
Balloon spacers in the management of massive rotator cuff tears: a focus on clinical outcomes
Introduction
Rotator cuff tears are among the most common injuries in orthopaedic patients presenting for evaluation of shoulder pain, motion loss, and disability following injury (1-4). Rotator cuff tears range in severity from partial-thickness to massive tears, with massive tears (≥5 cm or involving two or more tendons) constituting 10–40% of all clinically diagnosed tears (5-7). Furthermore, 6.5% to 30% of rotator cuff tears are characterized as irreparable. Irreparable tears are defined as tears in which direct repair back to the native footprint is not feasible even with soft tissue mobilization and release, or tears deemed certain to retear despite repair (2,6,8,9). Additional indicators of irreparable tears include static superior migration of the humeral head, a narrowed or absent acromiohumeral interval (AHI), and fatty infiltration affecting greater than 50% of the rotator cuff musculature (5). Despite the success of operative treatment for partial-thickness, small, and medium sized tears (10,11), the effective surgical management of painful, massive, and irreparable rotator cuff tears in patients without significant glenohumeral arthritis remains challenging (4,12).
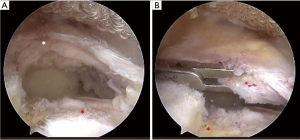
Various surgical options have been described for the treatment of patients with massive, irreparable rotator cuff tears (Figure 1A,B). These include subacromial decompression, bursectomy, biceps tenotomy or tenodesis, partial tendon repairs, synthetic graft interposition, superior capsular reconstruction, tendon transfers, and reverse total shoulder arthroplasty (RTSA) (6,13-18). However, no universally accepted treatment algorithm exists to effectively guide management for massive, irreparable tears. Savarese and Romeo (12) introduced the biodegradable subacromial balloon spacer (InSpace system; OrthoSpace, Caesarea, Israel) as a novel treatment option for patients with massive, irreparable rotator cuff tears. The balloon spacer can be used as a bridging option for future procedures such as superior capsular reconstruction, tendon transfer, or shoulder arthroplasty in younger, active patients, or as a definitive procedure in patients medically unfit for extended surgical procedures (12,19-21). The purpose of this review is to provide a concise overview of balloon spacer function, clinical outcomes and associated complications, and limitations for use in patients with massive, irreparable rotator cuff tears.
Function
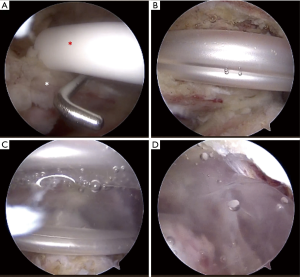
The OrthoSpace InSpaceTM implant is a biodegradable balloon-spacer made of a copolymer Poly (L-lactide-co-ε-caprolactone) (19). Spacer insertion into the subacromial space (SAS) is typically performed arthroscopically (Figure 2A,B,C,D) (9,12,19-28); however, the spacer may also be deployed under fluoroscopic guidance using local anesthesia (2,29). Biomechanical and cadaveric studies have shown that the balloon spacer functions in cuff-deficient shoulders to restore native biomechanics by depressing the humeral head into a more centralized position on the glenoid (30-32). By restoring the coupling forces of the subscapularis and teres minor, and improving muscle contraction capacity with elongation of the deltoid, the spacer allows for improved abduction and pain relief by increasing shoulder range of motion (ROM) before impingement (9,19,23,24). In their biomechanical study of 14 cadaveric shoulders (mean age of death, 67.9 years), Lobao et al. (30) found that the subacromial balloon spacer significantly depressed the humeral head at 0°, 30° and 60° of abduction compared to the irreparable, cuff-deficient state. Furthermore, the spacer significantly increased the deltoid load at 0°, 30° and 60° of abduction, while restoring glenohumeral contact pressures close to that of an intact rotator cuff state. Other research has investigated the effect of varying levels of balloon insufflation, referring to the amount of saline that is used to fill the balloon once it is introduced into the SAS. Singh et al. (31) found that balloon insufflation to 25 mL was the most effective volume in depressing the humeral head and restoring native glenohumeral joint position compared to volumes of 10 and 40 mL in eight cadaveric shoulders. Specifically, insufflation with 10 mL of saline was ineffective in depressing the humeral head compared to the intact state. In contrast, insufflation with 40 mL of saline was effective in depressing the humeral head; however, it also significantly translated the humeral head anteroinferiorly relative to the intact state.
