Charcot foot reconstruction—how do hardware failure and non-union affect the clinical outcomes?
Introduction
Charcot neuroarthropathy (CN) is a degenerative condition, associated with peripheral neuropathy that most commonly affects the bones and joints in the foot. This condition was first described by Jean-Martin Charcot (1), who linked it to syphilis. It was later, in 1936, that W Jordan established the association between diabetes and CN (2).
The World Health Organization estimated that the number of people with diabetes has risen from 108 million (4.7% of the adult population) in 1980 to 422 million (8.5%) in 2014 (3). It is estimated that up to 50% of the diabetic population will develop peripheral neuropathy (4). One of the most devastating complications of diabetes is CN and 0.2% of the diabetic population is estimated to be suffering from this (5).
Foot ulcers in the presence of peripheral neuropathy carry a high rate of complications (6). Walsh et. al. reported that patients with diabetic foot ulcers had a 5-year death rate of 42.2% (7), whereas van Baal identified in a cohort study that patients with acute CN had a decreased life expectancy of 14 years (8). CN and its complications are also known to have a negative impact on the mobility and quality of life of the individual (9-11).
Acute CN of foot is considered as a clinical emergency and the standard initial treatment is immobilization and offloading in a total contact cast until the inflammatory process is resolved, followed by gradual return to normal weight bearing in a brace or custom-made shoes. However, if offloading fails or the treatment is delayed, the combination of mechanical instability and/or foot deformity can lead to ulceration and osteomyelitis. This may result in a series of events that could lead to a major amputation. The lower extremity amputation rate in patients with CN has been reported to range from 3% to 9% (12).
Major amputation in a diabetic patient is known to carry a high mortality rate. In a study from New Zealand, 11% of those who underwent a major amputation died within 30 days of their procedure and 18% within 90 days (13). A systematic review from 2016 concludes that 5-year mortality after below knee amputation ranges from 40% to 82% and after above knee amputation from 40% to 90% when the amputation is secondary to diabetes and peripheral vascular disease (14).
There has recently been a greater emphasis on limb salvage by surgically correcting the most severely deformed Charcot feet, where conservative treatment has failed (15-18). The goals of this correction are to achieve a stable, plantigrade and functional foot that is resistant to ulceration. This is achieved by correction of the foot deformity/instability and stabilization using internal or external fixation methods so that the correction becomes permanent through bone fusion. In our unit, internal fixation is our preferred method. Over the last 10 years, we have seen a higher incidence of breakage of internal fixation devices, with varied outcomes (16-18). We noticed certain patterns in hardware failures and also observed that not all hardware failures resulted in foot or ankle instability or poor outcomes requiring removal of hardware or revision fixation.
A number of studies have reported implant failures as part of their clinical outcomes (19-22). However, to our knowledge, no investigation has been done on the mechanisms behind the metal work failures following CN reconstruction and the subsequent clinical outcomes. We have performed this study with the aim of determining the demographics of hardware failure in Charcot foot reconstructions using internal fixation methods, along with the risk factors, the clinical and radiographic outcomes in this group of patients.
Methods
All patients who had undergone Charcot foot reconstruction with internal fixation in our unit between October 2007 and December 2017 with a minimum follow-up of 12 months were included in this study. All surgical procedures were either performed by the senior author or undertaken under his direct supervision.
All Charcot reconstruction cases were prospectively logged in our unit’s database which provided us with the platform to work on relevant data collection and perform analysis. Ethical approval was not required for this retrospective analysis of prospectively collected data.
A total of 82 Charcot foot reconstructions were performed on 80 patients during this period. Two patients died within 12 months following the procedure due to unrelated conditions and therefore were removed from the study. Two patients had undergone staged bilateral procedures. In these cases, only the second foot was included in the study, as the post-operative mobility goal was possible only following the second foot reconstruction. After the removal of the two deceased patients and the 2 feet in the bilateral group, our study encompassed 78 feet.
