The evolution of pelvic endoprosthetic reconstruction after tumor resection
Background
Large skeletal defects in the pelvis typically exist in the setting of primary malignant tumors, extensive metastatic disease, major trauma, or end-stage revision arthroplasty. Extensive defects in the pelvis usually cause significant postoperative morbidity or functional impairment. The 1970s saw the advent of the limb-salvage procedure for malignant tumors in extremities (1,2), and the endoprosthesis was gradually accepted by orthopaedic oncologists and became popular by the 90s. In the early years of the limb-salvage era, reconstruction with custom-made endoprostheses, also known as megaprostheses, allowed for sparing of the extremity (3). The term “megaprosthesis” seems to have been first used in the International Workshop on Design and Application of Tumor Prosthesis, held in Mayo Clinic in 1981 (4). The meeting was also the start of the International Society of Limb Salvage (ISOLS). However, the disadvantages of the custom-made endoprosthesis, such as fabrication delay and difficulty in revision, hindered its application in clinical practice. The development of modular endoprostheses in the 1980s was regarded as a new era in complex reconstruction in orthopedic oncology. Modular megaprostheses consist of a number of different components in readily available sets that can be assembled in various combinations to best match the actual bone defect allowing for much easier revision during surgery.
With the improvement in preoperative imaging techniques, neoadjuvant treatments, and surgical techniques, limb-preserving procedures have become standard procedure for the pelvic girdle in the past few decades. Enneking and Dunham (2,5) proposed tumor classifications typically associated with four types of pelvic resections and/or reconstructions: type I, the ilium; type II, the periacetabulum; type III, the obturator; and type IV, the sacrum. For type 1, isolated resections of the ilium or ischium and pubis may not require reconstructive procedures to achieve excellent postoperative function. Type II resections require reconstruction to restore force transmission and weight bearing along anatomic axes. Adequate excision of type II tumors often requires complete excision of the skeletal hemipelvis and large parts of the soft tissue of the pelvis. Several different reconstruction options have been proposed after this type of resection, including ischiofemoral arthrodesis or pseudarthrosis, iliofemoral arthrodesis (6,7) or pseudarthrosis, massive allograft, autoclaved autograft, allograft prosthetic composite, custom-made endoprosthesis combined with hip arthroplasty (8-11), or the modular saddle prosthesis (12-15) and 3D-printing endoprosthesis (16,17). There are various options for reconstruction, each having their respective advantages and limitations. For instance, implantation of a megaprosthesis in the early years was shown to result in a high complication rate with poor functional results. Meanwhile, major complications of megaprosthetic reconstructions, such as infection, loosening, and dislocation, have occurred frequently, at a proportion of approximately 25–35% (6,10,18-22).
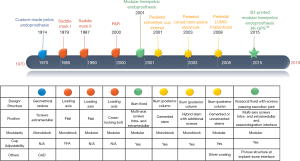
The main trend in the evolution of pelvic endoprosthesis is modularity and fixation mode (13,15,23-26). With the advent of megaprosthetic reconstruction in the pelvis, custom-made fashion was the main design focus in the early days (11,22,27). However, with development of endoprosthesis modularity for limb-salvage procedure in extremities, the modularity concept was introduced into pelvic endoprosthetic reconstruction (15,24). Nowadays, three main types of pelvic endoprostheses are used worldwide (Table 1).

Full table
Geometric pelvic endoprosthesis
The history of oncologic reconstruction of the pelvis using endoprosthesis is intimately associated with the development of joint arthroplasty and manufacture technology. Naturally, anatomical recovery was attempted in the early days using the available materials for orthopedic reconstruction. Here, we define this period as “geometry pelvic endoprostheses”. The focus of reconstruction was anatomical recovery without a well-established design style or fixation principles. The requisites for a successful implant design should be resistance to corrosion, high biocompatibility, biomechanical compatibility, early stability, along with the potential for osteointegration and soft tissue ingrowth for longevity. However, the approach of geometrical restoration in pelvic reconstruction did not provided satisfying outcomes (28).
