
The function and behavior of chondrogenic progenitor cells in osteoarthritis
Introduction
Osteoarthritis (OA) is the most common joint degenerative disease affecting about 240 million people worldwide (1). Clinically, significant pain and physical disability are severe outcomes and the subsequent cost will result in large socioeconomic burden (2). OA is primarily characterized by cartilage degradation with subsequent synovitis, subchondral bone sclerosis and osteophyte formation (3,4).
Unfortunately, it is difficult to restore the degraded cartilage due to the natural hypocellular, matrix-rich and avascular features. Cartilage repair is a challenge has been troubling researchers for decades. In 1994, Brittberg et al. firstly transplanted autologous chondrocytes onto cartilage defects (5). Recently, stem cells have attracted increasing attention for cartilage repair owing to their good proliferative and chondrogenic capacity (6). Whereas, some drawbacks are unavoidable in these cell-based therapies, for example, unsustainable chondrogenic phenotype of chondrocytes, fibrocartilage formation and poor integration of repaired tissues with native cartilage (7,8). The main reason is that none current seeding cells have yet addressed the challenge to maintain abundant number and exhibit good stemness at the same time. Therefore, an ideal seeding cell with sustained proliferative and superior chondrogenic capacity is needed for hyaline cartilage regeneration.
Chondrocytes have been considered the sole cell type in articular cartilage for a long time. In recent years, numerous investigations have shown that chondrogenic progenitor cells (CPCs) reside in cartilage showed stem cell properties (9,10). Similar to other tissues-derived stem cells, CPCs exhibit clonogenic capacity and multi-linage differentiation potential especially with high chondrogenic capacity (9,11). Currently, CPCs have been successfully isolated from human, bovine, equine and porcine articular cartilage (9,10,12-15). Growing evidences suggest that CPCs reside in cartilage are recommendable candidates for cartilage repair. However, the function and regulatory factors of cell behavior are not understood entirely. In this review, we compared the proliferative and chondrogenic abilities of CPCs with other tissues-derived stem cells. Then, we made a summary of the regulatory factors of CPCs’ behavior, mainly migration and chondrogenesis, to provide new insight for further investigations and CPCs-based cartilage repair therapies.
Characteristics to identify CPCs
Like the identification of other tissues-derived stem cells, self-renewal capacity, multilineage differentiation potential and surface markers are major characteristics to identify CPCs.
Self-renewal capacity is reflected by colony forming efficiency, in which cells are cultured in low density initially and then expand into large number cell colony, defined as a more than 32 cells cluster represents at least 5 population doublings (PDs). Koelling et al. observed 2–8% colony forming cells from late stage osteoarthritic cartilage (9). Further, after 10 days’ incubation, the colony size of migrated cells from the surface of artificial injured cartilage is greater than that from chondrocytes (12).
Multilineage differentiative potential is the most important characteristic to identify stem cells, in which osteogenic, chondrogenic and adipogenic differentiation are included. Under osteogenic differentiation culture, CPCs are positive for alkaline phosphatase and Alizarin red staining indicating CPCs’ osteogenic differentiation and calcification (9). In pellet culture system, CPCs tend to form pellets and these pellets are positive for Alcian blue and Safranin O-fast green (12) staining demonstrating cartilage proteoglycans production, as well as type II collagen (Col II) immunohistochemistry staining (14). After adipogenic differentiation, Oil-red O stained lipid droplet is observed in CPCs (16). This multi-differentiation characteristic proves CPCs are cells with stemness.
Cell surface molecules provide molecular typing of cells. Surface markers to definite CPCs mainly based on those have been found in other tissues-derived stem cells, such as bone marrow-derived mesenchymal stem cells (BMSCs). Currently, mainly identified positive and negative markers of CPCs are listed in Table 1. Whereas, there is none specific surface marker to trace CPCs and illustrate their biological behavior during the progression of OA indicating that further investigations should focus on this issue.
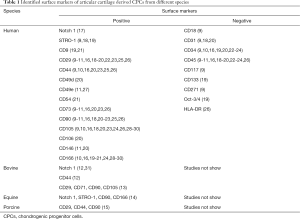
Full table
CPCs are superior seeding cells
Proliferative capacity
Due to low proportion of stem cells in tissues, expanding them to sufficient quantity in vitro is vital for stem cell-based therapies. Although with high proliferative capacity, the division of stem cells is limited because of cell senescence. Therefore, for better cartilage repair in cell-based therapy, seeding cells with better proliferative capacity is of great importance.
