reports on PJI due to S. viridans are limited and are not commonly found in the literature. Additionally, results from a retrospective study demonstrated evidence of increasing incidence of S. viridans infection (2). Thus, the purpose of this review article is to discuss the prevention, diagnosis and treatment of S. viridans PJI.
S. viridans
S. viridans exists as an overarching group of organisms with common laboratory characteristics. They are visualized as gram-positive cocci with chaining morphology. These organisms do not grow on 6.5% NaCl or bile esculin agar. Laboratory measures show that they are catalase negative, pyrrolidonyl arylamidase negative, optochin resistant and not bile soluble. The S. viridans umbrella is further separated into five subfamilies: S. mutans, S. salivarius, S. anginosus, S. sanguinis, and S. mitis (5-7). All S. viridans organisms are found primarily in the oral cavity and S. anginosus can also be found in the gastrointestinal tract (6).
Each subfamily of the S. viridans group has additional identifying features. The S. mutans species ferment mannitol and sorbitol, hydrolyze esculin and has a positive Voges-Proskauer test. S. salivarius has a positive Voges-Proskauer test and hydrolyzes esculin. S. anginosus is able to hydrolyze arginine and esculin, and results in a positive Voges-Proskauer test. S. sanguinus can hydrolyze arginine and esculin. On the other hand, S. mitis cannot hydrolyze arginine or esculin, cannot ferment mannitol or sorbitol, and has a negative Voges-Proskauer reaction (5-7) (Table 1).
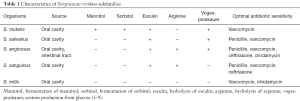
Full table
Although relatively rare and not seen often in the literature, organisms from almost each subfamily of S. viridans have been noted to cause PJI after primary TKA or THA. Researchers described either the specific subgroup—S. mutans (3,10), S. anginosus (11), S. sanguinus (12,13), or S. mitis (13-18)—or cited the overarching group S. viridans (3,18-31) as responsible for PJI in their patients. S. salivarius has not been specifically reported in the literature as the causative organism of PJI in TKA or THA.
Prevention
The best way to limit the consequences of PJI is to prevent the infection before it occurs. Although remote sources are considered to be a rare cause of PJI (32), specific subfamilies of S. viridans are thought to cause PJI due to spread from another part of the body. Specifically, S. mutans, which is normally found in the oral cavity (5,6,10), has the ability to spread hematogenously and infect a prosthetic joint. The literature on antibiotic prophylaxis prior to an oral procedure in patients with prosthetic joints is controversial. Prior to 1997, there was no consensus or professional recommendation on prophylactic measures. Some reports indicate a relationship between dental procedures and the onset of an S. mutans PJI (3,12,21,22,25). This relationship generated the idea of limiting the hematogenous spread from invasive oral procedures with preemptive antibiotic measures.
The American Academy of Orthopedic Surgeons (AAOS) and the American Dental Association (ADA) released a total of three joint statements regarding antibiotic prophylaxis in TJA patients prior to oral procedures. Additionally, the AAOS released a further individual statement on the practice (33-36). The first joint statement was released in 1997, and the report suggested that antibiotic prophylaxis prior to dental procedures was not routinely indicated in most patients. However, certain subpopulations were at higher risk for PJI and should be considered for prophylaxis. These included patients undergoing procedures that were at high-risk for hematogenous spread of infection, as well as immunocompromised patients, or patients with comorbidities with a high likelihood for infection that underwent TJA within two years of the oral procedure (34).
In 2003, the AAOS and ADA released the next update based on a combined literature analysis. The major difference was clarification of the recommendation based on the two-year postoperative time mark. Antibiotic prophylaxis was still recommended for high-risk dental procedures, but only as a routine measure for patients that underwent TJA within the last two years. In immunocompromised and comorbid patients, antibiotic prophylaxis was still recommended only before high-risk dental procedures, but with the added clarification that the duration since the index TJA should be disregarded (33,37). No antibiotic prophylaxis was recommended for all other patient groups: low risk dental procedures in immunocompromised or comorbid TJA patients, or TJA performed more than two years prior (33,37).
