
The role of anatomic ACL reconstruction in ACL revision surgery
Introduction
Anterior cruciate ligament (ACL) reconstruction has largely been considered a successful operation, but failure, particularly in young, competitive athletes, remains a difficult problem. Revision ACL surgery has been shown to be less successful than primary ACL reconstruction, with a failure rate 3 to 4 times that of primary reconstruction (1). Graft failure occurs in 6–25% of revision surgeries (2-6). Revision ACL reconstruction patients also have lower IKDC scores and lower return to sport rates than primary ACL reconstruction patients (2). Hence, there is a need to improve revision ACL reconstruction treatment. Anatomic revision ACL reconstruction can provide a biomechanically superior reconstruction (7) but requires thoughtful preoperative planning and sound intraoperative techniques as discussed in this review.
Revision ACL reconstruction
Early recurrence of instability has been shown to be related to poor surgical technique, biological failure of the graft, premature return to high-level sports, and improper rehabilitation (8). Late failure, occurring more than one year after ACL reconstruction, is hypothesized to be related to new isolated trauma or repetitive microtrauma on the graft (9,10). An independent third-party investigation of the Multicenter ACL Revision Study (MARS) data found that 60% of revision reconstruction cases were due to “technical cause of failure,” with improper positioning of the femoral tunnel being cited most often (11).
The increased complexity and inferior clinical outcomes of revision ACL reconstruction, as compared to primary ACL reconstruction, indicates a need to establish sound indications for revision surgery. The criteria for revision ACL surgery are continued symptomatic and functional instability in activities of daily living or sport with or without complete failure of the graft (12). Revision surgery can be appropriate in patients without overt graft rupture, but with a poorly positioned graft and recurrent symptomatic instability. A vertically oriented graft may lead to persistent rotatory instability that may limit an athlete’s ability to return to the previous level of competition (3). Malpositioned tunnels can affect the range of motion of the knee. Femoral tunnels placed too anteriorly or tibial tunnels placed too posteriorly can lead to loss of flexion and tibial tunnels placed too anteriorly may lead to graft impingement and loss of extension (13). The anatomic footprint of the native ACL has been well described with the posterior border 4mm from the articular cartilage, anterior border at the lateral intercondylar ridge, and approximate width between 10 and 18 mm depending on the patient (14,15). Radiographically, the center of the femoral insertion is located 24.8% of the long axis and 28.5% of the short axis as described in the widely used quadrant method (16). Prior femoral tunnel malposition is defined as tunnel placement partially or completely outside of the native femoral insertion.
Subjective patient factors are a large aspect of the criteria for revision surgery, therefore it is important to obtain a thorough history, as well as objective measures. Patients often present with pain, loss of motion, recurrent episodes of “giving out”, and decreased level of activity compared to their pre-injury state (12). The subjective symptoms can be corroborated on the physical examination, with a positive pivot shift, Lachman, or anterior drawer test, or more quantitatively using a KT-1000 arthrometer (12). Contraindications to revision surgery include active infection, lack of significant symptoms, unacceptable anesthesia or medical risks, and lack of functional deficits.
Anatomic reconstruction
Anatomic repair or reconstruction is a guiding principle in orthopedic surgery, and studies have demonstrated the benefits of anatomical reconstruction for multiple joints. Patients with displaced acetabular fractures who undergo anatomic reconstruction report higher postoperative Harris hip scores (17). In athletes, when anatomic reconstruction of the anterior talofibular ligament was not possible due to insufficient normal ligament, a hybrid anatomic procedure produced higher Foot and Ankle Outcome Scores than a non-anatomic repair (18). There is a growing body of evidence within the ACL literature to support the benefits of anatomic reconstruction (7,19-21). Anatomic reconstruction may be thought of as “the functional restoration of the ACL to its native dimensions, collagen orientation, and insertion sites” (22).
