
Bone-marrow-aspirate-concentrate for chondral defects: surgical techniques, clinical applications and basic science
Introduction
Mesenchymal stem cells (MSCs) often remain the cells of choice for many regenerative treatment strategies in orthopedics (1). On the one hand, MSC’s key role is attributable to their differentiation potential into diverse connective tissue cell types such as chondrocytes and osteocytes. On the other hand, a variety of regulatory features of MSCs or MSC’s paracrine factors has been described. Among those are immunomodulatory, antiapoptotic and coordinative functions for regenerative differentiation processes in host cells (2).
The basic concept for clinical MSC application is either (I) through intra-articular injection primarily in osteoarthritis patients or (II) surgical application for chondral or bone pathologies, mostly in combination with scaffolds.
MSCs can be found in a variety of tissues such as umbilical cord blood, peripheral blood, skeletal muscles and others (3). However, the most commonly used source for orthopedic procedures is bone marrow and fat tissue.
Some factors led to a trend to use autologous MSCs in a “point-of-care” use during surgeries. A major factor is regulatory hurdles regarding the manipulation of cells in the U.S. as well as in Europe (4). This stringent regulatory environment led to “minimally manipulative” approaches such as simple centrifugation of bone marrow aspirate in contrast to potentially more “manipulative” techniques such as ex vivo cell cultivation. Moreover, time-cost and safety benefits of a single surgery led to a significant increase of bone-marrow-aspirate-concentrate (BMAC) use as a source of MSCs in regenerative orthopedics.
However, literature about the exact application, preparation and indication remains scanty and heterogenous.
This article aims to bridge that gap by providing comprehensive information for practicing surgeons to ease patient selection and surgical application of BMAC for chondral defects.
BMAC—basic science
BMAC is obtained by density gradient based centrifugation of bone marrow aspirate (BMA). BMACs cellular composition primarily constitutes of mononuclear cells like bone marrow-mesenchymal stem cells (BM-MSCs), haematopoietic stem cells (HSCs), platelet-derived growth factors, cytokines and chemokines. Cassano et al. (5) investigated variances for MSC surfaces markers between the two commercially available manufacturing systems Magellan® (Isto Biologics, Massachusetts) and Harvest SmartPrep® (Terumo BCT, Inc., Colorado). They found negative markers for CD45 (lymphocytes), a small population of cells expressing CD34 (hematopoietic stem cell) and positive results for markers expressed on MSCs (CD271, CD34, CD73, CD146). Moreover, quantitative growth factors and cytokine levels found in this study revealed an increase in levels of TGFβ1 as a stimulant for chondrogenesis of BM-MSCs. One difference between the composition of the processed aspirate between the two systems was an increase in IL-1ra/IL-1β ratio denoting the inhibition of IL-1β catabolic activity in Magellan® BMAC preparations. However, with regards to clinical benefits, no evidence based recommendation for either systems can be made.
BMAC—patient selection
The current literature does not allow high-level evidence-based recommendations regarding exact indications. However, for chondral defects, the knee and ankle joint are best investigated and systematically reviewed (6,7). Furthermore, BMAC as an additive for other established treatments such as microfracturing and similar bone-marrow-stimulating techniques has to be distinguished from BMAC application in combination with scaffolds alone. The recommendations given are for the more common BMAC application in combination with scaffolds. These applications aim to regenerate hyaline-like cartilage comparable to autologous-chondrocyte-transplantation. In consequence, defect size and grade selection for BMAC are “in between” of recommendations for autologous chondrocyte transplantation and bone-marrow-stimulating techniques (8). Defect grades of III–IV (ICRS Score) and sizes greater than 1.5 cm2 up to 4 cm2 in the knee and greater than 1 cm2 in the ankle are the primary indications for BMAC application in combination with a scaffold. Table 1 gives an overview of indications- and contraindications.
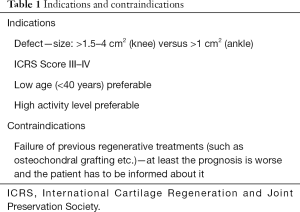
Full table
BMAC—preparation techniques
BMAC preparation involves isolation of bone marrow aspirate from the anterior or posterior iliac crest, most frequently with the listed commercial systems (Table 2). This involves a two-step centrifugation procedure and the resulting cellular composition is free from erythrocytes and left with nucleated cells such as bone marrow cells, concentrated platelets and sometimes with white blood cells in a smaller fraction.

Full table
An alternative to traditional needle systems is the Marrow CellutionTM System. This harvesting needle automatically leads to higher cell yields in the aspirate and thus provides the surgeon with BMAC that is applicable eventually without further centrifugation. This is achieved through a closed needle tip that prevents peripheral blood contamination, which is the main cause for the necessity of centrifugation (9).