In monolayer culture, migratory CPCs from late OA cartilage undergo 28–30 PD in 100 days (9). In fetal cartilage-derived CPCs, the PD time maintains at approximately 2.2±0.6 days up to passage 15, which is significantly longer than that in chondrocytes and BMSCs (32). Surprisingly, CPCs reach 1.3×1010 at 7th passage and 6.9×1012 at 11th passage, which is sufficient according to the requirement of hundreds of million cells in cell therapy protocols (32,33). BMSCs need to be expanded about 10 weeks to require hundreds of million cells (33) and CPCs reach thousands of million after 40 days (32). From this point of view, the proliferation capacity of CPCs is better than that of BMSCs.
Synovial fluid-derived MSCs (SF-MSCs) and synovium-derived MSCs (SD-MSCs) are also reported candidates for cartilage repair. However, SF-MSCs reach only 5.32×105 after 14 days’ expansion and SD-MSCs reach approximately 108 after 39 days’ expansion indicating inferior proliferative capacity of these two candidates than CPCs (32,34). Considering the difference of experimental conditions and cell source, more studies to compare these stem cells’ proliferative capacity directly are essential.
Above these observations, taking consideration of expanding times and the maximal passages, CPCs’ proliferation capacity is comparable with or better than other tissues-derived stem cells in vitro. However, it remains to be seen how OA condition will impact the proliferative potential of these seeding cells in vivo.
Chondrogenic capacity
Stable and persistent chondrogenic property of seeding cells is the key of cartilage repair. Since several seeding cells have been identified with chondrogenic property, as aforementioned, BMSCs, AD-MSCs and SD-MSCs, which candidate is the best for cartilage repair is still controversial.
In pellet culture system, both CPCs and BMSCs pellets are positive for Col II and aggrecan; however, the later also positive for collagen X, matrilin-1 and Runx2 indicating hypertrophic and osteogenic tendency, which is not or rarely observed in CPCs pellets (14). Under the same condition, CPCs exhibit superior chondrogenic differentiation and inferior osteogenic and adipogenic differentiation capacity than BMSCs (10,15,26), illustrating superior chondrogenic capacity of CPCs than that of BMSCs. After comparing CPCs with BMSCs, AD-MSCs and nasal septum-derived progenitors (NSPs), Shafiee et al. observed similar glycosaminoglycans secretion of CPCs and NSPs; besides, NSPs exhibited highest aggrecan and Sox9 expression levels followed by CPCs, AD-MSCs and BMSCs (35). Whereas, the Col II expression level in CPCs was less than that in NSPs and AD-MSCs (35). Therefore, they preferred NSPs than CPCs for cartilage repair. Considering the acquired convenience clinically, nasal septum cartilage is more difficult than knee articular cartilage illustrating less clinical application probability of NSPs.
SF-MSCs and SD-MSCs have been found with satisfactory chondrogenesis. However, because of large size of human knee joint cavity, it seems unlikely that SD-MSCs have the ability to reach the injury site to promote cartilage repair (36). In chondrogenic culture, SD-MSCs exhibit similar chondrogenic differentiation to BMSCs (37), indicating inferior chondrogenic potential to CPCs because BMSCs are not better than CPCs, as aforementioned. Collectively, whether the chondrogenic capacity of CPCs is better than SF-MSCs and SD-MSCs needs further and direct investigations.
Compared with other stem cells, settlement in cartilage is the absolute advantage of CPCs to promote cartilage repair. Future studies should pay more attention to the comparison of chondrogenic capacity of these seeding cells in natural OA joint environment or after injection to joint cavity. Moreover, enhancing chondrogenic capacity of CPCs prior to cartilage regeneration therapy by gene editing or small molecules worth consideration.
CPCs based therapy for cartilage repair in vivo
Given the superior proliferative and chondrogenic capacity of CPCs in vitro, several groups carried out preclinical or clinical studies to determine whether CPCs are effective seeding cells for cartilage defects repair in vivo (Table 2).