In the 1997 and 2003 statements, high-risk dental procedures were defined as dental extraction, periodontal procedures, dental implants, endodontic instrumentation beyond the tooth apex, subgingival placement of antibiotic fibers or strips, placement of orthodontic bands, intraligamentary local anesthetic injections and tooth cleaning with anticipated bleeding. Low-risk procedures include restorative dentistry, local anesthetic injections, intracanal endodontic treatments, placement of rubber dams, postoperative suture removal, placement of removable prosthetic or orthodontic appliances, taking oral impressions, fluoride treatments, oral radiographs, orthodontic appliance adjustment, and shedding of primary teeth. The risk stratification of high or low was based on likelihood of hematogenous spread of bacteria. Patients were considered immunocompromised if they had a history of rheumatoid arthritis, systemic lupus erythematous, other inflammatory polyarthropathy, or drug or radiation induced suppression. Comorbid medical conditions include previous PJI, malnutrition, hemophilia, human immunodeficiency virus (HIV), type 1 diabetes mellitus or malignancy (33,34,37).
The AAOS released a solo information statement in 2009 regarding the practice of antibiotic prophylaxis before oral procedures in patients with a history of TJA. The AAOS recommended that prophylactic antibiotics be given to all patients before all oral procedures—regardless of time since surgery or risk of hematoengous spread (37). The new statement was without consultation of any dental professional organization and was ultimately unsuccessful at replacing the combined 2003 report (35).
The most recent and current joint AAOS/ADA recommendation was published in 2013. The report utilized evidence based medicine and literature reviews to create recommendations regarding oral dental procedures and PJI. The workgroup submitted a total of three recommendations regarding the relationship of bacteremic spread from the oral cavity and PJI in patients with a prosthetic joint implant. The first recommendation stated that health care professionals should consider discontinuing the use of antibiotic prophylaxis prior to invasive dental procedures. The recommendation was graded as “limited,” implying that the quality of evidence was unconvincing and that strong studies do not show a clear advantage from either practice. With this in mind, the paper stressed that each provider must decide what is in the best interest of the patient after consideration of the limited recommendation, personal clinical judgment, and the wishes of the patient. The second conclusion formed by the workgroup was that they were unable to recommend for or against the use of topical oral antibiotics in patients with prosthetic joint implants. This statement received an “inconclusive” grade and it was suggested that providers be cognizant of any future publications that may sway the debate in one direction. The final recommendation that received consensus support was that all patients with prosthetic joint implants maintain appropriate oral hygiene. The consensus was formed around the expert opinions of the members of the group, and not based on published data (36).
When antibiotic prophylaxis is indicated, the 1997 and 2003 combined reports provide suggested treatment regimens to be administered one hour before the oral procedure. First line antibiotic therapy is two grams of amoxicillin, cephradine or cephalexin by mouth. If the patient is unable to tolerate oral medication, then 1 g of cefazolin or 2 g of ampicillin can be administered intravenously or intramuscularly. If the patient is allergic to beta-lactams, then 600 mg of clindamycin by mouth or intravenously is indicated (34,37).
Diagnosis
Studies suggest that history taking is the most effective way to determine the source of an infection (38). Although hematogenous seeding from a remote source infection is considered a rare cause of PJI (32), a thorough history will provide a complete medical picture for the clinician.
The current standard for diagnosing PJI is from the Musculoskeletal Infection Society (MSIS) criteria developed in 2011 (39). The criteria defined PJI if there was a sinus tract communicating with the prosthesis or if a pathogen was isolated via culture from two separate tissue or fluid samples. PJI was also diagnosed if 4 of 6 specific laboratory measures were met: (I) elevated serum erythrocyte sedimentation rate (ESR) and elevated serum C-reactive protein (CRP); (II) elevated synovial fluid leukocyte count; (III) elevated synovial fluid neutrophil percentage; (IV) presence of purulence in the affected joint; (V) isolation of microorganism in one culture of tissue or fluid; and/or (VI) greater than five neutrophils per high-power field in five high-power fields at ×400 magnification. However, the statement did concede that a PJI may exist without meeting the criteria listed (39). Subsequent to the MSIS criteria, the International Consensus Group on PJI developed a new definition of PJI, which removed the criteria of purulence and added leukocyte esterase as another marker of infection (40).
