Ceramic resurfacing: the future and challenges
The history of ceramics in resurfacing
A variety of materials have been used in resurfacing including early generation ceramics. Table 1 describes some of the earliest hip resurfacing devices which utilised alumina ceramic. The majority of these devices only used ceramic in the femoral side and many early devices had the limitation of a polyethylene acetabular cup. The majority of failures in these early hip resurfacing cases are cited for loosening caused by particle-induced-osteolysis. Polyethylene material properties are now better controlled through sterilisation in an inert atmosphere, cross-linking and use of antioxidants, but do still wear (5). There were no cases of femoral loosening reported for the cemented Furuya alumina ceramic resurfacing components (4) but the cementless all-ceramic Salzer device reported 5 incidences of femoral loosening out of a total of 16 implantations (3). Despite evidence that alumina ceramic in total hip replacement (THR) can fail due to component fracture when abnormally loaded (6), reports of fracture in these early design hip resurfacing devices has not been found. The alumina ceramic used in these early hip resurfacing designs was significantly weaker than the current generation of BIOLOX® delta.

Full table
In 1990, hip resurfacing re-emerged in the form of the McMinn Hip (made by Corin Medical—designed by Derek McMinn with Finsbury Orthopaedics) then in 1997 the Birmingham Hip Replacement (BHR, Midland Medical Technologies designed by McMinn with and made by Finsbury Orthopaedics, bought by Smith and Nephew in 2006 but manufactured by Finsbury Orthopaedics until 2008), learning from previous manufacturing and materials. Several other metal-on-metal (MoM) devices were subsequently released to the market following early success but most of these were subsequently withdrawn due to design differences. Outside of patient selection, the factors contributing to the success or failure of a MoM hip resurfacing device are now acknowledged to include: different bearing clearances, different metallurgy, poorer quality control of manufacturing tolerances, inadequate head coverage of the cup when implanted at steep inclination angles, different operative techniques, and accelerated commercial rollout with poor training. Currently we believe that only two MoM hip resurfacing devices remain CE marked: the BHR and the ADEPT® (MatOrtho, previously Finsbury Orthopaedics Ltd., UK). The MatOrtho ADEPT® had largely the same design team as the BHR and the same manufacturing processes as the early BHR. These two MoM hip resurfacing devices have shown excellent and consistent results up to 10 and 15 years, especially in active males under 55 years old with larger diameter femoral heads demonstrating survivorship of 96.3% (93.7% to 98.3%) at ten years and 94.1% (84.9% to 97.3%) at 14 years (7).
The potential reaction of the body to excessive Cobalt and Chromium of MoM bearings is however a continuing concern following reports of hypersensitivity reactions, aseptic lymphocyte-dominated vasculitis-associated lesions (ALVAL) and pseudotumour (8-10). Nevertheless hip resurfacing is used in limited numbers, mostly for active young male patients with good femoral bone, for whom these implants perform well. There are therefore several patient groups including women for whom resurfacing is not generally considered a good option despite good bone stock which is removed for conventional hip replacement. This has led to several investigations of alternative materials for use in resurfacing to replace MoM for all patients (11-13).
Design and development of ReCerf®
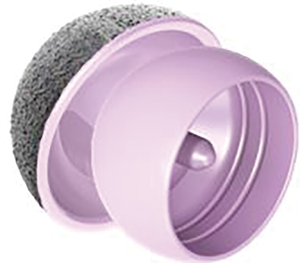
ReCerf® hip resurfacing (MatOrtho Ltd., UK) is exclusively manufactured for MatOrtho from BIOLOX® delta ceramic (CeramTec, Plochingen, Germany), Figure 1. BIOLOX® delta ceramic is a zirconia toughened alumina which is an established articulating surface used as a ceramic-on-ceramic (CoC) bearing surface in THR for over 16 years and has shown clinically low wear rates with excellent biocompatibility (14). BIOLOX® delta as a hip resurfacing bearing could make this a treatment option suitable for a greater range of patients. However this material as a bearing couple is clinically unproven in hip resurfacing devices but has been used in bearing diameters up to 48 mm as developed by MatOrtho’s predecessor, Finsbury Orthopaedics, in the DeltaMotion THR. The use of this material in larger diameters for hip resurfacing is an incremental step-wise innovation.