BMAC—surgical techniques
BMAC harvest
The primary recommendation for harvesting BMAC is the iliac crest, usually on the side of the chondral defect. The patient is positioned supine to ensure easy access to the iliac crest. The entry point might be chosen using the “zone model” of Hernigou et al. (10). This model is based on the thickness of the pelvic bone and recommends entry points in zones with thick underlying bone in order to ensure safe placement of the trocar. Accordingly, a possible solution is to choose an entry point approximately 2.5 cm distally from the anterior superior iliac spine—(performed like this, one should be in zone 2 according to this model, which is supposed to be safe. However, other approaches might equally secure). Afterwards, a 1-cm skin incision is made on top of this location and the underlying bone of the iliac crest is carefully exposed. A 11-gauge 119 mm needle is inserted about 3 centimeters (avoid going deeper than 6 cm!) into the marrow of the iliac crest in a favorable angle to not break through the pelvic wall. Try to aspirate around 5 mL. Repeat the aspiration by carefully fanning out to other locations until you aspirated a total bone-marrow of around 60 mL. A potential higher cell yield does in the authors’ opinion not justify the increased comorbidity of multiple entry points. The non-sterile assistant is handed the aspirate and centrifuges it in the proper device. This takes around 15 minutes. Centrifugation automatically removes most plasma and erythrocytes leaving nucleated cells with an average volume of 7 mL. With this 7 mL of BMAC, the scaffold of choice has to be seeded in a sterile manner. 2 mL will approximately soak 2 cm × 2 cm of Hyaluronan-based scaffolds sufficiently.
Scaffolds
Before use, the scaffold is taken out of the secondary packing and brought onto the steril table within its primary packing. One might want to tailor the scaffold carefully to perfectly fit the lesion size with a previously created template. There is no orientation to the scaffold. Both sides can equivalently be used for BMAC seeding. Afterwards, seed the scaffold with BMAC until it is fully soaked—approximately 1 mL per 1 cm2 is required.
Commonly used combination of scaffolds includes collagen type I and hyaluronic acid based matrices such as Hyalofastc (Table 3).

Full table
Hyaluronan based scaffolds are softer and easier to adjust to the defect. Usually, no further fixation is needed. Moreover, the chondrogenic feature of hyaluronan might be an argument to use it. However, in areas with greater mechanical stress such as the trochlea or for very big defects (greater than 4 cm2), collagen might be the material of choice. Collagen scaffolds usually need to be additionally fixated via suturing or fibrin glue.
A few studies compared hyaluronan and collagen based scaffolds, but could not show a general superiority for either one (Table 3).
Knee and ankle approaches
After joint inspection via standard arthroscopic portals, the chondral lesion is carefully debrided. The aim is to create stable borders to healthy surrounding cartilage. Subchondral sclerosis should be removed using round drills. The implantation of the seeded scaffold is usually performed arthroscopically. It is recommended to use a cannule or halfpipe-like instrument in order to guide the scaffold into the joint safely. However, a slight widening of the arthroscopic portals might be necessary. This surgical step might be challenging, and particular devices have been designed to aid. Within the joint, scaffolds are placed onto the lesion by using a forceps. If necessary, scaffolds can be overlapped to cover the lesion fully. Finally, if necessary, the scaffolds can be additionally secured into the defect by using fibrin glue.
In most instances, the scaffolds cannot be placed arthroscopically without destruction. In these cases the lesions has to be accessed openly by performing a medial- or lateral mini-arthrotomy, depending on the location. The tightness of the ankle joint poses an additional challenge in accessing the lesion site. In these cases, osteotomy of the malleoli might be inevitable. It is recommended to use a bi-planar osteotomy for better stability and put drilling holes for latter refixation before osteotomy (14).
Before closure, the joint should carefully be put through a full range of motion under visual control to test the scaffold’s stability. In case a tourniquet was used, stability testing should be performed before and after its release. Generally, portals are closed using a 3-0 non-absorbable suture. Alternatively, intracutaneous suturing is performed with a monocryl 4-0 resorbable suture. In case an arthrotomy was necessary, wound closure is performed layer by layer (VicrylTM non-absorbable 2-0, VicrylTM non-absorbable 3-0 colorless—if possible for aesthetic reasons—and intracutaneous skin suture using a StratafixTM monocryl suture). If possible, usage of a drain is avoided in order not to lose BMAC via suction. Local anesthetic like 1% xylocaine is only used around the site of BMAC harvesting. Intra-articular injection might negatively influence cartilage regeneration and is thus avoided.
Safety
BMAC treatments are considered to be safe. However, potential risks of BMAC treatments either come from donor site morbidities or from the site of administration of BMAC.
Most often, the iliac crest is the site auf BMAC harvest. Hernigou et al. (10) have developed a readily applicable zone system for BMAC harvesting in order to reduce risk associated with placing the trocar. Therefore they divided the iliac crest into 6 sectors with a common starting point in the center of the hip. Among the 480 studied trocar entry points, they described the association of trocar placements with risks for specific vital structures within the complex pelvic anatomy. One simple way of applying their findings is described in the surgical techniques above. Beside damaging crucial structures, BMAC harvesting is associated with other, general risks (Table 4).
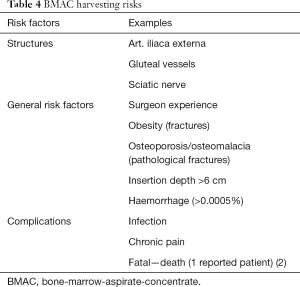
Full table
Chronic pain occurs in some patients after harvesting that might require prolonged treatment with neuropathic medication (15).