Balloon implantation has also been suggested to reduce the friction forces between the acromion and the humeral head, redistributing forces at the subacromial level (33), and facilitating smooth gliding of the humeral head under the acromion (2,12,29). This benefit has been examined in the context of balloon spacer implantation following partial rotator cuff repair. Using six fresh-frozen cadavers with balloon spacer implantation following supraspinatus repair, Chevalier et al. (33) reported that the balloon spacer significantly reduced both mean and peak pressures during shoulder abduction and adduction as measured by a subacromial compression sensor. It is hypothesized that by allowing for a wider load distribution in the SAS and normalizing focal pressures over the repair tissue, the balloon may effectively protect against retears by reducing wear over the repaired tissue (26). In addition, the reduction in pressure may in principle allow for improved motion postoperatively, allowing for more effective and successful rehabilitation (33).
Following implantation, the balloon spacer is estimated to remain inflated for approximately 6–12 months, after which time the spacer proceeds to degrade (12,23,25). This time frame allows patients to complete rehabilitation protocols for any arthroscopic procedure performed on the rotator cuff (12,23). Sustained results beyond 12 months despite balloon degradation are attributed to more effective strengthening during the rehabilitation period secondary to effective restoration of muscle balance and patterning from recentering of the humeral head (25,26).
Clinical
The balloon spacer has been utilized in a variety of capacities within the spectrum of surgical options for massive, irreparable rotator cuff tears. Most commonly, these other treatment options for massive, irreparable rotator cuff tears include partial repairs with or without patch augmentation, superior capsular reconstruction, or RTSA. The balloon spacer may serve as a bridging option for any of the above-mentioned procedures, or as a definitive treatment in patients with significant medical comorbidities that preclude other surgical options. As both the quality and duration of clinical outcomes data continues to improve, the role of the balloon spacer within this treatment algorithm will become better defined.
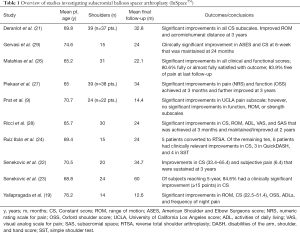
Overall, clinical studies are limited to small and short-term prospective trials; however, outcomes following spacer implantation are promising, including immediate postoperative improvements in various pain scores and validated functional outcomes that are often maintained or further improved at subsequent follow up timepoints ranging from 2 to 5 years. Additional details of individual outcomes studies are summarized in Table 1. Clinical outcomes evaluating the subacromial balloon spacer were first reported in the prospective, non-randomized investigation by Senekovic et al. (22) in 20 consecutive patients with massive, irreparable rotator cuff tears. The authors reported a significant increase in mean Constant score (CS) by 6 weeks postoperatively when compared to preoperative scores (P=0.010), and a mean overall improvement in CS of 31.5 points (P<0.0001) at 3-year final follow up. Outcome longevity was demonstrated by the same authors in their subsequent study of 24 patients with 5-year follow up data (23). CSs were again significantly improved at final follow up when compared to preoperative values (P<0.0001), while 84.6% of patients reaching the 5-year time point achieved a clinically significant improvement of at least 15 points in CS, with 61.5% improving at least 25 points. However, a total of 9 patients withdrew, died, or were lost to follow-up including 1 patient that converted to RTSA at 4 years postoperatively. Ricci et al. (28) also reported significant improvements in 30 patients based on CS evaluations at 6 months (P=0.0002), 12 months (P<0.0001) and 24 months (P<0.0001) following spacer implantation. Meanwhile, ROM and activities of daily living (ADL) were also found to significantly improve with a concurrent reduction in pain based visual analog scale (VAS) at 12 and 24 months. Radiographically, the mean AHI was significantly increased from baseline measurements at 3 months (P<0.0001), 6 months (P=0.0004), 12 months (P=0.00067) and 24 months (P=0.0007) time points.