Prior to the adoption of the standardized diagnostic criteria, there was a wide spectrum of diagnostic methods to establish the presence of a PJI. Specifically, the diagnosis of S. viridans joint infection was based on a multitude of factors. Many researchers utilized the presence of elevated serum inflammatory markers, specifically ESR and CRP, as signs of infection (13,14,16,18-20). Others relied on positive culture results from joint fluid aspiration or intraoperative tissue samples (10,13,16,18,19,21,24,27,28). To boost the sensitivity of the diagnosis, some researchers also incorporated the clinical picture of infection (19,28) and radiologic signs (19) in their assessment. However, since each study incorporated slightly different methods, it is difficult to provide a consensus on improved diagnostic reliability of S. viridans. The recent adoption and implementation of standard diagnostic criteria will help with the future analysis of PJI.
Treatment
The treatment regimens for suspected or confirmed PJI can vary depending on severity and symptom duration. The gold standard for PJI treatment is two-stage resection arthroplasty with subsequent reimplantation (14,24,26,41,42). However, surgeons can also opt to treat with antibiotic suppression alone, debridement with component retention, or more rarely, resection arthroplasty, knee arthrodesis or amputation (26,30,41). These secondary options have fallen out of favor due to the increased rates of treatment failure and high morbidity/mortality, and are only reserved for specific circumstances (41-45).
While it is preferred to wait until an organism has been definitively identified before starting an antibiotic treatment, empiric coverage is an option for extremely ill patients. Standard empiric coverage generally includes antimicrobial activity against Staphylococci and gram-negative bacilli. However, once the diagnosis of S. viridans is confirmed, it is imperative to change treatment and start the appropriate antimicrobial agent.
Each subfamily of S. viridans demonstrates maximum susceptibility to different antibiotics. Literature suggests that S. sanguinus is most sensitive to ceftriaxone, S. mitis to clindamycin and S. anginosus to both ceftriaxone and clindamycin (8). Additionally, all S. viridan group organisms are highly responsive to vancoymcin treatment (8,9) (Table 1). As is the situation with many organisms, S. viridans group organisms, and S. mitis specifically, have shown a growing and significant increase in its resistance to beta-lactams agents (5,9,46).
Unlike with other organisms, Streptococcus PJI does not have a standard or recommended therapy regimen (11). The absence of standardization may explain the lack of uniformity in treatment methods reported. In patients undergoing two-stage exchange arthroplasty, the most common antibiotics utilized in polymethylmethacrylate cement are vancomycin and tobramycin, as both antibiotics are heat stable and vancomycin is effective against S. viridans while tobramycin works synergistically with vancomycin (47). Postoperatively, dual antibiotic coverage often includes vancomycin and ceftazidime, linezolid, tobramycin, cefotaxime or gentamycin (14,18,19,23,28). One author suggested that Streptococcus species are more amenable to primary antibiotic suppression compared to other organisms (29). Alternatively, some surgeons have utilized oral rifampin and levofloxacin acutely, and then switched to amoxicillin for longer-term suppression (15). Antibiotic coverage for patients that undergo debridement surgery with component retention can receive either triple therapy of nafcillin, penicillin and gentamicin or dual coverage with penicillin and clindamycin (27,30).
Conclusions
The incidence of TJA is predicted to increase in the United States, and the associated increase in PJI may result in more cases of S. viridans. Although S. viridans is a relatively uncommon cause of PJI, the current literature on S. viridans PJI is scarce and lacks a comprehensive review. Four of the subfamilies have been specifically identified as a cause of PJI: S. mutans, S. anginosus, S. sanguinis and S. mitis. Since S. viridans is known to live in the oral cavity, antibiotic prophylaxis may be used prior to invasive dental procedures, but should be reserved for TJA patients with high likelihood for developing PJI. There is no published standard treatment regimen for either Streptococcal or S. viridans PJI, likely due to the low incidence of S. viridans PJI. The preferred treatment for S. viridans PJI is identical to PJI from more common organisms, including two-stage resection arthroplasty with different primary antibiotic treatment based on each subfamily of S. viridans.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2017.06.10). AFC serves as an unpaid Associate Editor of Annals of Joint from Jun 2016 to May 2018. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005-2030. J Bone Joint Surg Am 2007;89:780-5. [PubMed]