At the site of administration, infection is the major complication besides general surgery related risks. Subchondral cyst formation might also occur after the procedure, possibly requiring surgical intervention. Some studies reported joint stiffness due to intraarticular adhesions after administration of BMAC. However, arthroscopic lysis showed good results (16).
BMAC—postoperative care
After surgery, the joint is immobilized for 24 hours to secure scaffold adherence. COX-inhibitors should not be used for pain treatment to avoid potential adverse effects on cartilage regeneration.
Rehab protocol
To our knowledge, scientific evidence for rehabilitation protocols after BMAC application for chondral injuries is scarce. However, depending on the joint, this group uses locally designed rehab protocols for the ankle and follows recommendations from Pietschmann et al. after autologous chondrocyte transplantation (17). Protocols for rehabilitation are followed as presented in Table 5.

Full table
Conclusions/discussion
BMAC applications for chondral defects in combination with scaffolds are a promising alternative to other regenerative-joint preserving methods such as autologous chondrocyte transplantation. The clinical results after BMAC application and ACT seem to be comparable. The main advantage of BMAC applications is the single-step procedure and the avoidance of high costs related to cell cultivation. BMAC as a mere additive for bone-marrow-stimulation is considered by the authors to be a less critical treatment alternative. This opinion is based upon the thought, that the fibrocartilaginous regenerate after bone-marrow-stimulation alone is most often sufficient for small defects. Thus, donor-site comorbidity might not be vindicated. However, a lack of high-level systemic studies comparing BMAC to ACT or bone-marrow-stimulation techniques in all joints of interest makes a final evaluation of a potential superiority not yet possible, warranting further research in the field.
This article aimed to guide surgeons as to why, when and how to applicate BMAC for chondral defects and help to shape standardized BMAC procedures for further research.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: S Nehrer received travel funds from Anika Therapeutics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steinert AF, Rackwitz L, Gilbert F, et al. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 2012;1:237-47. [Crossref] [PubMed]
- Kristjánsson B, Honsawek S. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int 2014;2014:194318 [Crossref] [PubMed]
- Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J 2012;2:154-62. [PubMed]
- Coppens DGM, De Bruin ML, Leufkens HGM, et al. Global Regulatory Differences for Gene- and Cell-Based Therapies: Consequences and Implications for Patient Access and Therapeutic Innovation. Clin Pharmacol Ther 2018;103:120-7. [Crossref] [PubMed]
- Cassano JM, Kennedy JG, Ross KA, et al. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc 2018;26:333-42. [Crossref] [PubMed]
- Chahla J, Dean CS, Moatshe G, et al. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J Sports Med 2016;4:2325967115625481 [Crossref] [PubMed]
- Chahla J, Cinque ME, Shon JM, et al. Bone marrow aspirate concentrate for the treatment of osteochondral lesions of the talus: a systematic review of outcomes. J Exp Orthop 2016;3:33. [Crossref] [PubMed]
- Niemeyer P, Andereya S, Angele P, et al. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Tissue Regeneration" of the German Society of Orthopaedic Surgery and Traumatology (DGOU). Z Orthop Unfall 2013;151:38-47. [PubMed]
- Scarpone M, Kuebler D. Marrow cellution bone marrow aspiration system and related concentrations of stem and progenitor cells. Presented at: Allegheny Health Network Annual Orthopaedic Update. PA, USA, 8-10 April 2016.
- Hernigou J, Picard L, Alves A, et al. Understanding bone safety zones during bone marrow aspiration from the iliac crest: the sector rule. Int Orthop 2014;38:2377-84. [Crossref] [PubMed]
- Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med 2014;42:648-57. [Crossref] [PubMed]
- Gobbi A, Scotti C, Karnatzikos G, et al. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc 2017;25:2494-501. [Crossref] [PubMed]
- Enea D, Cecconi S, Calcagno S, et al. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015;22:30-5. [Crossref] [PubMed]
- Nehrer S, Domayer SE, Hirschfeld C, et al. Matrix-Associated and Autologous Chondrocyte Transplantation in the Ankle: Clinical and MRI Follow-up after 2 to 11 Years. Cartilage 2011;2:81-91. [Crossref] [PubMed]
- Bain BJ. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol 2005;58:406-8. [Crossref] [PubMed]
- Gobbi A, Chaurasia S, Karnatzikos G, et al. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage 2015;6:82-97. [Crossref] [PubMed]
- Pietschmann MF, Horng A, Glaser C, et al. Post-treatment rehabilitation after autologous chondrocyte implantation: State of the art and recommendations of the Clinical Tissue Regeneration Study Group of the German Society for Accident Surgery and the German Society for Orthopedics and Orthopedic Surgery. Unfallchirurg 2014;117:235-41. [Crossref] [PubMed]
Cite this article as: Neubauer M, Jeyakumar V, Muellner T, Nehrer S. Bone-marrow-aspirate-concentrate for chondral defects: surgical techniques, clinical applications and basic science. Ann Joint 2018;3:107.