Full table
Positive outcomes were further reported in a retrospective review of 37 patients (n=39 shoulder) undergoing spacer placement by Deranlot et al. (21), including a significant increase in mean adjusted CS (P<0.001) at a mean follow up of 32.8 months. Additional improvements beyond 12 months post-implantation were also noted, with significant improvement in mean adjusted CS at final follow up when compared to scores at 12 months (P=0.02). Mean shoulder ROM was also significantly improved in forward flexion (P=0.02), abduction (P=0.01) and external rotation (P=0.0001). On radiographic follow up, there was limited progression. The Hamada score advanced a single radiographic stage in 4 patients and 3 stages in 1 patient. The remaining shoulders available for follow up did not demonstrate any further increase in Hamada grade. However, the mean AHI was found to decrease from 8.2 mm prior to surgery to 6.2 mm at final follow up (P=0.002). A prospective study of 14 patients by Yallapragada et al. (19) also found that at a mean follow up of 12.6 months, patients experienced a significant improvement in forward elevation, abduction, and external rotation. Mean CS was also found to significantly increase at final follow up (P<0.001), with no patients reporting nocturnal pain following surgery.
When compared to conventional arthroscopic techniques alone, concomitant use of the balloon spacer has demonstrated equivocal outcomes in patients with massive, irreparable rotator cuff tears. Holschen et al. (25) compared the clinical results of patients managed with traditional arthroscopic techniques (subacromial debridement, synovectomy, bursectomy, biceps tenodesis/tenotomy ± partial reconstruction; n=11 patients) to patients undergoing debridement ± partial repair with implantation of a balloon spacer (n=12 patients). Patients receiving the balloon spacer had greater absolute improvements in American Shoulder and Elbow Surgeon (ASES) scores (P<0.001) and CSs (P<0.001) at a mean follow of 22.3 months compared to patients without spacer implantation at a mean of 30.6 months. However, the authors largely attributed these findings to lower preoperative shoulder function scores and shorter follow up for patients in the balloon spacer group, making direct comparisons difficult between the two groups.
Meanwhile, Piekaar et al. (27) examined results from 38 patients (n=39 shoulders) undergoing spacer placement. The majority of patients (n=23) underwent balloon implantation without any additional intervention. Concurrent biceps tenotomy was performed in 44% (n=17) of shoulders and partial repair was performed in 21% (n=8). When correcting for concurrent biceps tenotomy and/or partial repair with balloon implantation, no significant difference in subjective pain or Oxford shoulder score (OSS) were reported, as any additional intervention produced comparable outcomes compared to isolated balloon spacer implantation. Malahias et al. (26) compared a cohort of 31 patients treated with balloon spacer placement with non-anatomic cuff repair and biceps tenotomy (n=18 patients) versus patients undergoing spacer placement and biceps tenotomy without repair (n=13 patients). No significant differences were appreciated between baseline demographics or clinical characteristics between the two groups. The authors reported a non-significant difference in VAS, ASES, CS and ROM values between groups, concluding that partial repair with spacer placement was not superior to spacer placement alone.
Outcomes of spacer implantation have also been reported using fluoroscopy and local anesthetic. Gervasi et al. (29) reported on a series of 15 patients with 100% of patients demonstrating improvement in total CS and ASES beginning at 6 weeks postoperatively, with positive results sustained at 12 months of follow up. Moreover, 85% of patients had a clinically significant improvement of at least 15 points in CS at final follow up. These results demonstrate that similar benefits can be achieved in elderly and medically complicated patients with multiple comorbidities at high risk for complications during surgery and general anesthesia.
However, the clinical benefits associated with spacer utilization have been questioned in other investigations. Prat et al. (9) evaluated 22 patients (n=24 shoulders) treated with a subacromial balloon spacer with a mean study follow up of 14.4 months. While University of California Los Angeles (UCLA) score significantly increased (P=0.001) at final follow up, no significant differences in ROM were appreciated when compared to preoperative measurements. Moreover, no improvements in proximal migration of the humeral head were appreciated in any patients on postoperative radiographs, leading the authors to caution spacer use in patients with irreparable rotator cuff tears. Meanwhile, Ruiz Ibán et al. (24) followed 15 consecutive patients over a 24-month study period in which only 40% (n=6 patients) reported a successful outcome based on a minimally clinically important difference in the CS and the absence of surgical reinterventions. Thirty-three percent (n=5) of patients underwent RTSA at a median of 9.8 months following spacer implantation due to absence of clinical improvement or worsening of symptoms. The authors concluded that use of the balloon spacer device was not indicated for patients with massive irreparable rotator cuff tears, advocating for further study to better define the indications for subacromial balloon spacer use.