Custom-made megaprostheses
The first attempts to reconstruct resected pelvic bone and to restore the pelvic ring using endoprostheses began in the early 1970s (7). Scales and Rodney implanted a temporary spacer of acrylic cement and designed a steel prosthesis in the shape of a resected iliac bone, but the prosthesis was removed due to infection. In 1974, the first case pertaining to endoprosthetic reconstruction after pelvic tumor resection was reported in the literature and described a patient with chondrosarcoma (29). In the earlier period, the preoperative plan was determined by X-ray. Later, attempts were made to improve the accuracy of pelvic prosthesis design and production. The first report on endoprosthetic pelvis reconstruction was by Gradinger in 1993 (30). The prosthesis was custom-manufactured from plain x-rays with low accuracy for intraoperative orientation of the acetabulum. The anchorage into the remaining sacral ala or iliac bone was mainly provided by screws with additional plates or flanges. Ozaki et al. (10) reported a series of twelve cases of pelvic prosthetic reconstruction following tumor resection based on computer-aided design according to preoperative computed tomography (CT) scan. Deep infection occurred in 3 of the 12 patients. The overall survival of endoprosthesis at 3 years after surgery was 42%. Windhager et al. (31) reported a series of 21 consecutive cases of different reconstruction approaches: 9 of the 21 patients received a custom-made prosthesis with better functional results compared to saddle prosthesis and allografts.
Single-stage by preoperative CT scan and implant design or two-stage procedure by mold made from an excised pelvis were the two typical approaches during the period of time spanning the 1970s to the 1990s. Studies reported surgical-related complication rates between 42–50% (27,30,31) including deep infection, dislocation, loosening, and hardware breakage. The high complication rate and lack of flexibility during surgery led to the application of saddle prosthesis in periacetabular reconstruction (PAR).
Transitional period—saddle prosthesis
The saddle prosthesis was designed by Nieder in Germany in 1979 (32). Initially, this prosthetic concept was used for pelvic reconstruction of large acetabular defects following total hip arthroplasty. Since 1984, it has also been indicated as a replacement after resection of periacetabular tumors. The advantage of this method has been in the simplicity of its design, alleviating the need for an acetabular implant. However, the disadvantage is the need for postoperative immobilization to ensure soft tissue healing. Additionally, certain aspects of notch preparation are more challenging. Poor range of motion, dislocation, and progressive upward migration are common complications after saddle endoprosthesis reconstruction. In the literature, dislocation was reported to range from 2–20%. Heavy sutures will help secure the saddle component to the ilium. Saddle prosthesis was evolved from the Mark I, which was one piece (monoblock), to the Mark II. The Mark II prosthesis is a modular design that provides more flexibility during surgery. Studies on the subject support the superiority of modular implants. Also, the polyethylene sleeve can provide rotation range of motion.
Stryker PAR endoprosthesis was designed to be secured with internal fixation and bone cement applied to the remaining ilium to support a reconstructed acetabulum. To address the previous mechanical complications found in the Mark I and Mark II saddle prostheses, which included loosening, migration, and dissociation, PAR endoprosthesis was developed as a modular third-generation saddle prosthesis. The PAR endoprosthesis consists of a wide iliac wing component that is secured to the ilium with cross bolts and cement, a constrained bipolar ball-and-socket joint, and a modular standard or endoprosthetic femoral stem. The design of the prosthesis provided four times the amount of osseous support compared to the Mark II prosthesis. The complication rate of PAR endoprosthesis was reported to be 56%, and implant survivorship was 60% at 5 years; the dislocation rate was decreased to 12% (26).
The saddle prosthesis introduced concepts of modularity and clarified iliofemoral weight-bearing axis restoration for pelvic reconstruction by metallic endoprosthesis.
Structural pelvic endoprosthesis
Distinct from custom-made endoprostheses, structural endoprostheses were designed to reconstruct the key stress-loading axes rather than the geometry of pelvic bone. Currently, the two main types of structural pelvic endoprostheses are the modular hemipelvic endoprosthesis and the pedestal cup.