We collected the demographic characteristics and analysed statistically what the risk factors were associated with the hardware failure. We also compared the clinical and radiological outcomes between the hardware failure to the non-hardware failure group.
Surgical techniques
The patients who presented with an actively infected ulcer underwent two-stage reconstructions. The first stage consisted of aggressive surgical debridement of the ulcer and infected bone and removal of bone prominences, followed by osteotomy at the deformity site to provisionally restore the normal foot shape. Where appropriate, local antibiotic eluding calcium sulphate preparations were used to mount local delivery of high concentration antibiotic. Temporary stabilization of the osteotomized bones was achieved with judicious use of 3.2-mm threaded guide wires. Targeted intravenous antibiotics based on microbiological cultures from deep tissues and bone specimens were continued until there was clinical and serological evidence of infection eradication. Negative pressure wound therapy (NPWT) was used to manage the soft tissue defects. A period of six to ten weeks of treatment usually results in establishing sterile bone bed to proceed to the second-stage procedure.
The surgical principles used during the second stage of two-stage CN reconstructions and one-stage procedures were similar and often included percutaneous Achilles tendon lengthening to correct the equinus deformity, using Hoke triple hemisection technique (23). Our principle of internal fixation was “a durable long-segment rigid internal fixation with optimal bone opposition”. All joints intended for bone fusion were thoroughly prepared. The hindfoot deformity correction was achieved by performing wedge osteotomy at the center of rotation of angulation (CORA), commonly in the ankle or subtalar joint or both. A straight hindfoot intramedullary nail was used to provide compression across joint surfaces and secure fixation. We used Trigen (Smith & Nephew) and OxBridgeTM (Orthosolutions) hindfoot nails in this series and the choice of these was based on our standard practice at that time of surgery.
Where combined hindfoot and midfoot correction was required, the hindfoot correction proceeded the midfoot. The midfoot deformity was corrected by performing closing wedge osteotomy of the medial column (in most cases) or both columns. Whenever possible, we used a compression beam in the column, spanning from the metatarsal to talus or calcaneus, in order to restore the alignment, and an additional locking plate was used, in order to enhance the rotational stability of the fixation construct. The choice of the locking plates used was based on our standard practice the availability of these devices at the time of surgery. We used locking plates with varying thickness—1.6 mm (Marquardt utility PEDUS-R plate), 2 mm (Marquardt standard plate), 2.5 mm (Wright ORTHOLOCTM plate) and 4.6 mm (Synthes large fragment LCP® plate).
Primary wound closure was always aimed for except in circumstances where tension-free closure was not possible.
Post-operative care
The post-operative care was delivered by our multidisciplinary team comprising of orthopaedic surgeons, endocrinologists, vascular surgeons, podiatrists, diabetic foot practitioners, orthotists, physiotherapists and occupational therapists. The patients were kept non-weight bearing in a total contact cast for a minimum of 3 months postoperatively. Weight bearing was commenced after clinical and radiological evidence of satisfactory bony union, consisting of a minimum of 50% of bone surface area across the fusion sites. Weight bearing in a bi-valved total contact cast was continued until appropriate custom-footwear was ready.
Outcome measures
The clinical outcomes included post-operative complications, weight bearing status and the time taken for weight bearing. The radiological outcomes included bone fusion and metal work breakage. Any subsequent surgical procedure to address post-operative complications and evolving problems were also recorded. We defined major hardware failure as breakage of hardware component such as plate, nail, beam or bolt, in the post-operative radiographs; and radiological union when trabecular bone forms across the intended fusion site on plain X-rays or CT scan.
All patients had regular postoperative radiographs taken during the follow-up period. It was challenging to systematically calculate the timing of hardware failure. All patients were neuropathic and most of them did not experience any symptoms due to metal work breakage. We recorded the date of the X-ray taken when the breakage was first discovered. We then calculated the number of months from the date of surgery till this date. Therefore, for this study, the timing of breakage meant the time when the breakage was first discovered.