Biomechanical studies show that non-anatomic transtibial single-bundle reconstruction does not fully restore rotatory stability of the knee (23). The degree of rotational stability at 30 degrees of flexion is comparable between the anatomically reconstructed ACL and a native ACL, while a non-anatomically reconstructed ligament shows increased rotatory instability (7,24,25). Similarly, the non-anatomically reconstructed ACL also experiences increased AP laxity at 30 degrees flexion when an internal rotatory force is applied (26). Validated computer models have compared graft forces and stability of anatomic to non-anatomic transtibial reconstructions and showed that anatomic reconstruction provides improved AP stability (27). This increased instability may contribute to the lack of athletic confidence after ACL reconstruction that prevents some athletes from return to sport (28). A biomechanical analysis of in situ forces on cadaveric knees showed that anterior tibial translation of anatomic double-bundle ACL reconstructions was significantly closer to that of the intact knee than single bundle reconstruction (7). Furthermore, the in situ force on the anatomically reconstructed ACL normalized to the intact ACL (97%±9% of the intact ACL), while the single bundle reconstruction only restored 89%±13% of the in situ force (7). This difference is accentuated when the knees were placed in 30 degrees of flexion with a rotatory load. Anatomic reconstructions restored 91%±35% of the forces the intact ACL experienced, while the single bundle reconstruction only restored 60%±40% of intact ACL forces (7). Forces not felt by the ACL graft may be absorbed by the surrounding tissues, including the menisci and articular cartilage, and disruptions of normal knee kinematics may consequently contribute to an increased risk of knee osteoarthritis (OA) seen long term (29).
There is a large body of clinical evidence which supports anatomic reconstruction over non-anatomic reconstruction for improved patient outcomes, objective knee stability, and reduced risk for graft failure and revision surgery (30,31). In a randomized controlled trial comparing non-anatomic ACL reconstruction to anatomic single- and double-bundle reconstruction, anatomic reconstruction showed significant improvement in Lysholm scores, IKDC scores, objective knee anterior stability, and rotatory instability compared to non-anatomic reconstruction (32). Anatomic ACL reconstruction best restores rotatory stability to the native ACL and represents the optimal biomechanical option in revision ACL reconstruction.
One-stage versus two-stage reconstruction
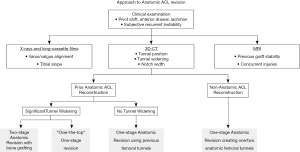
Thorough preoperative planning for revision ACL reconstruction with clinical examination and imaging, including X-ray, magnetic resonance imaging (MRI), and computed tomography (CT), is essential for successful surgery. Preoperative planning is greatly facilitated by three-dimensional CT (3D-CT) modeling, from which tunnel position, tunnel widening, and hardware position can be most accurately determined (Figure 1). In particular, surgical technique is dictated by the location of the tunnels relative to the anatomic insertion site of the native ACL. The tunnel location is broadly divided into three categories: (I) non-anatomic—tunnels completely outside of the anatomic footprints, (II) anatomic—tunnels completely within the anatomic footprints, or (III) semi-anatomic—tunnels partially overlapping the anatomic footprints (33).
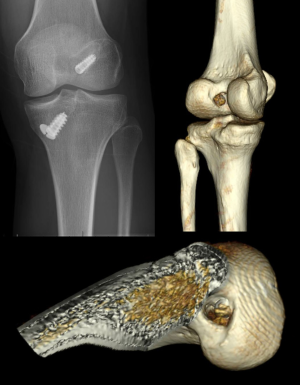
With non-anatomic tunnels, which are seen most commonly (11), the technique of primary anatomic ACL reconstruction can be employed without modification, while ensuring adequate bony bridge between tunnels. When tunnels are anatomic, evidence of tunnel widening must be considered. In the absence of widening, anatomical tunnels may be reused in the revision reconstruction. Graft debridement, tunnel re-drilling, and dilation are first completed to remove soft tissues, which may interfere with graft-bone healing, and create solid bony walls for the new tunnel. If significant widening or osteolysis exists (>16 mm for a single-bundle tunnel), bone loss may be addressed in a two-stage technique using allograft or autograft bone to promote ingrowth (34). Traditionally, staged revision ACL reconstruction has been performed 4–6 months following bone grafting of tunnels.