Modular pelvic endoprosthesis
The modular design was initially discussed by Guo et al. (33) in 2007 (Figure 1). There are two main components: the iliac fixation part that serves as the main fixation structure between the prosthesis and residual bone; and the acetabular cup, which connects to the iliac fixation component by Morse taper. The pedestal cup or ice-cream endoprosthesis is an iliac-based fixation without structure which restores the anterior pelvic ring. The advantage of a modular pelvic endoprosthesis is the smaller size, which facilitates soft-tissue coverage and may reduce the infection rate. As for the fixation strategy, multi-axial fixation by screws is used in Guo’s modular pelvic endoprosthesis and has a low breakage and loosening rate. Pedestal endoprosthesis has a cemented or press-fit fixation technique, which is similar to a megaprosthesis used for extremities. However the lack of channel structure and cortical bone in the ilium and iliosacral area leads to difficulties in applying such a fixation concept. It seems that it is well accepted that the anterior pelvic ring should be left open in prosthetic reconstruction due to the rigidity at the pubic connection area. Both intramedular-fixation (pedestal endoprosthesis) and extramedular-fixation (iliac-flange-fixation) exclude anterior ring restoration.
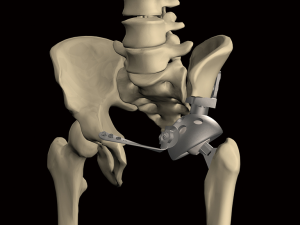
Compared with the megaprosthesis used for extremities, fixation of a metallic pelvic implant remains unsatisfactory due to the special skeletal structure of the ilium. Better fixation may reduce biomechanical failures. Metal implants with an interconnected pore structure exhibit the potential to facilitate bone ingrowth and the possibility for reducing stiffness. The electron beam melting (EBM) processing technique may be a novel approach for enhancing osseointegration of the bone-implant interface thus achieving durable prosthetic fixation (Figure 2). A prosthetic surface with 3D-printed macropores may support bone formation deep within the porous network with a high level of bone-implant contact. In an attempt to reduce mechanical failures, an EBM-based modular hemipelvic endoprosthesis was introduced in 2015 and was part of the Global Pelvic SystemTM (GPSTM) (AK Medical, Beijing, China). The design was similar to a previously reported modular hemipelvic endoprosthesis (first generation); however, the main fixation structure evolved from the residual iliac bone to the strong iliosacral part, which can provide more biomechanical compatibility with less shear stress on the screws (Figure 3). The bone-contact surface of the endoprosthesis was a 1.5-mm-thick layer of titanium porous structure to enhance the osseointegration by bone ingrowth. The lateral wing of the iliac fixation component was modified to match the anatomical surface of the lateral cortex of the ilium.

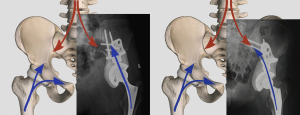
Iliac stem endoprosthesis
The pedestal cup (Zimmer, Freiburg, Germany) was originally designed for severe acetabular revision. It was first used in an oncological condition in 2001 in Vienna, Austria, as reported by Hipfl et al. (13). The so-called iliac stemmed-cone prostheses are effectively modified versions of the McMinn acetabular reconstruction component. In their retrospective review of a series of 48 cases reconstructed by stemmed pedestal cup (Schoellner cup, Zimmer Biomet Inc.), a complication rate of 40% was found along with a median follow-up of 6.6 years. Deep infection was the most common complication which affected 17% of the patients. The mean function score by MSTS 93 was 71%.
The “ice-cream” cone reconstruction of the pelvis was developed in 2003 by Stanmore Implants and the system was named the “coned hemi-pelvis”. The concept was based on the old design of the McKee-Farrar stemmed hip replacement and has become known as the “ice-cream” cone prosthesis, as it looks like an inverted ice-cream cone. The prosthesis is inserted into the remnant of the pelvis and surrounded by antibiotic-laden bone cement. In one study of this method, the overall complication rate was 37% with dislocation being the most common type (14.8%), followed by deep infection (11.1%) (34).
A modification type of pedestal endoprosthesis, LUMiC® (implantcast, Germany) was introduced in 2003. The LUMiC® prosthesis is a modular device, built of a separate cemented or uncemented stem with an HA-coated acetabular cup. The cup is also available with silver coating for anti-infection effect. The cup is connected to the stem by sawteeth allowing for rotational adjustment of the cup position after implantation of the stem. A 6-year multicenter study including 47 patients showed a 13% dislocation rate for single-time dislocations and a 9% rate for recurrent dislocations. Infection was the most common type of complication (28%).