Statistical analysis
Baseline demographic characteristics, biochemical results, and outcomes were analyzed using Statistical Package for the Social Sciences (SPSS). We performed Kruskal-Wallis tests to compare the two patient groups (with or without hardware failure) on quantitative measures (creatinine levels and age of the patients) and Chi-square tests for the qualitative parameters (gender, obesity, preoperative ulceration, single- or two-stage procedure, perceived unsalvageable state of limb, i.e., prior recommendation for major amputation, level of reconstruction and peripheral vascular disease). A significant difference between the two patient groups on each measure would be reflected in a P value above 0.05. Additionally, we calculated univariable logistic regression for all measures.
Results
Of the 78 patients (45 male), 28 underwent hindfoot, 25 midfoot and 25 both hindfoot and midfoot correction. All patients presented with associated peripheral neuropathy—73 (94%) due to diabetes, and 5 (6%) from other neurological conditions. The mean age was 56.5 years (SD, ±11.59), ranging from 27 to 80. Forty-five (58%) of the patients had a body mass index (BMI) higher than 30. Twenty-one (27%) patients had renal chronic renal disease, defined as creatinine levels higher than 120 µmol/L. The mean duration of follow-up in our cohort is 31.8 months (12–91 months).
All 78 patients presented with chronic severe deformity/instability in midfoot, hindfoot or both. Forty (51%) patients presented with chronic non-healing ulcers, of which 18 (23%) were actively infected requiring a two-stage procedure. Thirty-seven (47%) had prior recommendation for major amputation as treatment by the clinicians in the referring center. Ten (13%) patients had received endovascular procedures to optimize limb vascularization prior to undergoing Charcot foot reconstruction. Limb salvage was achieved in all patients.
Incidence of hardware failure
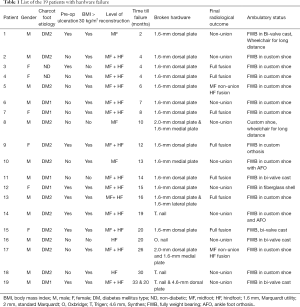
In this cohort, we observed that 19 patients (24%) had major hardware failure. These patients are listed in Table 1. Nail breakage occurred in 4 and plate(s) failure in 15 patients. Table 2 lists the demographics of broken hardware. One of the patients experienced both plate and nail breakage and, similarly, one had both midfoot bolt and plate failure.
Full table

Full table
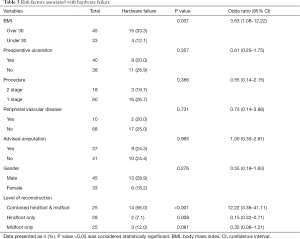
The extent of fixation and patients’ BMI were found to play a significant role on the incidence of hardware failure. We found that 14/25 (56%) of patients who underwent combination hindfoot and midfoot surgery had hardware failure, compared to those with solely hindfoot 2/28 (7%) or midfoot 3/25 (12%) (P<0.001). This suggests that combined hindfoot and midfoot group had 12 times higher risk of metal breakage compared to single segment procedures (95% CI: 3.36–41.11). Similarly, we observed that patients with a BMI of over 30 were 3.5 times more likely to have a hardware failure (95% CI: 1.08–12.22, P=0.038).
Other variables such as age, gender, history of peripheral vascular disease, presence of ulcer and the need for single or staged reconstruction procedure did not have any influence on the risk of hardware failure. We found no higher risk of metal work failure in those feet previously deemed unsalvageable at the referring center (Table 3).
Full table
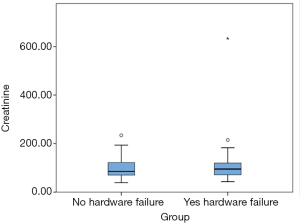
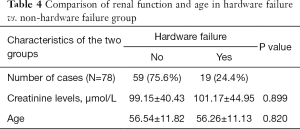
We tested our hypothesis on renal impairment contributing to non-union and/or hardware failure. The creatinine data was entered in a box plot (Figure 1) and one outlier with extremely high level (630 µmol/L) was excluded from the analysis. We could conclude that there was no significant difference in the creatinine level of the reconstructed patients with or without hardware failure (Table 4).