Semi-anatomic tunnels represent a unique challenge. The surgeon must decide whether a one-stage or two-stage reconstruction is the best approach for the patient. One-stage techniques include eccentric drilling of the previous tunnel, diverging tunnel creation, using larger bone blocks, or an over-the-top (OTT) technique. The OTT ACL reconstruction technique, in which the graft is routed through the notch, around the posterosuperior lateral femoral condyle, and directly contacts the lateral wall, permits one-stage revision regardless of tunnel location. This technique is especially helpful in the case of semi-anatomic tunnels where obtaining adequate residual bone stock may not be possible. In a recent systematic review of primary ACL reconstruction, the OTT technique was found to yield comparable outcomes to traditional all-inside, transtibial, and anteromedial portal drilling techniques (35). While this trend was also seen for revision ACL reconstruction, only three case series of revision OTT ACL reconstruction (comprised of 60 total patients), have been reported (35). No studies directly comparing OTT against alternative techniques for revision ACL reconstruction have been performed. Instead of the OTT technique, divergent tunnel creation can be used to provide a cone of adequate bone for graft incorporation in order to avoid tunnel convergence and minimize the risk of graft pullout (36). Utilization of grafts with large bone blocks, or the inclusion of impacted bone graft adjacent to the graft in the tunnel, may permit one-stage procedures in the context of mild to moderate osteolysis (37,38). Alternatively, the use of a rectangular tunnel technique may permit placement of the graft within the anatomic footprint despite an overlapping primary tunnel, as the rectangular bone block is smaller in area than the traditional oval-shaped bone block (39). Additionally, in the revision of double bundle ACL reconstructions, it may be possible to drill a new tunnel corresponding to one bundle while preserving the other tunnel for the second bundle (33,40).
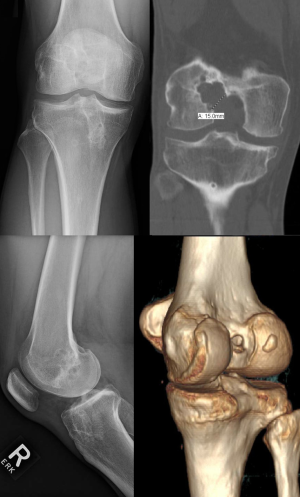
On the other hand, a staged approach with bone grafting remains a viable strategy (Figure 2). Staged procedures have been reported to restore knee laxity measurements similar to that achieved with primary ACL reconstruction, but are associated with worse subjective outcomes. This is likely due in part to secondary meniscal and/or chondral lesions acquired in the interim between bone grafting and revision ACL reconstruction (41,42). However, a recent cohort study comparing one-stage versus two-stage revision ACL reconstruction found no differences between groups in objective and subjective outcomes at 2-year follow-up (43). Nevertheless, presumed equivalence between treatment strategies may be questioned as the one-stage revision group was comprised of patients with bone tunnels completely outside of the native insertion sites, while the two-stage revision group underwent bone grafting as they had enlarged bone tunnels (>16 mm) or those that would critically overlap with anatomic tunnels (43).
The Pittsburgh approach to anatomic revision ACL reconstruction
Preoperative evaluation
In our experience, the key for successful anatomic revision ACL reconstruction is a thorough preoperative workup. When presenting with clinical signs of ACL reconstruction failure, the patient undergoes radiographic evaluation with a series of knee radiographs, a hip-to-ankle long cassette, CT including 3D reconstruction, and MRI to evaluate multiple anatomic factors for preoperative planning (Figure 3). Knee radiographs and the hip-to-ankle long cassette are used to assess mechanical varus/valgus alignment of the knee, tibial slope, and signs of OA. The tibial slope is an important and sometimes overlooked factor. Increased lateral tibial posterior slope is associated with risk of ACL reconstruction graft rupture (44,45). Posterior slope is measured in all revision cases as part of the routine preoperative planning, and preparations are made for correction if necessary. MRI allows for evaluation of graft integrity and concurrent cartilage, meniscal, and ligamentous injuries, which may require repair. 3D-CT helps to define prior tunnel position and evaluate the amount of tunnel osteolysis or widening. We have also used 3D printed models of the knee, which provide even further detail in defining the previous tunnels and intercondylar notch size.
Operative considerations
Review of prior operative reports allows for identification of the previously used graft and hardware, which may require removal intra-operatively. Fluoroscopy and a variety of screwdrivers may be necessary for previous hardware removal and should always be available the day of revision surgery.