The design evolution of the pedestal pelvic endoprosthesis is summarized in Table 2. The change of design over time reflects the main concepts of pelvic endoprosthesis: modularity and adjustability of the acetabular cup. Figure 4 shows the main phases of the development of the pelvic endoprosthesis.
Conclusions
Modern modular pelvic megaprostheses have allowed limb-preserving surgery to be the preferred choice, as they facilitate efficient reconstruction of extensive defects after tumor removal. Improvements in the design of the implants have reduced the rate of mechanical complications. In addition, the functional outcome after surgery appears to be satisfactory, offering a good quality of life to the patient. While research should focus mainly on the elimination of non-mechanical events, such as infection, there are still some mechanical drawbacks present in modern designs, such as bone-implant integration and the reattachment of soft tissues. Introduction of 3D-printing technology may provide some solutions to certain problems. Indeed, additional improvements and advances in the field that will further improve the results of surgery are eagerly anticipated.
Acknowledgments
Funding: One of the authors (T Ji) received funding from the National Key Research and Development Program of China (2016YFB1101502), the Capital Health Research and Development of Special (No. 2018-2-4088), and the National Natural Science Foundation of China (No. 81872180). Author (W Guo) received funding from the Beijing Municipal Science and Technology Project (Z161100000116100).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Joint for the series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends”. The article has undergone external peer review.
Conflict of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.06.01). The series “Reconstruction in Orthopaedic Oncology - Frontier and Future Trends” was commissioned by the editorial office without any funding or sponsorship. TJ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Joint from Jun 2018 to May 2020. WG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;241-6. [PubMed]
- Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am 1978;60:731-46. [Crossref] [PubMed]
- Schwartz AJ, Kabo JM, Eilber FC, et al. Cemented distal femoral endoprostheses for musculoskeletal tumor: improved survival of modular versus custom implants. Clin Orthop Relat Res 2010;468:2198-210. [Crossref] [PubMed]
- Gkavardina A, Tsagozis P. The use of megaprostheses for reconstruction of large skeletal defects in the extremities: a critical review. Open Orthop J 2014;8:384-9. [Crossref] [PubMed]
- Enneking WF. Local resection of malignant lesions of the hip and pelvis. 1966. Clin Orthop Relat Res 2002;3-11. [Crossref] [PubMed]
- Hugate R Jr, Sim FH. Pelvic reconstruction techniques. Orthop Clin North Am 2006;37:85-97. [Crossref] [PubMed]
- Johnson JT. Reconstruction of the pelvic ring following tumor resection. J Bone Joint Surg Am 1978;60:747-51. [Crossref] [PubMed]
- Dai KR, Yan MN, Zhu ZA, et al. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty 2007;22:981-6. [Crossref] [PubMed]
- Kitagawa Y, Ek ET, Choong PF. Pelvic reconstruction using saddle prosthesis following limb salvage operation for periacetabular tumour. J Orthop Surg (Hong Kong) 2006;14:155-62. [Crossref] [PubMed]
- Ozaki T, Hoffmann C, Hillmann A, et al. Implantation of hemipelvic prosthesis after resection of sarcoma. Clin Orthop Relat Res 2002;197-205. [Crossref] [PubMed]
- Müller PE, Durr HR, Wegener B, et al. Internal hemipelvectomy and reconstruction with a megaprosthesis. Int Orthop 2002;26:76-9. [Crossref] [PubMed]
- Tang X, Guo W, Yang R, et al. Acetabular Reconstruction With Femoral Head Autograft After Intraarticular Resection of Periacetabular Tumors is Durable at Short-term Followup. Clin Orthop Relat Res 2017;475:3060-70. [Crossref] [PubMed]
- Hipfl C, Stihsen C, Puchner SE, et al. Pelvic reconstruction following resection of malignant bone tumours using a stemmed acetabular pedestal cup. Bone Joint J 2017;99-B:841-8. [Crossref] [PubMed]