Full table
Radiological outcomes
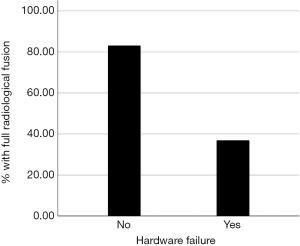
We observed that, while 49 out of 59 patients (83%) with intact hardware achieved full union at the osteotomy site, only 7 out to 19 (37%) with broken hardware achieved full radiological union (P<0.001, Figure 2). Patients with hardware failure are 88% less likely to unite radiologically, compared to intact hardware group (95% CI: 0.04–0.37).
Clinical outcomes
All patients in the hardware failure group were able to ambulate. There were four patients in the intact hardware group who were wheelchair bound. The reasons reported were bilateral Charcot foot, calcaneal stress fracture, non-healing ulcer and one other non-specific comorbidity respectively.
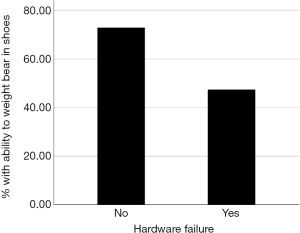
In the hardware failure group, only 9 (47%) were able to weight bear in shoes, a statistically significant difference from the 43 (73%) of the non-hardware failure patients (P=0.040, Figure 3). Those with hardware failure were 66% less likely to be able to weight bear in custom made shoes compared to the non-hardware failure group (95% CI: 0.12–0.98).
Need of cast/orthosis
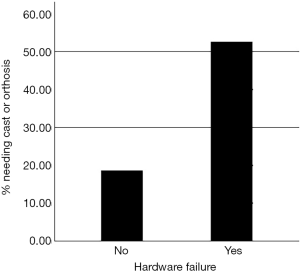
Ten (53%) of the patients from the hardware failure group needed a cast or orthosis to ambulate, compared to 11 (19%) of the non-hardware failure group (P=0.004). This indicated a significant difference in the need for bivalve cast or orthosis in the hardware failure group compared to the non-failure group. Those with hardware failure were almost 4 times more likely to need a bivalve cast or orthosis to ambulate (95% CI: 1.29–11.96) (Figure 4).
Time from surgery till breakage
The time to hardware failure was found to be very variable in our patient group. The timings of the failure have been categorized into the following groups and included in Table 5: breakage before 3 months, between 3 and 6 months, between 6 and 12 months and after 12 months postoperatively (Figures 5,6). All nail breakage happened after 12 months. Table 1 lists the number of months until failure for each patient.

Full table
The patients who had clinically stable feet and were able to ambulate in shoes had a lower mean time for hardware failure compared to those that we considered less stable (patients that needed a cast or an orthosis). The average breakage time for the shoe-group was 9.44 months (SD =7.30), while it was 16.73 months (SD =6.94) for the cast/orthosis-group. This difference was statistically significant (P=0.036).
Patterns of plate breakage
Fourteen out of the 18 broken plates were positioned on the dorsomedial aspect of the foot. Six of these broke at the ankle level and 8 at the talonavicular level. Nine out of the 18 plates that broke were used in bridging mode and 9 in neutralization mode.
Further surgery
Eight (42%) out of the hardware failure cases required further surgery during the follow-up period, compared to 19 (32%) of the non-hardware failure cases and this difference was not statistically significant (P=0.089). All had ulcer debridement in addition to hardware revision [2], screw removal [2] and exostectomy [4].
Discussion
The high incidence of nonunion of arthrodesis after fixation in patients with CN has previously been well documented (15,24) with reported rates of nonunion up to 34%. Pre-operative ulceration, vitamin D deficiency, obesity, and impaired immune response are known factors that challenge the outcome (5). It is known that the patients with neuropathy have difficulty in complying with long periods of non-weight bearing post-operatively. Hence this group of patients should receive a robust and durable fixation construct, compared to non-neuropathic non-diabetic patients.