Revision ACL reconstruction is complicated by the previous tunnel position, with or without signs of tunnel widening or osteolysis. Previous anatomic tunnels require hardware removal, drilling of the tunnels to remove soft tissues, and placement of new fixation. In patients who present with significant tunnel widening and anatomically placed tunnels, the OTT reconstruction is preferred as discussed previously, but alternatively a two-stage procedure with bone grafting can be considered. Although the OTT technique is our preference in this case, there is no significant difference in patient satisfaction or clinical outcomes between one-stage and two-stage revision ACL reconstruction (43). If the prior reconstruction was non-anatomic with misplaced femoral tunnels and adequate bone stock is available for anatomic tunnels, the patient can undergo a one-stage revision ACL reconstruction with the creation of anatomic tunnels. In this situation, previous hardware can frequently be left in place. Inside-out drilling through an anteromedial portal is preferred for anatomic femoral tunnels. A narrow intercondylar notch may make this challenging, in which case an outside-in approach may facilitate the creation of the anatomic femoral tunnels.
Graft choice for revision ACL reconstruction is similar to primary ACL reconstruction with the exception that the prior ACL reconstruction(s) may have used one or more of the potential autograft options. Patellar tendon, quadriceps tendon, and hamstring tendon autografts have all been successfully implemented in revision procedures (46). Contralateral autograft harvest is an option in the setting of multiple revisions, although this is avoided when possible. Allograft does not have the morbidity associated with autograft harvest, but has an increased risk of graft failure in young, active patients (47,48). Achilles tendon allograft, with the ability to obtain a larger bone block, may be beneficial in cases of tunnel osteolysis (49). The calcaneus bone block can be rotated to match the osteolysis, and can be used for the OTT technique for femoral-sided fixation. Potential graft options should be discussed thoroughly with the patient during the preoperative visit.
Concurrent meniscal and ligamentous injuries must be carefully evaluated. Meniscus injuries occur more frequently in the setting of revision ACL reconstruction than in primary ACL reconstruction (50,51). Meniscus injuries should be repaired whenever possible in revision ACL reconstructions to aid in restoring rotatory stability (52,53). Missed posterolateral instability has been found to be a factor in failed ACL reconstructions and should be evaluated prior to revision (54). In the search for continued improvement in rotatory stability, the exact role for lateral extra-articular augmentation procedures is still being defined. We consider adding a modified Lemaire procedure (iliotibial band tenodesis) to anatomic revision ACL reconstruction in the settings of a large quantitative pivot shift, generalized ligamentous laxity, failed anatomic ACL reconstruction without identifiable cause, or a lateral femoral condyle bony morphology that we predict will be highly unstable (55,56).
Postoperative rehabilitation
Rehabilitation following anatomic revision ACL reconstruction follows our institutions standardized protocols for primary ACL reconstructions (57). The early postoperative period focuses on minimizing pain and swelling, restoring range of motion, and progressing to full weight bearing. Patients begin ankle pumps, isometric quadriceps contractions, and straight leg raises postoperative day one with frequent use of cryotherapy for the control of swelling and pain. Patients are restricted to crutches for 4 to 6 weeks but are allowed to unlock their brace for ambulation if no meniscal repair was performed. Active range of motion is progressively restored during the first 4 to 6 weeks, and focus is placed on regaining quadriceps strength with straight leg raises, isometric quadriceps contractions, and the use of adjunctive high-intensity electrical stimulation. High-intensity electrical quadriceps stimulation has been shown to improve patient outcomes and increase quadriceps strength following ACL reconstruction (58). Closed-chain weight bearing exercises and limited-arc (60–90o) open-chain exercises start as quadriceps strength improves. Aerobic low-impact training is added during the first three months postoperatively, including stationary bicycle or low-speed treadmill walking. As stability and balance improve, running is initiated four to six months postoperatively, usually first on a treadmill. Patients undergo slow progression if they do not develop new knee swelling or increased pain. Agility training and cutting drills can be started as soon as six months postoperatively if patients progress well through running training. Patients complete return to sport testing in conjunction with our physical therapy colleagues. Typical anatomic revision ACL reconstruction sees progression through rehabilitation and return to sport 9 to 12 months postoperatively.