- Bjerke-Kroll BT, Christ AB, McLawhron AS, et al. Periprosthetic joint infections treated with two-stage revision over 14 years: An evolving microbiology profile. J Arthroplasty 2014;29:877-82. [PubMed]
- Waldman BJ, Mont MA, Hungerford DS. Total knee arthroplasty infections associated with dental procedures. Clin Orthop Relat Res 1997;164-72. [PubMed]
- Marculescu CE, Berbari EF, Cockerill FR, et al. Unusual aerobic and anaerobic bacteria associated with prosthetic joint infections. Clin Orthop Relat Res 2006;55-63. [Crossref] [PubMed]
- Doern CD, Burnham CD. It’s not easy being green: The viridans group Streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol 2010;48:3829-35. [PubMed]
- Coykendall AL. Classification and identification of the viridans streptococci. Clin Microbiol Rev 1989;2:315-28. [Crossref] [PubMed]
- Centers for disease control and prevention. Identification of Other Streptococcus Species: Streptococcus General Methods. Available online: https://www.cdc.gov/streplab/strep-doc/general-methods-section2.html
- Tuohy M, Washington JA. Antimicrobial susceptibility of ciridans group Streptococci. Diagn Microbiol Infect Dis 1997;29:277-80. [Crossref] [PubMed]
- Smith A, Jackson MS, Kennedy H. Antimicrobial susceptibility of viridan group streptococcal blood isolates to eight antimicrobial agents. Scand J Infect Dis 2004;36:259-63. [PubMed]
- Sonohata M, Kitajima M, Kawano S, et al. Acute hematogenous infection of revision total hip arthroplasty by oral bacteria in a patient without a history of dental procedures: case report. Open Orthop J 2014;8:56-9. [PubMed]
- Seng P, Vernier M, Gay A, et al. Clinical features and outcomes of bone and joint infections with streptococcal involvement: 5-year experience of interregional reference centres in the south of France. New Microbes New Infect 2016;12:8-17. [Crossref] [PubMed]
- Bartzokas CA, Johnson R, Jane M, et al. Relation between mouth and haematogenous infection in total joint replacements. BMJ 1994;309:506-8. [Crossref] [PubMed]
- Rasouli MR, Harandi AA, Adeli B, et al. Revision Total Knee Arthroplasty: Infection Should Be Ruled Out in All Cases. J Arthroplasty 2012;27:1239-43.e1-e2. [Crossref] [PubMed]
- Ben-Lulu O, Farno A, Gross AE, et al. A modified cement spacer technique for infected total hip arthroplasties with significant bone loss. J Arthroplasty 2012;27:613-9. [Crossref] [PubMed]
- Barba T, Wach J, Lustin S, et al. Metallosis-associated prosthetic joint infection. Med Mal Infect 2015;45:484-7. [Crossref] [PubMed]
- Parvizi J, Ghanem E, Menashe S, et al. Periprosthetic infection: What are the diagnostic challenges? J Bone Joint Surg Am 2006;88:138-47. [PubMed]
- Mortazavi SM, Schwartzenberger J, Austin MS, et al. Revision total knee arthroplasty infection: Incidence and predictors. Clin Orthop Relat Res 2010;468:2052-9. [Crossref] [PubMed]
- Hart WJ, Jones RS. Two-stage revision of infected total knee replacements using articulating cement spacers and short-term antibiotic therapy. J Bone Joint Surg Br 2006;88:1011-5. [PubMed]
- Joel J, Graham SM, Peckham-Cooper A, et al. Clinical results of linezolid in arthroplasty and trauma MRSA related infections. World J Orthop 2014;5:151-7. [Crossref] [PubMed]
- Buttaro MA, Tanoira I, Comba F, et al. Combing C-reactive protein and interleukin-6 may be useful to detect periprosthetic hip infection. Clin Orthop Relat Res 2010;468:3263-7. [PubMed]
- Grogan TJ, Dorey F, Rollins J, et al. Deep sepsis following total knee arthroplasty: Ten-year experience at the University of California at Los Angeles Medical Center. J Bone Joint Surg Am 1986;68:226-34. [Crossref] [PubMed]
- Lindqvist C, Slatis P. Dental bacteremia – a neglected cause of arthroplasty infections? Three hip cases. Acta Orthop Scand 1985;56:506-8. [Crossref] [PubMed]