- Flecher X, Appy B, Parratte S, et al. Use of porous tantalum components in Paprosky two and three acetabular revision. A minimum five-year follow-up of fifty one hips Int Orthop 2017;41:911-6. [Crossref] [PubMed]
- Bus MP, Szafranski A, Sellevold S, et al. LUMiC® Endoprosthetic Reconstruction After Periacetabular Tumor Resection: Short-term Results. Clin Orthop Relat Res 2017;475:686-95. [Crossref] [PubMed]
- Fang C, Cai H, Kuong E, et al. Surgical applications of three-dimensional printing in the pelvis and acetabulum: from models and tools to implants. Unfallchirurg 2019;122:278-85. [Crossref] [PubMed]
- Liang H, Ji T, Zhang Y, et al. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Joint J 2017;99-B:267-75. [Crossref] [PubMed]
- Aljassir F, Beadel GP, Turcotte RE, et al. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res 2005;36-41. [Crossref] [PubMed]
- Zeifang F, Buchner M, Zahlten-Hinguranage A, et al. Complications following operative treatment of primary malignant bone tumours in the pelvis. Eur J Surg Oncol 2004;30:893-9. [Crossref] [PubMed]
- Hillmann A, Hoffmann C, Gosheger G, et al. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg 2003;123:340-4. [Crossref] [PubMed]
- Natarajan MV, Bose JC, Mazhavan V, et al. The Saddle prosthesis in periacetabular tumours. Int Orthop 2001;25:107-9. [Crossref] [PubMed]
- Wirbel RJ, Schulte M, Maier B, et al. Megaprosthetic replacement of the pelvis: function in 17 cases. Acta Orthop Scand 1999;70:348-52. [Crossref] [PubMed]
- Sculco PK, Ledford CK, Hanssen AD, et al. The Evolution of the Cup-Cage Technique for Major Acetabular Defects: Full and Half Cup-Cage Reconstruction. J Bone Joint Surg Am 2017;99:1104-10. [Crossref] [PubMed]
- Ji T, Guo W, Yang RL, et al. Modular hemipelvic endoprosthesis reconstruction--experience in 100 patients with mid-term follow-up results. Eur J Surg Oncol 2013;39:53-60. [Crossref] [PubMed]
- De Paolis M, Biazzo A, Romagnoli C, et al. The Use of Iliac Stem Prosthesis for Acetabular Defects following Resections for Periacetabular Tumors. ScientificWorldJournal 2013;2013:717031 [Crossref] [PubMed]
- Menendez LR, Ahlmann ER, Falkinstein Y, et al. Periacetabular reconstruction with a new endoprosthesis. Clin Orthop Relat Res 2009;467:2831-7. [Crossref] [PubMed]
- Bruns J, Luessenhop SL, Dahmen G Sr. Internal hemipelvectomy and endoprosthetic pelvic replacement: long-term follow-up results. Arch Orthop Trauma Surg 1997;116:27-31. [Crossref] [PubMed]
- Brown TS, Salib CG, Rose PS, et al. Reconstruction of the hip after resection of periacetabular oncological lesions: a systematic review. Bone Joint J 2018;100-B:22-30. [Crossref] [PubMed]
- Schöllner D, Ruck W. Proceedings: Pelvic prosthesis--an alternative to hemipelvectomy in tumor patients. Z Orthop Ihre Grenzgeb 1974;112:968-70. [PubMed]
- Gradinger R, Rechl H, Ascherl R, et al. Partial endoprosthetic reconstruction of the pelvis in malignant tumors. Orthopade 1993;22:167-73. [PubMed]
- Windhager R, Karner J, Kutschera HP, et al. Limb salvage in periacetabular sarcomas: review of 21 consecutive cases. Clin Orthop Relat Res 1996;265-76. [Crossref] [PubMed]
- Nieder E, Keller A. The Saddle Prosthesis Mark II, Endo-Modell®. In: Yamamuro T. editor. New Developments for Limb Salvage in Musculoskeletal Tumors Tokyo: Springer, 1989:481-90.
- Guo W, Li D, Tang X, et al. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res 2007;180-8. [PubMed]
- Fisher NE, Patton JT, Grimer RJ, et al. Ice-cream cone reconstruction of the pelvis: a new type of pelvic replacement: early results. J Bone Joint Surg Br 2011;93:684-8. [Crossref] [PubMed]
- Dominkus M, Kotz R. Pelvic Prosthesis. In: Sim FH, Choong PF, Weber KL. editors. Orthopaedic Oncology and Complex Reconstruction. Lippincott Williams & Wilkins, 2011:43-54.
Cite this article as: Ji T, Guo W. The evolution of pelvic endoprosthetic reconstruction after tumor resection. Ann Joint 2019;4:29.