Multiple revision ACL reconstruction
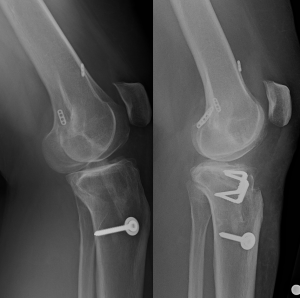
In the multiple revision patient, it is even more important to determine the reason for failure to ensure the same mistakes are not repeated. The surgeon must be prepared to expand the scope of possible interventions. For example, we present a case of a 41-year-old female with a history of a failed primary hamstring autograft ACL reconstruction, followed by a failed bone-patellar tendon-bone allograft revision ACL reconstruction (Figure 4). She presented with knee pain and recurrent instability. As part of her preoperative evaluation, posterior tibial slope was measured at 17°. Therefore, in addition to anatomic revision ACL reconstruction, an anterior tibial closing wedge osteotomy was performed to decrease the excessive posterior tibial slope and decrease the strain on the ACL (59). Multiple revision cases, such as this example, highlight the importance of a thorough preoperative plan and execution.
Outcomes and complications
The Multicenter ACL Revision Study (MARS) is a multicenter consortium collecting prospective data on revision ACL reconstruction. This study has provided the most significant data to the revision ACL reconstruction literature. Patient outcomes, including IKDC-Pain, -ADL, -Sports and -Symptoms, as well as MARX scores, have been shown to be significantly lower 2 years after revision ACL reconstruction as compared with primary ACL reconstruction (60). The use of autograft for revision ACL reconstruction was shown to have improved IKDC scores as compared to allograft (61). In addition, while IKDC and WOMAC scores are improved 2 years postoperatively from revision ACL reconstruction, Marx activity levels are significantly decreased (61).
In the MARS cohort, complications and the need for additional surgery following revision ACL reconstruction occurred in 11% of patients at 2-year follow-up (44). Arthrofibrosis requiring lysis of adhesions or synovectomy occurred in 1.4% of revision cases, while the need for meniscus surgery was the most frequent subsequent surgery in 4.1% of revision cases. Infection was reported in 0.35% of cases. Risk factors for subsequent surgery after revision ACL reconstruction included grade 4 cartilage damage at the time of revision, use of allograft, two-stage revision, and younger patient age (44). Bony cyst formation has been attributed to the use of bioabsorbable sutures (62). Overall, outcomes following revision ACL reconstruction are inferior to primary ACL reconstruction, but with adequate preoperative planning, establishing appropriate patient expectations, and high quality postoperative rehabilitation, complications can be minimized.
Conclusions
Anatomic revision ACL reconstruction is a complex procedure involving more comprehensive clinical examination, radiographic assessment, and preoperative testing than primary ACL reconstruction. Difficulties with revision ACL reconstruction include prior tunnel placement, tunnel widening and osteolysis, limb malalignment, concomitant injuries, and graft choice. Multiple techniques can be employed to allow for one-stage reconstruction including the use of the OTT technique, new anatomic tunnel placement, grafts with larger bone blocks, and divergent tunnel placement. While patient reported outcomes and return to sport following revision ACL reconstruction are lower than with primary ACL reconstruction, anatomic revision ACL reconstruction allows for restoration of knee stability and improved patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Takeshi Muneta) for the series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2018.11.14). The series “Anatomic Reconstruction of Anterior Cruciate Ligament - Concept, Indication, and Its Efficacy” was commissioned by the editorial office without any funding or sponsorship. RT reports educational support from Johnson&Johnson, outside the submitted work. SJM reports educational support from Smith&Nephew, outside the submitted work. VM reports personal fees from Smith&Nephew, other from ISAKOS, other from KSSTA, outside the submitted work. In addition, VM has a patent quantified injury diagnostics issued, outside of the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am 2012;94:531-6. [Crossref] [PubMed]