Several principles and guidelines have been developed, including the concept of “Super construct” (25). A super construct is defined by four principles; one of them being that fusion is extended beyond the zone of injury to include joints that are not affected to improve fixation. In our analysis, we found a significantly higher rate of hardware failure among combined midfoot-hindfoot reconstructions, compared to isolated hindfoot or midfoot corrections. It is the senior author’s opinion that when multiple joints in the foot and ankle are prepared and fixed to achieve full bone fusion; as these bones possess variable bone healing rates and responses, this may lead to different timescales of bone fusion and consolidation across these joints. This may contribute to abnormal load concentrations and higher mechanical forces operating beyond the tolerance rates of hardware used in these patients resulting in breakage.
Radiological evidence of osseous fusion is considered the ideal favorable outcome in Charcot foot reconstruction. However, Wiewiorski and colleagues stated that joint stability and complete osseous fusion is not always the desired outcome for Charcot midfoot reconstruction (26). The osseous fusion at a specific joint might later be complicated by neuropathic changes of an adjacent joint due to stress concentration. However, it is still considered as strongly desirable to achieve full bone fusion during Charcot reconstruction as unstable non-union can lead to recurrence of deformity or instability. In order to achieve full bone fusion, it is recommended that all joints intended for fusion should be thoroughly prepared when correcting the medial or lateral column (27). We do not think inadequate preparation was responsible for our non-union rate as it is our default technique to thoroughly prepare all joint included in the fixation.
The position of the plates used in midfoot medial column fusion has also been subject to discussion in the literature. Medial column plating has been advocated by several investigators to restore alignment, enhancing construct stability and promoting earlier post-operative functional recovery in midfoot reconstruction (28). LC Schon recognized that application of plates in a plantar location offered mechanical advantages, despite technical difficulties in applying the device in this location (29). The senior author uses plantar plate if the Charcot changes are isolated to Lisfranc articulation and the fusion does not need to extend to the Talonavicular joint. This situation is less common in severe Charcot foot deformities and the plantar plate fixation was possible only in two patients in this series. Rest of them had full length medial column plate fixation placed on the medial or dorsomedial surfaces depending on the deformity pattern and soft tissue cover available.
The senior author had a preference to use thinner medial column locking plates as thick plates, in his experience, carried a higher risk of wound complications and difficulty with wound closure. However, in this series, we found a higher incidence of breakage of thinner plates. We believe that dedicated locking plates of adequate thickness, designed specifically for Charcot foot reconstructions, will potentially reduce the rate of hardware failure and wound complications, and improve fusion rates.
Our experience has shown that patients with a BMI of over 30 carried a higher risk of hardware failure. This group of patients with significant neuropathy often didn’t realize that they were load bearing on the operated leg during the non-weight bearing phase of treatment. We feel that this mechanism had a significant role in the higher rate of metal work breakage among high BMI patients. Moreover, one patient with high BMI had early breakage of metal work in 2 months postoperatively and it is highly likely that this was due to early load bearing that the patient was not fully aware of due to neuropathy.
The Eurodiale study team concluded that infection and peripheral artery disease have a major impact on healing rates in patients with diabetic ulcers (30). In our series, we found no evidence of increased rate of hardware failure in our two-stage reconstructions and therefore we feel that we had achieved infection clearance during the first stage of the procedure and there was no evidence of recurrence or persistence of infection following the usage of internal fixation during the second stage. Likewise, preoperative ulceration did not have higher rate of hardware failure. Our pathway for Charcot reconstruction included prior revascularization in the presence of significant vascular compromise that is performed about 4–8 weeks before the deformity correction. All patients with significant vascular compromise in this series had vascular optimization prior to reconstruction. We do not think critical ischemia played a significant role in the metal work failure in our series.
Diabetic nephropathy is known to affect vitamin D metabolism which can lead to abnormalities in bone turn-over (31) and higher risk of non-union. We tested the hypothesis of chronic renal disease possibly contributing to higher incidence of non-union and hardware failure in our patients. However, we did not find any such risk. Similarly, higher age did not seem to be associated with higher rate of hardware failure.