Complications/limitations
The complication rate related to subacromial spacer balloon arthroplasty has been reported to be between 3% to 16.7% of patients (9,21-24,29,30,34). A list of reported complications in the literature are included in Table 2. Most commonly, complications included spacer migration; however, this did not uniformly require additional interventions (19) such as spacer removal and/or replacement (9,21). Malahias et al. (26) reported that two patients (6.5%) required balloon revision surgeries postoperatively; however, no additional details regarding complications or revision surgeries were provided. Holschen et al. (25) reported on a single patient with increased pain in the shoulder 12 months following surgery with magnetic resonance imaging showing a deflated balloon spacer, which had transformed into scar tissue. Other reported complications attributed to balloon spacer implantation include transient neural damage with forearm dysesthesia (9), two cases of superficial wound infection (9,27), and a single case of late deep wound infection requiring balloon removal 2 months following implantation (9). In patients with unsatisfactory outcomes following balloon implantation, RTSA is generally performed, with a reported conversion rate ranging between 5% to 33% (22-24,29).

Full table
Caution must be exercised to avoid overstuffing the SAS. Applying excess pressure over a partially repaired tendon or potentially depressing the humeral head too far inferiorly may negate the benefits of the spacer (35). Moreover, the possibility of early mechanical failure with spacer rupture and reabsorption prior to 12 months has been reported. Ruiz Ibán et al. (24) found that in their five cases of patients requiring conversion to RTSA, no residual evidence of the spacer was appreciated in any patient, despite two patients undergoing conversion to RTSA at 6 months and one patient at 7 months. While not currently reported in the literature, the presence of infection should be considered in the setting of early balloon reabsorption or failure.
In order to minimize complications during subacromial balloon spacer implantation, proper patient selection is critical (Table 3). Ideal patients include those with massive and irreparable rotator cuff tears with an intact or reparable subscapularis and restricted ROM secondary to pain (26). Spacer placement is not recommended in patients with irreparable subscapularis tears (26,27), as higher spacer inflation volumes (25 and 40 mL) have been shown to result in anterior spacer translation in the presence of subscapularis insufficiency (32). Other contraindications to spacer use include patients with a known allergy to the device material (polylactide and/or ε-caprolactone), those with an active or latent infection within the shoulder, or patients with signs of tissue necrosis within the subacromial area (12). Variable definitions of pseudoparalysis exist in the setting of irreparable, massive rotator cuff tears including active shoulder elevation of less than 90° (36), active elevation less than 45° (37), or no active elevation with maintained or restricted passive elevation (38-40). Overall, the balloon spacer has been shown to produce inferior results in the pseudoparalytic shoulder. Holschen et al. (25) found that use of the balloon spacer in two patients with painless pseudoparalytic shoulders resulted in continued poor function with minimal clinical or functional improvement. Malahias et al. (26) excluded balloon implantation in patients with pseudoparalysis. However, in both studies, pseudoparalysis was mentioned but not defined. As such, more precise and consistent definitions of pseudoparalysis are warranted to better define the potential role of balloon spacer arthroplasty in the pseudoparalytic shoulder. Other authors have also advocated against spacer use in patients with significant osteoarthritis (OA) with preserved passive shoulder motion (2,20,22,27). In addition, due to the rehabilitation required following placement, the spacer may not be ideal for patients lacking motivation or those unable to follow a rehabilitation protocol (26).