- O’Neill DB. Revision arthroscopically assisted anterior cruciate ligament reconstruction with previously unharvested ipsilateral autografts. Am J Sports Med 2004;32:1833-41. [Crossref] [PubMed]
- Wilde J, Bedi A, Altchek DW. Revision Anterior Cruciate Ligament Reconstruction. Sports Health 2014;6:504-18. [Crossref] [PubMed]
- Battaglia MJ, Cordasco FA, Hannafin JA, et al. Results of revision anterior cruciate ligament surgery. Am J Sports Med 2007;35:2057-66. [Crossref] [PubMed]
- Creighton RA, Bach BR. Revision anterior cruciate ligament reconstruction with patellar tendon allograft: Surgical technique. Sports Med Arthrosc Rev 2005;13:38-45. [Crossref]
- Weiler A, Schmeling A, Stöhr I, et al. Primary versus single-stage revision anterior cruciate ligament reconstruction using autologous hamstring tendon grafts: A prospective matched-group analysis. Am J Sports Med 2007;35:1643-52. [Crossref] [PubMed]
- Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 2002;30:660-6. [Crossref] [PubMed]
- George MS, Dunn WR, Spindler KP. Current concepts review: Revision anterior cruciate ligament reconstruction. Am J Sports Med 2006;34:2026-37. [Crossref] [PubMed]
- Harner CD, Giffin JR, Dunteman RC, et al. Evaluation and treatment of recurrent instability after anterior cruciate ligament reconstruction. Instr Course Lect 2001;50:463-74. [PubMed]
- Johnson DL, Fu FH. Anterior cruciate ligament reconstruction: why do failures occur? Instr Course Lect 1995;44:391-406. [PubMed]
- Morgan JA, Dahm D, Levy B, et al. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg 2012;25:361-8. [Crossref] [PubMed]
- Johnson DL, Swenson TM, Irrgang JJ, et al. Revision anterior cruciate ligament surgery: experience from Pittsburgh. Clin Orthop Relat Res 1996;100-9. [Crossref] [PubMed]
- Kamath GV, Redfern JC, Greis PE, et al. Revision anterior cruciate ligament reconstruction. Am J Sports Med 2011;39:199-217. [Crossref] [PubMed]
- Steiner ME, Murray MM, Rodeo SA. Strategies to improve anterior cruciate ligament healing and graft placement. Am J Sports Med 2008;36:176-89. [Crossref] [PubMed]
- Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res 1975;216-31. [Crossref] [PubMed]
- Bernard M, Hertel P, Hornung H, et al. Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 1997;10:14-21; discussion 21-2. [PubMed]
- Mears DC, Velyvis JH, Chang CP. Displaced acetabular fractures managed operatively: Indicators of outcome. Clin Orthop Relat Res 2003;173-86. [Crossref] [PubMed]
- Kennedy JG, Smyth NA, Fansa AM, et al. Anatomic lateral ligament reconstruction in the ankle: A hybrid technique in the athletic population. Am J Sports Med 2012;40:2309-17. [Crossref] [PubMed]
- Kaz R, Starman JS, Fu FH. Anatomic Double-Bundle Anterior Cruciate Ligament Reconstruction Revision Surgery. Arthroscopy 2007;23:1250.e1-3. [Crossref] [PubMed]
- Maeyama A, Hoshino Y, Kato Y, et al. Anatomic double bundle ACL reconstruction outperforms any types of single bundle ACL reconstructions in controlling dynamic rotational laxity. Knee Surg Sports Traumatol Arthrosc 2018;26:1414-9. [Crossref] [PubMed]
- Asai S, Maeyama A, Hoshino Y, et al. A comparison of dynamic rotational knee instability between anatomic single-bundle and over-the-top anterior cruciate ligament reconstruction using triaxial accelerometry. Knee Surg Sports Traumatol Arthrosc 2014;22:972-8. [Crossref] [PubMed]
- van Eck CF, Gravare-Silbernagel K, Samuelsson K, et al. Evidence to support the interpretation and use of the anatomic anterior cruciate ligament reconstruction checklist. J Bone Joint Surg Am 2013;95:e153 [Crossref] [PubMed]