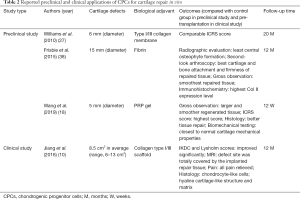
Full table
In 2010, Williams et al. implanted CPCs or chondrocytes, loaded on type I/III collagen membrane, onto the full-thickness osteochondral defects (FTOD) on the goat lateral femoral condyle (27). After 20 months, they observed comparable ICRS score between the CPCs and chondrocytes implantation groups (27). Later, Frisbie et al. implanted CPCs plus fibrin onto equine FTOD models and observed significantly better repaired tissue and less central osteophyte formation than control group after 12 months (38). Recently, platelet-rich plasma (PRP) attracted much attention, Wang et al. reported significantly better histological and biomechanical results in repaired tissues of PRP + CPCs group than PRP + BMSCs and PRP + chondrocytes groups after implantation onto the cartilage defects of New Zealand White rabbits (18). Further, Jiang et al. implanted CPCs onto cartilage defect sites of 15 patients and observed significantly better clinical scores and MRI evaluation; besides, the pain of all patients has been relieved (10). With the assistance of biological adjuvants, these preclinical and clinical studies achieved satisfactory outcomes demonstrating that CPCs are appropriate seeding cells for cartilage defects repair in vivo.
Regulatory factors of behavior
The etiology of OA is intricate, in which injury, inflammation, obesity and aging are included. In OA, many disease related factors, such as IL-1β and TNF-α (19), influence the biological behavior of CPCs. In this part, we summarized investigations that focus on the mechanism of regulation of CPCs’ behavior.
The regulation of migration
Diminished migratory capacity of resident stem cells will result in unsatisfactory endogenous tissue regeneration. As illustrated in Figure 1, many factors that participated in OA affect the migratory capacity of CPCs evidently.
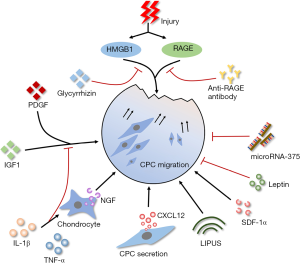
CPCs are responsive to the tissue injury related intracellular components. After cartilage explants impacted by blunt injury, high mobility group box chromosomal protein 1 (HMGB1) and receptor for advanced glycation end products (RAGE) strongly promote the migration of CPCs to the injury site (12). Besides, CPCs are showed significantly increased migration upon the stimulation of HMGB1 and cell lysates (12,13). Supernatant from cultured blunt trauma cartilage also promote site-directed migration of CPCs significantly (19). This indicates inherent ability of CPCs migrate to the injury site. Further, CPCs also migrate ex vivo for penetrating from surface layer into deep layer of late-stage OA cartilage tissues (9). Whereas, whether CPCs can migrate from deep layer into surface layer to promote the repair of surface cartilage lesions is still unknown.
Under inflammatory conditions, the migration of CPCs is affected. IL-1β and TNF-α inhibit the migration of CPCs under the stimulation of chemo-attractive factors (platelet derived growth factor/PDGF, insulin-like growth factor/IGF-1) or supernatant from cultured blunt trauma cartilage (19). However, Jiang et al. found that IL-1β upregulated the nerve growth factor (NGF) expression in chondrocytes and NGF promoted the migration of CPCs (16). Interestingly, CPCs are able to secret C-X-C chemokine ligand-12 (CXCL-12) to attract inflammatory cells or other progenitor cells (13). It can be speculated that the effect of inflammatory microenvironment on migratory capacity of CPCs is comprehensive. Studies to clarify the correlation between OA inflammation and CPCs’ migration will provide new therapeutic targets.
In addition, the migratory capacity of CPCs is influenced by a series of factors except injury and inflammation. Low-intensity pulsed ultrasound (LIPUS) has been shown with the promotion of CPCs’ migration via focal adhesion kinase activation (39). This may provide an effective physiotherapeutic to delay OA progression. Leptin, a fat metabolism related factor, mediates the association between adiposity and cartilage thinning (40). Zhao et al. found that leptin decreased the migratory ability and chondrogenic potential of CPCs and increased the osteogenic potential at the same time (11), indicating systemic metabolic factors influence the behavior of CPCs. MicroRNA-375 also has an inhibitory effect on migration through the negative regulation of cadherin-7 (41).