Full table
Lastly, the clinical benefit of the balloon spacer is controversial given the high number of concomitant procedures (subacromial bursectomy, debridement, biceps tenotomy/tenodesis and/or partial rotator cuff repairs) often performed during implantation. Multiple prior investigations have demonstrated the effectiveness of subacromial debridement and bursectomy (41), biceps tenotomy (42), and partial repair (43,44) in reducing pain and improving functional outcomes for patients with massive, irreparable rotator cuff tears. As such, the direct clinical benefits of the balloon spacer versus additional arthroscopic interventions performed at the time of implantation remains unclear (27,30). In addition, the long-term outcomes following spacer implantation are largely unknown. While Senekovic et al. (23) reported outcomes 5-year following implantation, the patient dropout rate was 37.5%, suggesting a potentially significant selection bias in the final reported outcomes. Finally, the clinical results from a number of studies must be interpreted with caution and within the context of author conflicts of interest with the device manufacturers (2,9,22,23,26,29).
Conclusions
The subacromial balloon spacer represents a simple, minimally invasive device that may be inserted arthroscopically or under fluoroscopy using local anesthesia. Despite a limited number of clinical studies, the spacer has been shown to improve clinical outcomes, ROM, and pain, with questionable impact on restoring AHI when used as either a temporary measure or as a definitive treatment option. The procedure carries some inherent risk of short-term complications including infection and spacer migration. As much as a third of patients may also progress to conversion to RTSA. However, the balloon spacer remains a potentially attractive option for properly selected patients given the short rehabilitation period, potential for prolonged symptom relief, and in the event of spacer failure, the ability to pursue all remaining salvage procedures. However, appropriate selection is critical and should be avoided in patients with significant glenohumeral arthritis, irreparable subscapularis tears, as well as patients with active or latent infections and those allergic to device materials. Furthermore, given the limited quantity and duration of current data, its role in the pseudoparalytic shoulder warrants further investigation given the wide variety of definitions used in the literature. When interpreting clinical outcomes, the isolated contribution of the spacer remains largely unknown as the spacer is traditionally utilized with other arthroscopic procedures that have demonstrated independent clinical benefit. As such, prospective, randomized controlled trials using large patient cohorts with long-term follow up are warranted to establish the effectiveness and exact contribution of the balloon spacer compared with other arthroscopic interventions for the treatment of patients with massive, irreparable rotator cuff tears.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Adnan Saithna) for the series “Current and Emerging Concepts in the Management of Rotator Cuff Tears” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (http://dx.doi.org/10.21037/aoj-20-35). The series “Current and Emerging Concepts in the Management of Rotator Cuff Tears” was commissioned by the editorial office without any funding or sponsorship. NNV reports other from American Orthopaedic Society for Sports Medicine, other from American Shoulder and Elbow Surgeons, other from Arthrex, Inc., other from Arthroscopy, other from Arthroscopy Association of North America, other from Breg, other from Cymedica, other from Knee, other from Minivasive, other from Omeros, other from Orthospace, other from Ossur, other from SLACK Incorporated, other from Smith and Nephew, other from Vindico Medical-Orthopedics Hyperguide, other from Wright Medical Technology, Inc., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Castagna A, Garofalo R, Maman E, et al. Comparative cost-effectiveness analysis of the subacromial spacer for irreparable and massive rotator cuff tears. Int Orthop 2019;43:395-403. [Crossref] [PubMed]