- Fu FH, van Eck CF, Tashman S, et al. Anatomic anterior cruciate ligament reconstruction: a changing paradigm. Knee Surg Sports Traumatol Arthrosc 2015;23:640-8. [Crossref] [PubMed]
- Guenther D, Irarrazaval S, Bell KM, et al. The Role of Extra-Articular Tenodesis in Combined ACL and Anterolateral Capsular Injury. J Bone Joint Surg Am 2017;99:1654-60. [Crossref] [PubMed]
- Bell KM, Rahnemai-Azar AA, Irarrazaval S, et al. In situ force in the anterior cruciate ligament, the lateral collateral ligament, and the anterolateral capsule complex during a simulated pivot shift test. J Orthop Res 2018;36:847-53. [PubMed]
- Lim HC, Yoon YC, Wang JH, et al. Anatomical versus non-anatomical single bundle anterior cruciate ligament reconstruction: A cadaveric study of comparison of knee stability. Clin Orthop Surg 2012;4:249-55. [Crossref] [PubMed]
- Wang L, Lin L, Feng Y, et al. Anterior cruciate ligament reconstruction and cartilage contact forces - A 3D computational simulation. Clin Biomech (Bristol, Avon) 2015;30:1175-80. [Crossref] [PubMed]
- Lentz TA, Zeppieri G, George SZ, et al. Comparison of physical impairment, functional, and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med 2015;43:345-53. [Crossref] [PubMed]
- Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med 2004;32:975-83. [Crossref] [PubMed]
- Ahldén M, Samuelsson K, Sernert N, et al. The Swedish National Anterior Cruciate Ligament Register: a report on baseline variables and outcomes of surgery for almost 18,000 patients. Am J Sports Med 2012;40:2230-5. [Crossref] [PubMed]
- Sadoghi P, Kropfl A, Jansson V, et al. Impact of tibial and femoral tunnel position on clinical results after anterior cruciate ligament reconstruction. Arthroscopy 2011;27:355-64. [Crossref] [PubMed]
- Hussein M, Van Eck CF, Cretnik A, et al. Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction: 281 cases with 3- to 5-year follow-up. Am J Sports Med 2012;40:512-20. [Crossref] [PubMed]
- Hofbauer M, Murawski CD, Muller B, et al. Revision surgery after primary double-bundle ACL reconstruction: AAOS exhibit selection. J Bone Joint Surg Am 2014;96:e30 [Crossref] [PubMed]
- Maak TG, Voos JE, Wickiewicz TL, et al. Tunnel widening in revision anterior cruciate ligament reconstruction. J Am Acad Orthop Surg 2010;18:695-706. [Crossref] [PubMed]
- Sarraj M, de Sa D, Shanmugaraj A, et al. Over-the-top ACL reconstruction yields comparable outcomes to traditional ACL reconstruction in primary and revision settings: a systematic review. Knee Surg Sports Traumatol Arthrosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Laidlaw MS, Buyukdogan K, Werner BC, et al. Management of bone deficiency in revision anterior cruciate ligament reconstruction. Ann Jt 2017;2:38. [Crossref]
- Ra HJ, Ha JK, Kim JG. One-stage Revision Anterior Cruciate Ligament Reconstruction With Impacted Bone Graft After Failed Primary Reconstruction. Orthopedics 2013;36:860-3. [Crossref] [PubMed]
- Tomihara T, Hashimoto Y, Taniuchi M, et al. One-stage revision ACL reconstruction after primary ACL double bundle reconstruction: is bone–patella tendon–bone autograft reliable? Knee Surg Sports Traumatol Arthrosc 2017;25:1653-61. [Crossref] [PubMed]
- Shino K, Mae T, Take Y, et al. One-stage revision anatomic anterior cruciate ligament reconstruction with rectangular tunnel technique. Asia Pac J Sports Med Arthrosc Rehabil Technol 2015;2:43-8. [PubMed]
- Hofbauer M, Muller B, Murawski CD, et al. Strategies for revision surgery after primary double-bundle anterior cruciate ligament (ACL) reconstruction. Knee Surg Sports Traumatol Arthrosc 2013;21:2072-80. [Crossref] [PubMed]
- Thomas NP, Kankate R, Wandless F, Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med 2005;33:1701-9. [Crossref] [PubMed]
- Franceschi F, Papalia R, Del Buono A, et al. Two-stage procedure in anterior cruciate ligament revision surgery: A five-year follow-up prospective study. Int Orthop 2013;37:1369-74. [Crossref] [PubMed]
- Mitchell JJ, Chahla J, Dean CS, et al. Outcomes after 1-stage versus 2-stage revision anterior cruciate ligament reconstruction. Am J Sports Med 2017;45:1790-8. [Crossref] [PubMed]