However, among these mentioned factors, which are the most specific and effective ones on CPCs’ migration in OA condition is still unknown. Besides, the functional role of migrated CPCs in the progression of OA is not elucidated clearly. Further studies should be undertaken for deeper understanding of the relationship between regulatory factors and the functional role of migrated CPCs.
The regulation of chondrogenesis
The chondrogenesis of CPCs is influenced by the cytokines that involved in OA (Figure 2). Diminishing the negative effect of adverse cytokines will provide better application prospects.
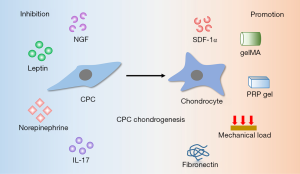
Like migratory capacity is influenced by inflammatory factors, chondrogenic potential is also affected. When CPCs are cultured in 3D pellets, NGF promotes their extracellular matrix (ECM) catabolism significantly and thus result in less matrix accumulation (16). IL-17 has been found significantly higher in OA synovia (42). Schminke et al. found that IL-17 promoted IL-6, MMP3 and Runx2 expression in rheumatoid arthritis cartilage-derived CPCs and anti-human IL-17 antibody blocked this effect significantly (23).
Vascular endothelial growth factor (VEGF), a crucial regulator of angiogenesis, contributes to the pathogenesis of OA. CPCs can stimulate the expression of VEGF themselves and in nearby cells via stromal cell-derived factor-1α (SDF-1α) signaling pathway (43). However, sustained release of SDF-1α promotes stem cells homing and chondrogenic differentiation and then promote cartilage repair (44). After loading recombinant human SDF-1α in fibrin/hyaluronic acid hydrogel, Yu et al. found improved recruitment of CPCs to cartilage defects and then resulted in satisfactory repaired tissue that similar to native cartilage (45). Therefore, it is essential to consider the dual function of SDF-1α, enhancing chemotaxis and chondrogenesis of CPCs and inducing expression of VEGF, at the same time for cartilage repair therapy. The combination of providing SDF-1α and inhibiting VEGF may bring us surprising results.
Systemic factors also influence the chondrogenic capacity. High dose of leptin diminishes the chondrogenic potential and increases osteogenic potential of CPCs, promoting cell senescence via the p53/p21 pathway activation and Sirt1 pathway inhibition at the same time (11). Elevated catecholamines are detected in synovial fluid of OA patients and norepinephrine inhibits chondrogenesis and accelerates hypertrophy of CPCs (46). In addition, mechanical load promotes CPCs to express chondrogenic markers (aggrecan and Col II) massively (47).
Although the effects of regulatory factors on chondrogenic capacity of CPCs are not clarified entirely, it can be expected that these investigations will provide a theoretical basis for CPCs-based cartilage repair therapy. Future studies should pay more attention to the regulatory effects of CPCs’ chondrogenesis in vivo and maintain the chondrogenic capacity by therapeutic interventions.
Biomaterials regulate CPCs’ behavior
Because of ineluctable limitations of current OA therapies, for example, the formation of fibrous tissue and poor integration of repaired tissue with native cartilage (8), cartilage repair engineering has attracted considerable attention recent years. Three dimensional biomaterials are favorably received with the support of adherent, proliferative and differentiated environment for seeding cells and compatible of various solutes (48). This will provide valuable methods for CPCs in cartilage repair engineering.
Gelatin methacryloyl (gelMA) hydrogel has gained increasing attention because it combines mechanical tunability and biofunctionality and can accelerate tissue repair process. Levato et al. embedded CPCs into gelMA and found that CPCs acted like chondrocytes in cartilage superficial zone with highly expressed PRG4, which was the key of joint lubrication (20). Besides, CPCs in gelMA expressed less Col X, a hypertrophic marker, than BMSCs illustrating better cartilage repair outcomes (20). In addition, as mentioned above, PRP gels as a carrier to transport CPCs achieved satisfactory cartilage repair in rabbit cartilage defect models (18).
Fibronectin, a type of ECM protein, is highly used in tissue repair engineering as a glue and has an outstanding capacity of transmitting information between cells and ECM. After stimulated by fibronectin, the progenitor cells isolated from subchondral cortico-spongious in late-stage OA specimen exhibit increased expression of Col II, SOX-9, aggrecan and decreased expression of collagen I (49). Tao et al. found that fibronectin promotes the migratory, proliferative and chondrogenic capacities of CPCs through integrin α5β1 signaling pathway and the rate of CD105-positive cells increased significantly after intra-articular injection of FN/Pluronic F-127 hydrogel in early OA model (24). Interestingly, CPCs are able to engulf fibronectin fragments and internalize cell debris like macrophages (50) suggesting that CPCs not only affected by microenvironment but have the ability to rebuild it.