Our assessment of clinical outcomes included the number of further surgical procedures during the follow-up period. This higher rate may represent the progressive nature of the disease in this high-risk group of patients. The incidence of further procedures was not found to be statistically significant between the hardware failure (42%) compared to the non-failure (32%) groups (P=0.430).
The limitations of this study include its retrospective nature, and the fact that the use of hardware was not randomized. Another drawback is that the definition of a good clinical outcome we used was not based on patient reported outcome scores. Instead we had chosen to define this as the ability to ambulate in a shoe or brace.
Conclusions
This study demonstrated the outcomes following Charcot foot reconstructions using internal fixation in a large group of patients with a minimum follow up of 12 months. Our results have shown that one- or two-stage reconstruction using internal fixation in patients with severe Charcot foot and/or ankle deformity can promote healing of chronic ulcers and achieve independent mobilization in spite of limb threatening predispositions.
The study has shown that the hardware failure rate among this group of patients is high. Factors such as BMI >30 kg/m2 and combined hindfoot and midfoot reconstructions seem to be the predictors for hardware failure. We have also noted a marked tendency for the thin plates to break. However, those patients with hardware failure, albeit still able to ambulate, required more supportive footwear in comparison to those with intact hardware. There was no significant difference in revision surgery rates between these two groups.
Despite higher incidence of hardware failure, the outcomes following Charcot midfoot and hindfoot reconstructions were still satisfactory. Considering the complexity of the deformities and the associated high medical comorbidities in this patient group, we believe limb salvage itself was a challenging goal that we have successfully reached in all our patients. The outcomes could be improved further, if specific dedicated durable hardware devices are developed for such procedures.
Acknowledgments
Åshild Kummen for writing assistance and statistics work. Prashanth Vas for advice on statistics.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.01.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional ethic committee approval was not required as this study was part of our ongoing audit work. Informed consent was obtained from all patients for the prospective data collection used in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Charcot JM. Sur quelques arthropathies qai paraisant dependre d’une lesion du cervau on de la moelle epinere. Arch Physiol 1868;1:161-78.
- Jordan W. Neuritic manifestations in diabetes mellitus. Arch Intern Med 1936;57:307-36. [Crossref]
- World Health Organization. Fact sheet on diabetes. [Published October 30th 2018, accessed October 1st 2019]. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes
- World Health Organization. Fact sheet on diabetes [Published February 2018, accessed October 2019] Available online: https://www.who.int/nmh/publications/fact_sheet_diabetes_en.pdf
- Rogers LC, Frykberg RG, Armstrong DG, et al. The Charcot foot in diabetes. Diabetes Care 2011;34:2123-9. [Crossref] [PubMed]
- Wukich DK, Sadoskas D, Vaudreuil NJ, et al. Comparison of diabetic Charcot patients with and without foot wounds. Foot Ankle Int 2017;38:140-8. [Crossref] [PubMed]
- Walsh JW, Hoffstad OJ, Sullivan MO, et al. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33:1493-8. [Crossref] [PubMed]
- van Baal J, Hubbard R, Game F, et al. Mortality associated with acute Charcot foot and neuropathic foot ulceration. Diabetes Care 2010;33:1086-9. [Crossref] [PubMed]
- Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications 2009;23:409-26. [Crossref] [PubMed]