ReCerf® was designed with an experienced group of hip resurfacing surgeons and based on the clinically proven ADEPT®, to be available in 2 mm increments from sizes 40 to 64 mm bearing diameter. The resurfacing heads feature three internal pockets and two circumferential grooves to maximise primary cementation stability. Head thickness and stem size increase with bearing diameter. The stem is proportional to head diameter to maximise bone volume and reduce the potential for stress shielding. The cups utilise direct fixation to bone through a titanium and hydroxyapatite coating (DeltaFix®) with an inference fit at the equator and a constant 163° coverage angle across all sizes. The coating is applied using vacuum plasma spray on the as-fired ceramic as characterized as to ISO 13179-1 and 13379-2 for titanium and hydroxyapatite coatings, respectively. Tensile and shear adhesion tests to ASTM F1147 and ASTM F1044 showed bonding strengths comparable or greater than bonding to medical grade titanium alloy under static and fatigue conditions. Of further interest was that the adhesion bond strengths were comparable between as-fired and polished surfaces. The ReCerf® bone to coating interface is effectively therefore the same as found on most modern modular lined cups and the coating adhesion tests allay potential concerns of debonding although longer term clinical data is required.
Despite the long-term intended use of the device only two ISO standards (ISO 14242 and ISO 7206-12) were identified as relevant for testing for regulatory body’s assessment under the medical device directive (MDD). Additional finite element and mechanical tests were therefore developed to ensure the safety of the device. Over the three year development cycle, 15 finite element studies and some 40 mechanical tests were conducted with CeramTec and Aurora Medical Ltd, Southampton UK. Numerous cadaver labs were conducted with the development surgeon team. These tests were designed to mitigate risks associated with fracture, fixation, stress shielding, wear, and surgical technique.
Novel simulation tests developed included comparisons of the BIOLOX® delta ceramic ReCerf® and CoCrMo ADEPT® devices to consider the materials’ behaviour and deformations during cadaveric implantation (12,13). Finite element studies were conducted comparing bone remodelling when implanted with a CoCrMo or BIOLOX® delta monobloc cup (15). The remodeling stimulus of the acetabular and femoral bone when implanted with BIOLOX® delta was of particular importance as the ceramic has a Young’s modulus 1.6 times greater than CoCrMo which could lead to increased stress shielding. However, finite element studies showed that the bone strain remained similar whether the device implanted was metal or ceramic suggesting that it is only magnitudes of Young’s modulus differences which change the bone’s response (15). Tests considering the ceramic material in isolation and head/cup design were also conducted showing BIOLOX® delta to be low wearing at diameters of 64mm even under microseparation conditions (16). Worst-case edge loaded impact tests were performed with the equivalent of car crash impacts, known to fracture the pelvis (17). New instrumentation was required including a patented cup impaction cap and handle which allowed for direct grip of the outer diameter of the cup without requiring compromise to the cup integrity or damaging pelvic bone, Figure 2. Finite Element Analysis of the stresses induced on the ceramic cup by the novel impactor, mechanical tests and surgeon cadaver sessions indicated suitability for safe clinical use.

Clinical and regulatory perspective
All testing with critical safety factors applied demonstrated suitability for the intended use of ReCerf® and demonstrated equivalence against the predecessor device, ADEPT®. Many regulatory systems worldwide are based on the European regulatory framework and require CE mark for entry into these markets. After a lengthy review period by the MatOrtho’s regulating notified body the CE mark application was rejected on the grounds of no other all-ceramic resurfacing device being available. This despite initially being advised that the CE mark was achievable through equivalency routes with independently approved monitoring of an almost identical predecessor design, accompanied with a substantial testing programme, and use of the most biocompatible proven ceramic material couple offering a metal ion free solution to the most widely documented concern with resurfacing technology. Major changes in the regulatory landscape in Europe were being introduced early and now require a fully approved limited Clinical Investigation before wider EU release. This requires a dataset having much reduced patient numbers to that proposed in the CE submission that voluntarily proposed a 500 patient limit reviewed by Beyond Compliance prior to wider release. Companies who seek to innovate medical devices now face serious challenges to any further innovation as discussions with the competent authorities and notified bodies have failed to answer what constitutes a viable introduction to prove safety and performance for orthopaedic devices. The increasing scrutiny of the impending EU medical device regulations (MDR) is already presenting further challenges to introduce innovation and threaten the viability of existing successful devices. The British Orthopaedic Association has itself highlighted the real risk that products will be more expensive, some will disappear due to commercial viability alone and the requirement for clinical investigations may not produce a relevant clinical or ethical route to relevant scientific data (18).