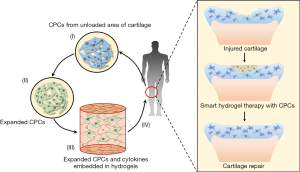
These illustrate the tremendous potential of biomaterials in cartilage repair therapy as carriers of CPCs to supply enough seeding cells. The future CPCs-based cartilage repair engineering for OA will meet the following process: (I) isolate CPCs from unloaded area of articular cartilage; (II) expand CPCs to increase quantity and enhance chondrogenic capacity in vitro; (III) embed CPCs into scaffolds with therapeutics, for instance, cytokines; (IV) transplant CPCs and therapeutics loaded scaffolds onto cartilage defects with less invasive manners (Figure 3).
Conclusions
CPCs are responsible for cartilage homeostasis. When compared with other tissues-derived stem cells, CPCs exhibit brilliant proliferative and chondrogenic abilities. However, among the multi-factors affecting the behavior of CPCs, which are the most specific and effective factors are still unknown. In order to fully utilize CPCs in cartilage repair therapy, future investigations should focus on the following aspects. First, maintaining the superior proliferative and chondrogenic capacity of CPCs throughout the CPCs-based cartilage repair therapy. Second, illustrating the positive and negative influences of multifactor on CPCs systemically. Avoiding or diminishing the negative effect of some adverse factors will play a synergistic role in CPCs based cartilage repair. Third, understanding the mechanisms of the effect of biomaterials on CPCs. At last, strategies to promote resident CPCs migrate to injury site and maintain cartilage homeostasis efficiently to realize endogenous cartilage repair will be the key of noninvasive or minimally invasive cartilage repair therapy.
Acknowledgments
Funding: This work was supported by National Key R&D Program of China (2018YFC1105904), Key Program of NSFC (81730067), Major Project of NSFC (81991514), Jiangsu Provincial Key Medical Center Foundation, Jiangsu Provincial Medical Outstanding Talent Foundation, Jiangsu Provincial Medical Youth Talent Foundation and Jiangsu Provincial Key Medical Talent Foundation.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.03.01). QJ serves as an Editor-in-Chief of Annals of Joint from March 2016 to February 2021. DS serves as an unpaid Executive Editor-in-Chief of Annals of Joint from March 2016 to February 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage 2018;26:319-25. [Crossref] [PubMed]
- Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet 2015;386:376-87. [PubMed]
- Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 2010;6:625-35. [Crossref] [PubMed]
- Mobasheri A, Rayman MP, Gualillo O, et al. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol 2017;13:302-11. [Crossref] [PubMed]
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N Engl J Med 1994;331:889-95. [Crossref] [PubMed]
- Pas HI, Winters M, Haisma HJ, et al. Stem cell injections in knee osteoarthritis: a systematic review of the literature. Br J Sports Med 2017;51:1125-33. [Crossref] [PubMed]
- Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 2007;59:339-59. [Crossref] [PubMed]
- Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 2000;21:431-40. [Crossref] [PubMed]
- Koelling S, Kruegel J, Irmer M, et al. Migratory Chondrogenic Progenitor Cells from Repair Tissue during the Later Stages of Human Osteoarthritis. Cell Stem Cell 2009;4:324-35. [Crossref] [PubMed]
- Jiang Y, Cai Y, Zhang W, et al. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl Med 2016;5:733-44. [Crossref] [PubMed]
- Zhao X, Dong Y, Zhang J, et al. Leptin changes differentiation fate and induces senescence in chondrogenic progenitor cells. Cell Death Dis 2016;7:e2188 [Crossref] [PubMed]