- Christensen JJ, Krych AJ, Engasser WM, et al. Lateral Tibial Posterior Slope Is Increased in Patients With Early Graft Failure After Anterior Cruciate Ligament Reconstruction. Am J Sports Med 2015;43:2510-4. [Crossref] [PubMed]
- Lee CC, Youm YS, Cho S, Do , et al. Does Posterior Tibial Slope Affect Graft Rupture Following Anterior Cruciate Ligament Reconstruction? Arthroscopy 2018;34:2152-5. [Crossref] [PubMed]
- Wright RW, Huston LJ, Spindler KP, et al. Descriptive epidemiology of the multicenter ACL revision study (MARS) cohort. Am J Sports Med 2010;38:1979-86. [Crossref] [PubMed]
- Engelman GH, Carry PM, Hitt KG, et al. Comparison of allograft versus autograft anterior cruciate ligament reconstruction graft survival in an active adolescent cohort. Am J Sports Med 2014;42:2311-8. [Crossref] [PubMed]
- Ellis HB, Matheny LM, Briggs KK, et al. Outcomes and revision rate after bone-patellar tendon-bone allograft versus autograft anterior cruciate ligament reconstruction in patients aged 18 years or younger with closed physes. Arthroscopy 2012;28:1819-25. [Crossref] [PubMed]
- Burnham JM, Herbst E, Pauyo T, et al. Technical Considerations in Revision Anterior Cruciate Ligament Reconstruction for Operative Techniques in Orthopaedics. Oper Tech Orthop 2017;27:63-9. [Crossref] [PubMed]
- Trojani C, Sbihi A, Djian P, et al. Causes for failure of ACL reconstruction and influence of meniscectomies after revision. Knee Surg Sports Traumatol Arthrosc 2011;19:196-201. [Crossref] [PubMed]
- Magnussen RA, Trojani C, Granan LP, et al. Patient demographics and surgical characteristics in ACL revision: a comparison of French, Norwegian, and North American cohorts. Knee Surg Sports Traumatol Arthrosc 2015;23:2339-48. [Crossref] [PubMed]
- Allen CR, Wong EK, Livesay GA, et al. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res 2000;18:109-15. [Crossref] [PubMed]
- Musahl V, Citak M, O’Loughlin PF, et al. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med 2010;38:1591-7. [Crossref] [PubMed]
- Gersoff WK, Clancy WG. Diagnosis of acute and chronic anterior cruciate ligament tears. Clin Sports Med 1988;7:727-38. [PubMed]
- Pfeiffer TR, Burnham JM, Hughes JD, et al. An Increased Lateral Femoral Condyle Ratio Is a Risk Factor for Anterior Cruciate Ligament Injury. J Bone Joint Surg Am 2018;100:857-64. [Crossref] [PubMed]
- Williams A, Ball S, Stephen J, et al. The scientific rationale for lateral tenodesis augmentation of intra-articular ACL reconstruction using a modified ‘Lemaire’ procedure. Knee Surg Sports Traumatol Arthrosc 2017;25:1339-44. [Crossref] [PubMed]
- Hensler D, van Eck CF, Fu FH, et al. Anatomic Anterior Cruciate Ligament Reconstruction Utilizing the Double-Bundle Technique. J Orthop Sports Phys Ther 2012;42:184-95. [Crossref] [PubMed]
- Fitzgerald GK, Piva SR, Irrgang JJ. A Modified Neuromuscular Electrical Stimulation Protocol for Quadriceps Strength Training Following Anterior Cruciate Ligament Reconstruction. J Orthop Sports Phys Ther 2003;33:492-501. [Crossref] [PubMed]
- Waiwaiole A, Gurbani A, Motamedi K, et al. Relationship of ACL Injury and Posterior Tibial Slope With Patient Age, Sex, and Race. Orthop J Sports Med 2016;4:2325967116672852 [Crossref] [PubMed]
- Wright R, Spindler KP, Huston LJ, et al. Revision ACL Reconstruction Outcomes: MOON Cohort. J Knee Surg 2011;24:289-94. [Crossref] [PubMed]
- MARS Group. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the multicenter ACL revision study (MARS) cohort. Am J Sports Med 2014;42:2301-10. [Crossref] [PubMed]
- Martinek V, Friederich NF. Tibial and pretibial cyst formation after anterior cruciate ligament reconstruction with bioabsorbable interference screw fixation. Arthroscopy 1999;15:317-20. [Crossref] [PubMed]
Cite this article as: Tisherman R, De Groot J, Rothrauff B, Byrne K, Meredith SJ, Musahl V. The role of anatomic ACL reconstruction in ACL revision surgery. Ann Joint 2018;3:103.