- Gervasi E, Cautero E, Dekel A. Fluoroscopy-guided implantation of subacromial "biodegradable spacer" using local anesthesia in patients with irreparable rotator cuff tear. Arthrosc Tech 2014;3:e455-8. [Crossref] [PubMed]
- Green A. Chronic massive rotator cuff tears: evaluation and management. J Am Acad Orthop Surg 2003;11:321-31. [Crossref] [PubMed]
- Berth A, Neumann W, Awiszus F, et al. Massive rotator cuff tears: functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol 2010;11:13-20. [Crossref] [PubMed]
- Bedi A, Dines J, Warren RF, et al. Massive tears of the rotator cuff. J Bone Joint Surg Am 2010;92:1894-908. [Crossref] [PubMed]
- Gerber C, Wirth SH, Farshad M. Treatment options for massive rotator cuff tears. J Shoulder Elbow Surg 2011;20:S20-9. [Crossref] [PubMed]
- Zumstein MA, Jost B, Hempel J, et al. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am 2008;90:2423-31. [Crossref] [PubMed]
- Warner JJ. Management of massive irreparable rotator cuff tears: the role of tendon transfer. Instr Course Lect 2001;50:63-71. [PubMed]
- Prat D, Tenenbaum S, Pritsch M, et al. Sub-acromial balloon spacer for irreparable rotator cuff tears: Is it an appropriate salvage procedure? J Orthop Surg (Hong Kong) 2018;26:2309499018770887 [Crossref] [PubMed]
- Bennett WF. Arthroscopic repair of massive rotator cuff tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy 2003;19:380-90. [Crossref] [PubMed]
- Park JY, Chung KT, Yoo MJ. A serial comparison of arthroscopic repairs for partial- and full-thickness rotator cuff tears. Arthroscopy 2004;20:705-11. [Crossref] [PubMed]
- Savarese E, Romeo R. New solution for massive, irreparable rotator cuff tears: the subacromial "biodegradable spacer". Arthrosc Tech 2012;1:e69-74. [Crossref] [PubMed]
- Anley CM, Chan SK, Snow M. Arthroscopic treatment options for irreparable rotator cuff tears of the shoulder. World J Orthop 2014;5:557-65. [Crossref] [PubMed]
- Greenspoon JA, Petri M, Warth RJ, et al. Massive rotator cuff tears: pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg 2015;24:1493-505. [Crossref] [PubMed]
- Burkhart SS, Denard PJ, Adams CR, et al. Arthroscopic superior capsular reconstruction for massive irreparable rotator cuff repair. Arthrosc Tech 2016;5:e1407-18. [Crossref] [PubMed]
- Ferguson DP, Lewington MR, Smith TD, et al. Graft utilization in the augmentation of large-to-massive rotator cuff repairs: a systematic review. Am J Sports Med 2016;44:2984-92. [Crossref] [PubMed]
- Bacle G, Nove-Josserand L, Garaud P, et al. Long-term outcomes of reverse total shoulder arthroplasty: a follow-up of a previous study. J Bone Joint Surg Am 2017;99:454-61. [Crossref] [PubMed]
- Omid R, Lee B. Tendon transfers for irreparable rotator cuff tears. J Am Acad Orthop Surg 2013;21:492-501. [Crossref] [PubMed]
- Yallapragada RK, Apostolopoulos A, Katsougrakis I, et al. The use of a subacromial spacer-inspace balloon in managing patients with irreparable rotator cuff tears. J Orthop 2018;15:862-8. [Crossref] [PubMed]
- Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskelet Surg 2018;102:247-55. [Crossref] [PubMed]
- Deranlot J, Herisson O, Nourissat G, et al. Arthroscopic subacromial spacer implantation in patients with massive irreparable rotator cuff tears: clinical and radiographic results of 39 retrospectives cases. Arthroscopy 2017;33:1639-44. [Crossref] [PubMed]
- Senekovic V, Poberaj B, Kovacic L, et al. Prospective clinical study of a novel biodegradable sub-acromial spacer in treatment of massive irreparable rotator cuff tears. Eur J Orthop Surg Traumatol 2013;23:311-6. [Crossref] [PubMed]
- Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg 2017;137:95-103. [Crossref] [PubMed]
- Ruiz Ibán MA, Lorente Moreno R, Ruiz Díaz R, et al. The absorbable subacromial spacer for irreparable posterosuperior cuff tears has inconsistent results. Knee Surg Sports Traumatol Arthrosc 2018;26:3848-54. [Crossref] [PubMed]