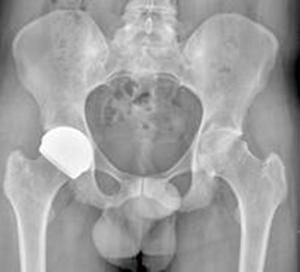
Other countries’ regulatory authorities have recognised the potential for ReCerf® to treat patients for whom other hip replacement devices are not appropriate when considering age, bone preservation, gender and metal sensitivity. Approved use of ReCerf® has been granted via Humanitarian access to a small number of trained hip resurfacing surgeons and this has allowed access to this device for patients seeking more optimised treatment than that currently available under standard regulatory pathways. The countries in which ReCerf® has been implanted to date are Australia, South Africa, Canada and Belgium. This humanitarian access route has been directly denied by the MHRA in the UK. It remains unclear what number of cases are required to show the show safety of a device and the new MDR regulations require constant monitoring of clinical outcomes throughout the lifetime of the device thus increasing the challenges to obtain European approvals. To date (December 2019) 117 implantations have taken place with several patients approaching up to 1 year post-surgery and reporting a return to activity, Figure 3. There has been 1 revision for acetabular component malposition at 3 months.
Discussion
Metal-on-Metal hip resurfacing use peaked in the late 2000s before issues surrounding the effects of metal wear debris became apparent. With patients living longer and having more active lifestyles, the typical expectations of a hip replacement have changed to include increased longevity, minimally invasive surgical options and increased functionality. There is a growing body of evidence that suggests hip resurfacing can offer better function in patients with similar aspirations for sports, more normal gait and push off forces when compared to a THR (19-24). Hip resurfacing also has lower risk of infection and dislocation as compared to THR (25). The registry data results and long term surgeon series are now starting to demonstrate fewer revisions than were or might have been expected (26). Resurfacing is therefore a suitable treatment for many patients but the CoCrMo material currently used limits the population of patients who can receive this device and a more benign ceramic material with the potential for lower wear than any prior hip resurfacing articulation could benefit all.
In summary, MatOrtho Limited has developed an innovative device that could combine the benefits of hip resurfacing with the proven material properties of BIOLOX® delta ceramics. ReCerf® Hip Resurfacing System is an all-ceramic hip resurfacing device based on MoM hip resurfacing and this includes similar functional geometries, cup coverage angle and use of the same instrumentation and operative technique. The proven hip resurfacing design (ADEPT®, MatOrtho Ltd.) has 13 years of successful clinical use and the BIOLOX® delta ceramic material has excellent outcomes in THR over 16 years. Combining proven design and this material may offer patients an alternative treatment to those currently available but which have limitations.
The cost to develop a novel device is substantially increasing, even for one, as in this case, very similar to an existing device (Adept®). ReCerf® received substantial input from several disciplines such as engineers (design, test, production), material scientists and clinicians over 3 years development and underwent extensive pre-clinical testing, much beyond standardised testing requirements for long-term devices and to date, there have been 117 ReCerf® implantations worldwide with no implant related adverse effects. A further two years has now been lost trying to navigate an unclear regulatory system as regulations change. Regulatory frameworks in the EU now make it increasingly challenging to make any progress for new devices, even when in this case changes are incremental, use no novel materials or processes and are specifically designed to reduce the known risks associated with metal. The requirement for a very limited clinical investigation is contributing to a further delay for patients receiving innovative medical treatments that need careful studies with statistically sensible numbers.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (George Grammatopoulos and Paul E. Beaulé) for the series “Hip Resurfacing for the Young Arthritic Hip” published in Annals of Joint. The article has undergone external peer review.
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2019.12.11). The series “Hip Resurfacing for the Young Arthritic Hip” was commissioned by the editorial office without any funding or sponsorship. DdV is a salaried employee of MatOrtho Ltd. LR is a salaried employee of MatOrtho Ltd. MT has a patent ReCerf/DeltaROM cup GB1522842.2A issued, a patent ReCerf/DeltaROM cup US15/538870 pending, and a patent ReCerf Head PCT/GB2018/054182 pending and is the founder and chairman of MatOrtho Ltd. SC has a patent ReCerf/DeltaROM cup GB1522842.2A issued, a patent ReCerf/DeltaROM cup US15/538,870 pending, and a patent ReCerf Head PCT/GB2018/053182 pending and is a salaried employee of MatOrtho Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cotella L, Railton GT, Nunn D, et al. ICLH double cup arthroplasty, 1980-1987. J Arthroplasty 1990;5:349-57. [Crossref] [PubMed]