- Seol D, McCabe DJ, Choe H, et al. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum 2012;64:3626-37. [Crossref] [PubMed]
- Yu Y, Zheng H, Buckwalter JA, et al. Single cell sorting identifies progenitor cell population from full thickness bovine articular cartilage. Osteoarthritis Cartilage 2014;22:1318-26. [Crossref] [PubMed]
- McCarthy HE, Bara JJ, Brakspear K, et al. The comparison of equine articular cartilage progenitor cells and bone marrow-derived stromal cells as potential cell sources for cartilage repair in the horse. Vet J 2012;192:345-51. [Crossref] [PubMed]
- Xue K, Xia W, Zhang X, et al. Isolation and identification of stem cells in different subtype of cartilage tissue. Expert Opin Biol Ther 2015;15:623-32. [Crossref] [PubMed]
- Jiang Y, Hu C, Yu S, et al. Cartilage stem/progenitor cells are activated in osteoarthritis via interleukin-1β/nerve growth factor signaling. Arthritis Res Ther 2015;17:327. [Crossref] [PubMed]
- Ustunel I, Ozenci AM, Sahin Z, et al. The immunohistochemical localization of notch receptors and ligands in human articular cartilage, chondroprogenitor culture and ultrastructural characteristics of these progenitor cells. Acta Histochem 2008;110:397-407. [Crossref] [PubMed]
- Wang K, Li J, Li ZL, et al. Chondrogenic Progenitor Cells Exhibit Superiority Over Mesenchymal Stem Cells and Chondrocytes in Platelet-Rich Plasma Scaffold-Based Cartilage Regeneration. Am J Sports Med 2019;47:2200-15. [Crossref] [PubMed]
- Joos H, Wildner A, Hogrefe C, et al. Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage. Arthritis Res Ther 2013;15:R119. [Crossref] [PubMed]
- Levato R, Webb WR, Otto IA, et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater 2017;61:41-53. [Crossref] [PubMed]
- Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther 2004;6:R422-R432. [Crossref] [PubMed]
- Zhang X, Qi L, Chen Y, et al. The in vivo chondrogenesis of cartilage stem/progenitor cells from auricular cartilage and the perichondrium. Am J Transl Res 2019;11:2855-65. [PubMed]
- Schminke B, Trautmann S, Mai B, et al. Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage. Eur J Immunol 2016;46:440-5. [Crossref] [PubMed]
- Tao T, Li Y, Gui C, et al. Fibronectin Enhances Cartilage Repair by Activating Progenitor Cells Through Integrin α5β1 Receptor. Tissue Eng Part A 2018;24:1112-24. [Crossref] [PubMed]
- Xue K, Zhang X, Qi L, et al. Isolation, identification, and comparison of cartilage stem progenitor/cells from auricular cartilage and perichondrium. Am J Transl Res 2016;8:732-41. [PubMed]
- Oda T, Sakai T, Hiraiwa H, et al. Osteoarthritis-derived chondrocytes are a potential source of multipotent progenitor cells for cartilage tissue engineering. Biochem Biophys Res Commun 2016;479:469-75. [Crossref] [PubMed]
- Williams R, Khan IM, Richardson K, et al. Identification and Clonal Characterisation of a Progenitor Cell Sub-Population in Normal Human Articular Cartilage. PLoS One 2010;5:e13246 [Crossref] [PubMed]
- Pretzel D, Linss S, Rochler S, et al. Relative percentage and zonal distribution of mesenchymal progenitor cells in human osteoarthritic and normal cartilage. Arthritis Res Ther 2011;13:R64. [Crossref] [PubMed]
- Alsalameh S, Amin R, Gemba T, et al. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 2004;50:1522-32. [Crossref] [PubMed]
- Mazor M, Cesaro A, Ali M, et al. Progenitor Cells from Cartilage: Grade Specific Differences in Stem Cell Marker Expression. Int J Mol Sci 2017; [Crossref] [PubMed]
- Dowthwaite GP, Bishop JC, Redman SN, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci 2004;117:889-97. [Crossref] [PubMed]
- Choi WH, Kim HR, Lee SJ, et al. Fetal Cartilage-Derived Cells Have Stem Cell Properties and Are a Highly Potent Cell Source for Cartilage Regeneration. Cell Transplant 2016;25:449-61. [Crossref] [PubMed]
- Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol 2000;28:707-15. [Crossref] [PubMed]
- Lee WJ, Hah YS, Ock SA, et al. Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp Cell Res 2015;333:273-88. [Crossref] [PubMed]
- Shafiee A, Kabiri M, Langroudi L, et al. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A 2016;104:600-10. [Crossref] [PubMed]
- McGonagle D, Baboolal TG, Jones E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol 2017;13:719. [Crossref] [PubMed]
- Gale AL, Linardi RL, McClung G, et al. Comparison of the Chondrogenic Differentiation Potential of Equine Synovial Membrane-Derived and Bone Marrow-Derived Mesenchymal Stem Cells. Front Vet Sci 2019;6:178. [Crossref] [PubMed]
- Frisbie DD, McCarthy HE, Archer CW, et al. Evaluation of Articular Cartilage Progenitor Cells for the Repair of Articular Defects in an Equine Model. J Bone Joint Surg Am 2015;97:484-93. [Crossref] [PubMed]
- Jang KW, Ding L, Seol D, et al. Low-Intensity Pulsed Ultrasound Promotes Chondrogenic Progenitor Cell Migration via Focal Adhesion Kinase Pathway. Ultrasound Med Biol 2014;40:1177-86. [Crossref] [PubMed]
- Stannus OP, Cao YL, Antony B, et al. Cross-sectional and longitudinal associations between circulating leptin and knee cartilage thickness in older adults. Ann Rheum Dis 2015;74:82-8. [Crossref] [PubMed]
- Song J, Kim D, Chun CH, et al. MicroRNA-375, a new regulator of cadherin-7, suppresses the migration of chondrogenic progenitors. Cell Signal 2013;25:698-706. [Crossref] [PubMed]
- Liu Y, Peng H, Meng Z, et al. Correlation of IL-17 Level in Synovia and Severity of Knee Osteoarthritis. Med Sci Monit 2015;21:1732-6. [Crossref] [PubMed]
- Wang S, Zhou C, Zheng H, et al. Chondrogenic progenitor cells promote vascular endothelial growth factor expression through stromal-derived factor-1. Osteoarthritis Cartilage 2017;25:742-9. [Crossref] [PubMed]
- Chen Y, Wu T, Huang S, et al. Sustained Release SDF-1α/TGF-β1-Loaded Silk Fibroin-Porous Gelatin Scaffold Promotes Cartilage Repair. ACS Appl Mater Interfaces 2019;11:14608-18. [Crossref] [PubMed]
- Yu Y, Brouillette MJ, Seol D, et al. Use of Recombinant Human Stromal Cell–Derived Factor 1α-Loaded Fibrin/Hyaluronic Acid Hydrogel Networks to Achieve Functional Repair of Full-Thickness Bovine Articular Cartilage Via Homing of Chondrogenic Progenitor Cells. Arthritis Rheumatol 2015;67:1274-85. [Crossref] [PubMed]
- Jenei-Lanzl Z, Grässel S, Pongratz G, et al. Norepinephrine Inhibition of Mesenchymal Stem Cell and Chondrogenic Progenitor Cell Chondrogenesis and Acceleration of Chondrogenic Hypertrophy. Arthritis Rheumatol 2014;66:2472-81. [Crossref] [PubMed]
- Neumann AJ, Gardner OFW, Williams R, et al. Human Articular Cartilage Progenitor Cells Are Responsive to Mechanical Stimulation and Adenoviral-Mediated Overexpression of Bone-Morphogenetic Protein 2. PLoS One 2015;10:e0136229 [Crossref] [PubMed]
- Liu Z, Tang ML, Zhao JP, et al. Looking into the Future: Toward Advanced 3D Biomaterials for Stem-Cell-Based Regenerative Medicine. Adv Mater 2018;30:e1705388 [Crossref] [PubMed]
- Jiang C, Ma P, Ma B, et al. Plasma-Derived Fibronectin Stimulates Chondrogenic Differentiation of Human Subchondral Cortico-Spongious Progenitor Cells in Late-Stage Osteoarthritis. Int J Mol Sci 2015;16:19477-89. [Crossref] [PubMed]
- Zhou C, Zheng H, Buckwalter JA, et al. Enhanced phagocytic capacity endows chondrogenic progenitor cells with a novel scavenger function within injured cartilage. Osteoarthritis Cartilage 2016;24:1648-55. [Crossref] [PubMed]
Cite this article as: Lv Z, Li J, Xu X, Jiang Q, Shi D. The function and behavior of chondrogenic progenitor cells in osteoarthritis. Ann Joint 2020;5:33.