- Swearingen MC, Granger JF, Sullivan A, et al. Elution of antibiotics from poly(methyl methacrylate) bone cement after extended implantation does not necessarily clear the infection despite susceptibility of the clinical isolates. Pathog Dis 2016;74:ftv103-4. [PubMed]
- Radoicić D, Popović Z, Barjaktarović R, et al. Infected total knee arthroplasty treatment outcome analysis. Vojnosanit Pregl 2012;69:504-9. [Crossref] [PubMed]
- LaPorte DM, Waldman BJ, Mont MA, et al. Infections associated with dental procedures in total hip arthroplasty. J Bone Joint Surg Br 1999;81:56-9. [Crossref] [PubMed]
- Hanssen AD, Trousdale RT, Osmon DR. Patient outcome with reinfection following reimplantation for the infected total knee arthroplasty. Clin Orthop Relat Res 1995;55-67. [PubMed]
- Jacobs MA, Hungerford DS, Krackow KA, et al. Revision of Septic Total Knee Arthroplasty. Clin Orthop Relat Res 1989;159-66. [PubMed]
- Darley ES, Bannister GC, Blom AW, et al. Role of early intravenous to oral antibiotic switch therapy in the management of prosthetic hip infection treated with one- or two-stage replacement. J Antimicrob Chemother 2011;66:2405-8. [Crossref] [PubMed]
- Everts RJ, Chambers ST, Murdoch DR, et al. Successful antimicrobial therapy and implant retention for streptococcal infection of prosthetic joints. ANZ J Surg 2004;74:210-4. [Crossref] [PubMed]
- Hyman JL, Salvati EA, Laurencin CT, et al. The arthroscopic drainage, irrigation, and debridement of late, acute total hip arthroplasty Infections: Average 6-year follow-up. J Arthroplasty 1999;14:903-10. [Crossref] [PubMed]
- Chen CE, Wang JW, Juhn RJ. Total hip arthroplasty for primary septic arthritis of the hip in adults. Int Orthop 2008;32:573-80. [Crossref] [PubMed]
- Uçkay I, Lübbeke A, Emonet S, et al. Low incidence of haematogenous seeding to total hip and knee prostheses in patients with remote infections. J Infect 2009;59:337-45. [Crossref] [PubMed]
- American Dental Association. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dent Assoc 2003;134:895-9. [Crossref] [PubMed]
- . Advisory statement. Antibiotic prophylaxis for dental patients with total joint replacements. American Dental Association; American Academy of Orthopaedic Surgeons. J Am Dent Assoc 1997;128:1004-8. [Crossref] [PubMed]
- Little JW, Jacobson JJ, Lockhart PB. The dental treatment of patients with joint replacements: A position paper from the American Academy of Oral Medicine. JADA 2010;141:667-71. [Crossref] [PubMed]
- Watters W, Rethman MP, Hanson NB, et al. Prevention of orthopedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg 2013;21:180-9. [Crossref] [PubMed]
- Young H, Hirsh J, Hammerberg EM, et al. Dental disease and periprosthetic joint infection. J Bone Joint Surg Am 2014;96:162-8. [Crossref] [PubMed]
- Bouvet C, Tchernin D, Seirafi M, et al. No need to search for the source of haematogenous arthroplasty infections. Swiss Med Wkly 2011;141:w13306-5. [PubMed]
- Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 2011;469:2992-4. [Crossref] [PubMed]
- Parvizi J. Gehrke, International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty 2014;29:1331. [Crossref] [PubMed]
- Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: Treatment options. Orthopedics 2010;33:659. [PubMed]
- Cochran AR, Ong KL, Lau E, et al. Risk of reinfection after treatment of infected total knee arthroplasty. J Arthroplasty 2016;31:156-61. [Crossref] [PubMed]
- Brandt CM, Sistrunk WW, Duff MC, et al. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis 1997;24:914-9. [Crossref] [PubMed]
- Crockarell JR, Hanssen AD, Osmon DR, et al. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am 1998;80:1306-13. [PubMed]
- Klouche S, Lhotellier L, Mamoudy P. Infected total hip arthroplasty treated by an irrigation-debridement/component retention protocol: A prospective study in a 12-case series with minimum 2 years’ follow-up. Orthop Traumatol Surg Res 2011;97:134-8. [PubMed]
- Kennedy HF, Gemmel CG, Bagg J, et al. Antimicrobial susceptible of blood culture isolates of viridans streptococci: relationship to a change in empirical antibiotic therapy in febrile neturopenia. J Antimicrob Chemother 2001;47:693-6. [Crossref] [PubMed]
- Chen AF, Parvizi J. Antibiotic-loaded bone cement and periprosthetic joint infection. J Long Term Eff Med Implants 2014;24:89-97. [Crossref] [PubMed]
Cite this article as: Mathur A, Chen AF. Streptococcus viridans periprosthetic joint infections. Ann Joint 2017;2:36.