- Holschen M, Brand F, Agneskirchner JD. Subacromial spacer implantation for massive rotator cuff tears: clinical outcome of arthroscopically treated patients. Obere Extrem 2017;12:38-45. [Crossref] [PubMed]
- Malahias MA, Brilakis E, Avramidis G, et al. Satisfactory mid-term outcome of subacromial balloon spacer for the treatment of irreparable rotator cuff tears. Knee Surg Sports Traumatol Arthrosc 2019;27:3890-6. [Crossref] [PubMed]
- Piekaar RSM, Bouman ICE, van Kampen PM, et al. The subacromial balloon spacer for massive irreparable rotator cuff tears: approximately 3 years of prospective follow-up. Musculoskelet Surg 2020;104:207-14. [Crossref] [PubMed]
- Ricci M, Vecchini E, Bonfante E, et al. A clinical and radiological study of biodegradable subacromial spacer in the treatment of massive irreparable rotator cuff tears. Acta Biomed 2017;88:75-80. [PubMed]
- Gervasi E, Maman E, Dekel A, et al. Fluoroscopy-guided biodegradable spacer implantation using local anesthesia: safety and efficacy study in patients with massive rotator cuff tears. Musculoskelet Surg 2016;100:19-24. [Crossref] [PubMed]
- Lobao MH, Canham RB, Melvani RT, et al. Biomechanics of Biodegradable Subacromial Balloon Spacer for Irreparable Superior Rotator Cuff Tears: Study of a Cadaveric Model. J Bone Joint Surg Am 2019;101:e49 [Crossref] [PubMed]
- Singh S, Reeves J, Langohr GDG, et al. The effect of the subacromial balloon spacer on humeral head translation in the treatment of massive, irreparable rotator cuff tears: a biomechanical assessment. J Shoulder Elbow Surg 2019;28:1841-7. [Crossref] [PubMed]
- Singh S, Reeves J, Langohr GDG, et al. The subacromial balloon spacer versus superior capsular reconstruction in the treatment of irreparable rotator cuff tears: a biomechanical assessment. Arthroscopy 2019;35:382-9. [Crossref] [PubMed]
- Chevalier Y, Pietschmann MF, Thorwachter C, et al. Biodegradable spacer reduces the subacromial pressure: a biomechanical cadaver study. Clin Biomech (Bristol, Avon) 2018;52:41-8. [Crossref] [PubMed]
- Carver TJ, Kraeutler MJ, Smith JR, et al. Nonarthroplasty surgical treatment options for massive, irreparable rotator cuff tears. Orthop J Sports Med 2018;6:2325967118805385 [Crossref] [PubMed]
- Szöllösy G, Rosso C, Fogerty S, et al. Subacromial spacer placement for protection of rotator cuff repair. Arthrosc Tech 2014;3:e605-9. [Crossref] [PubMed]
- Boileau P, Chuinard C, Roussanne Y, et al. Reverse shoulder arthroplasty combined with a modified latissimus dorsi and teres major tendon transfer for shoulder pseudoparalysis associated with dropping arm. Clin Orthop Relat Res 2008;466:584-93. [Crossref] [PubMed]
- Burks RT, Tashjian RZ. Should we have a better definition of pseudoparalysis in patients with RTC tears. Arthroscopy 2017;33:2281-3. [Crossref] [PubMed]
- Gschwend N, Ivosevic-Radovanovic D, Patte D. Rotator cuff tear-relationship between clinical and anatomopathological findings. Arch Orthop Trauma Surg 1988;107:7-15. [Crossref] [PubMed]
- Oh JH, Kim SH, Shin SH, et al. Outcome of rotator cuff repair in large-to-massive tear with pseudoparalysis: a comparative study with propensity score matching. Am J Sports Med 2011;39:1413-20. [Crossref] [PubMed]
- Denard PJ, Lädermann A, Jiwani AZ, et al. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy 2012;28:1214-9. [Crossref] [PubMed]
- Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy 2008;24:743-8. [Crossref] [PubMed]
- Boileau P, Baque F, Valerio L, et al. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am 2007;89:747-57. [Crossref] [PubMed]
- Porcellini G, Castagna A, Cesari E, et al. Partial repair of irreparable supraspinatus tendon tears: clinical and radiographic evaluations at long-term follow-up. J Shoulder Elbow Surg 2011;20:1170-7. [Crossref] [PubMed]
- Burkhart SS, Nottage WM, Ogilvie-Harris DJ, et al. Partial repair of irreparable rotator cuff tears. Arthroscopy 1994;10:363-70. [Crossref] [PubMed]
Cite this article as: Knapik DM, Williams BT, Verma NN. Balloon spacers in the management of massive rotator cuff tears: a focus on clinical outcomes. Ann Joint 2021;6:19.