- Dhawan V, Spratt KF, Pinzur MS, et al. Reliability of AOFAS Diabetic Foot Questionnaire in Charcot arthropathy: stability, internal consistency and measurable difference. Foot Ankle Int 2005;26:717-31. [Crossref] [PubMed]
- Pinzur MS. Benchmark analysis of diabetic patients with neuropathic (Charcot) foot deformity. Foot Ankle Int 1999;20:564-7. [Crossref] [PubMed]
- Saltzman CL, Hagy ML, Zimmerman B, et al. How effective is intensive nonoperative initial treatment of patients with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res 2005;185-90. [Crossref] [PubMed]
- Gurney JK, Stanley J, Rumball-Smith J, et al. Postoperative Death After Lower-Limb Amputation in a National Prevalent Cohort of Patients With Diabetes. Diabetes Care 2018;41:1204-11. [Crossref] [PubMed]
- Thorud JC, Plemmons B, Buckley CJ, et al. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg 2016;55:591-9. [Crossref] [PubMed]
- Papa J, Myerson M, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg Am 1993;75:1056-66. [Crossref] [PubMed]
- Siebachmeyer M, Boddu K, Bilal A, et al. Outcome of one-stage correction of deformities of the ankle and hindfoot and fusion in Charcot neuroarthropathy using a retrograde intramedullary hindfoot arthrodesis nail. Bone Joint J 2015;97-B:76-82. [Crossref] [PubMed]
- Butt DA, Hester T, Bilal A, et al. The medial column Synthes Midfoot Fusion Bolt is associated with unacceptable rates of failure in corrective fusion for Charcot deformity: Results from a consecutive case series. Bone Joint J 2015;97-B:809-13. [Crossref] [PubMed]
- Vasukutty N, Jawalkar H, Anugraha A, et al. Correction of ankle and hind foot deformity in Charcot neuroarthropathy using a retrograde hind foot nail-The Kings' Experience. Foot Ankle Surg 2018;24:406-10. [Crossref] [PubMed]
- Ford SE, Cohen BE, Davis WH, et al. Clinical Outcomes and Complications of Midfoot Charcot Reconstruction With Intramedullary Beaming. Foot Ankle Int 2019;40:18-23. [Crossref] [PubMed]
- Ramadani F, Härägus H, Radu P, et al. Complex reconstruction with internal locking plate fixation for Charcot arthropathy. Orthopade 2015;44:33-8. [Crossref] [PubMed]
- Harkin EA, Schneider AM, Murphy M, et al. Deformity and Clinical Outcomes Following Operative Correction of Charcot Ankle. Foot Ankle Int 2019;40:145-51. [Crossref] [PubMed]
- Richter M, Mittlmeier T, Rammelt S, et al. Intramedullary fixation in severe Charcot osteo-neuroarthropathy with foot deformity results in adequate correction without loss of correction - Results from a multi-centre study. Foot Ankle Surg 2015;21:269-76. [Crossref] [PubMed]
- Hoke M. An operation for the correction of extremely relaxed flat feet. J Bone Joint Surg 1931;13:773-83.
- Frey C, Halikus NM, Vu-Rose T, et al. A review of ankle arthrodesis. Foot Ankle Int 1994;15:581-4. [Crossref] [PubMed]
- Sammarco VJ. Superconstructs in the Treatment of Charcot Foot Deformity: Plantar Plating, Locked Plating, and Axial Screw Fixation. Foot Ankle Clin 2009;14:393-407. [Crossref] [PubMed]
- Wiewiorski M, Yasui T, Miska M, et al. Solid bolt fixation of the medial column in Charcot midfoot arthropathy. J Foot Ankle Surg 2013;52:88-94. [Crossref] [PubMed]
- Siddiqui NA, LaPorta G. Midfoot Charcot Reconstruction. Clin Podiatr Med Surg 2018;35:509-20. [Crossref] [PubMed]
- Capobianco CM, Stapleton JJ, Zgonis T. The role of an extended medial column arthrodesis for Charcot midfoot neuroarthropathy. Diabet Foot Ankle 2010; [Crossref] [PubMed]
- Schon LC, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop Relat Res 1998;116-31. [Crossref] [PubMed]
- Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008;51:747-55. [Crossref] [PubMed]
- Miller PD. Chronic kidney disease and the skeleton. Bone Res 2014;2:14044. [Crossref] [PubMed]
Cite this article as: Kummen I, Phyo N, Kavarthapu V. Charcot foot reconstruction—how do hardware failure and non-union affect the clinical outcomes? Ann Joint 2020;5:25.