- Wagner H. Surface replacement arthroplasty of the hip. Clin Orthop Relat Res 1978;102-30. [PubMed]
- Salzer M, Knahr K, Locke H, et al. Cement-free bioceramic double-cup endoprosthesis of the hip-joint. Clin Orthop Relat Res 1978;80-6. [PubMed]
- Furuya K, Tsuchiya M, Kawachi S. Socket-cup arthroplasty. Clin Orthop Relat Res 1978;41-4. [PubMed]
- Bracco P, Bellare A, Bistolfi A, et al. Ultra-High Molecular Weight Polyethylene: Influence of Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017;10:791-813. [Crossref] [PubMed]
- Traina F, De Fine M, Di Martino A, et al. Fracture of Ceramic Bearing Surfaces following Total Hip Replacement: A Systematic Review. Biomed Res Int 2013;2013:157247 [Crossref] [PubMed]
- Matharu GS, McBryde CW, Pynsent WB, et al. The outcome of the Birmingham Hip Resurfacing in patients aged < 50 years up to 14 years post-operatively. Bone Joint J 2013;95-B:1172-7. [Crossref] [PubMed]
- Fary C, Thomas GER, Taylor A, et al. Diagnosing and investigating adverse reactions in metal on metal hip implants. BMJ 2011;343:d7441. [Crossref] [PubMed]
- Nawabi DH, Gold S, Lyman S, et al. MRI predicts ALVAl and tissue damage in metal-on-metal hip arthroplasty. Clin Orthop Relat Res 2014;472:471-81. [Crossref] [PubMed]
- Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2008;90:847-51. [Crossref] [PubMed]
- Farrier AJ, Moore L, Manning W, et al. Comparing the cup deformation following implantation of a novel ceramic-on-ceramic hip resurfacing bearing to a metal standard in a cadaveric model. Proc Inst Mech Eng H 2019;233:603-10. [Crossref] [PubMed]
- Farrier AJ, Moore L, Manning W, et al. Comparison study of temperature and deformation changes in the femoral component of a novel ceramic-on-ceramic hip resurfacing bearing to a metal standard, using a cadaveric model. Proc Inst Mech Eng H 2019;233:1318-26. [Crossref] [PubMed]
- Treacy RBC, Holland JP, Daniel J, et al. Preliminary report of clinical experience with metal-on-highly-crosslinked-polyethylene hip resurfacing. Bone Joint Res 2019;8:443-50. [Crossref] [PubMed]
- Gamble D, Jaiswal PK, Lutz I, et al. The Use of Ceramics in Total Hip Arthroplasty. Orthop Rheumatol 2017;4:555636
- De Villiers D, Dickinson A, Taylor A, et al. Bone Remodelling Study of Metal and all-Ceramic Acetabular Resurfacing Cups, Paper presented at International Society for Technology in Arthroplasty. Annual Meeting; Toronto; 2019 Oct 2-5.
- De Villiers D, Collins S. Wear of a Ceramic Resurfacing Hip Prosthesis under Standard and Microseparation Conditions, Paper presented at International Society for Technology in Arthroplasty Annual Meeting; Toronto; 2019 Oct 2-5
- Avila C, Taylor A, Collins S. Resistance of a Novel Ceramic Acetabular Cup to Critical Impact Loads. Orthop Proc 2019;101-B Supp 4.
- British Orthopaedic Association. BOA Position statement on Medical Device Regulation from 2020 [published 8 November 2019, accessed 14 November 2019]. Available online: https://www.boa.ac.uk/uploads/assets/89d8178a-6d09-4512-86083b79bfaeb019/mdr-boa-position-statement-finaldocx.pdf
- Barrack RL, Ruh EL, Berend ME, et al. Do Young, Active Patients Perceive Advantages After Surface Replacement Compared to Cementless Total Hip Arthroplasty? Clin Orthop Relat Res 2013;471:3803-13. [Crossref] [PubMed]
- Sandiford N, Muirhead-Allwood SK, Skinner JA. Return to sporting activity after Birmingham hip resurfacing arthroplasty: Mid-term results. Indian J Orthop 2015;49:595-601. [Crossref] [PubMed]
- Haddad FS, Konan S, Tahmassebi J. A prospective comparative study of cementless total hip arthroplasty and hip resurfacing in patients under the age of 55 years A Ten-Year Follow-Up. Bone Joint J 2015;97-B:617-22. [Crossref] [PubMed]
- Girard J, Miletic B, Deny A, et al. Can patients return to high-impact physical activities after hip resurfacing? A prospective study. Int Orthop 2013;37:1019-24. [Crossref] [PubMed]
- Plate JF, Issa K, Wright C, et al. Patient activity after total hip arthroplasty: a comparison of three different bearing surfaces. J Long Term Eff Med Implants 2013;23:315-21. [Crossref] [PubMed]
- Aqil A, Drabu R, Bergmann JH, et al. The gait of patients with one resurfacing and one replacement hip: a single blinded controlled study. Int Orthop 2013;37:795-801. [Crossref] [PubMed]
- England, Wales, Northern Ireland and Isle of Man National Joint Registry 16th Annual Report, 2019.
- Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2018.
Cite this article as: de Villiers D, Richards L, Tuke M, Collins S. Ceramic resurfacing: the future and challenges. Ann Joint 2020;5:12.