
Preclinical models of elbow injury and pathology
Introduction
Musculoskeletal tissues and joints are critical for daily living. While decades of research have focused on the physiology and pathophysiology of major musculoskeletal joints (e.g., knee and shoulder), the elbow remains relatively understudied. The elbow is a complex joint in terms of both anatomy and functionality. Due to its complex nature, the elbow is challenging to study and prone to injury and secondary pathologies. Thus, an urgent need exists to understand the etiology and pathogenesis of elbow pathologies and to develop novel therapeutic strategies to treat such conditions.
Clinically, the elbow is subjected to various pathologic conditions: injury/dislocation, joint stiffness and contracture, immobilization, overuse, post-burn contracture, arthritis, and osteophytes/heterotopic ossification. Each elbow condition can occur as a distinct entity or concomitantly with other symptoms. Significant advancements have been achieved in how many of these elbow conditions are treated; however, difficult challenges persist and will require continued work to elucidate novel therapeutic strategies. A comprehensive presentation of the various therapies currently in clinical use is beyond the scope of this review but is discussed in many references used herein.
Although elbow pathologies have been clinical problems for decades, researchers have only recently started to identify and unravel the etiology and pathogenesis for some of these conditions. Unfortunately, most elbow conditions are studied at the late-disease stages when irreversible damage has already occurred in the elbow (i.e., contracted elbow); warranting future work to understand early disease stages. It is challenging and often not feasible to study joint conditions in humans, in part, because humans’ disease progresses relatively slowly and obtaining normal and diseased tissue is difficult. To overcome these limitations, preclinical models (in vivo, in situ, ex vivo, in vitro) can serve as valuable alternatives because of greater experimental control, faster disease progression, and increased access to tissue. Unfortunately, no preclinical model can recapitulate every aspect of human elbow anatomy and pathology; nonetheless, each model can provide valuable information towards at least some understanding of elbow pathologies.
Currently, only a handful of preclinical models have been used to investigate elbow injury and pathology, and as such there are many opportunities for advances in this area. This review will discuss relevant considerations for using animal models to simulate human conditions, current preclinical models of elbow diseases, then conclude by offering some ideas for potential future directions.
Elbow anatomy in humans and other mammalian species
The human elbow is one of the most complex and highly congruent joints, where three distinct bones (humerus, radius, and ulna) join to form three articulating surfaces (1-8). These surfaces are surrounded by a joint capsule and numerous ligaments (1-8), as well as several muscles (1,2,4,5,8,9). Intertwined among these tissues of the elbow is an extensive network of nerves (4,8,10-13) and vasculature (14). Collectively, these periarticular soft tissues allow for elbow stability and the motions of flexion-extension (normal range: ~0° to 150°; functional range: ~30° to 140°) and pronation-supination (normal range: ~155°–175° total motion between ~75°–85° pronation and ~80°–90° supination; functional range: ~100° total motion between 50° pronation and 50° supination) (1,2,5-8,15,16). The elbow is vital to countless daily activities, such as opening a door, typing, eating, personal hygiene, or using a telephone (5-7,15,16). The ability of the elbow to enable these activities is unlike other musculoskeletal joints (e.g., knee and hip) and is largely unique to humans, particularly in how pronation and supination is utilized. During normal daily activities, the elbow experiences a wide range of forces that largely originate from the periarticular tissues stabilizing the elbow (1,2,4-8,17). During heaving lifting and dynamic loading, the elbow experiences stress as high as 3−6× bodyweight (5,6,15,17,18). Overall, the elbow presents itself as a complex and challenging joint to study in humans, as well as in other mammalian species.
Given the complexity of the elbow joint, it is important to highlight some of the similarities and differences of the elbow between humans and other commonly used mammalian species for biomedical research (e.g., horses, primates, dogs, rats, rabbits, and mice). Many mammals other than humans, whether bipedal or quadrupedal, have elbows that are similar in anatomy to humans and can perform the flexion-extension motion. However, elbows of most mammals cannot pronate and supinate to the same extent as human elbows do. A few exceptions include some non-human primates and a specific breed of rats (i.e., Long-Evans) that can pronate and supinate over ranges that are similar to humans (19,20). As a result, these species perhaps are more clinically relevant to study the human elbow, although other species may still be valuable for research questions that are not as dependent on this type of motion. Regardless, the selection of species and the joint is critical to consider when designing preclinical models to study the elbow’s normal physiology and pathophysiology.
Current preclinical models of elbow injury and pathology
In the following sections, common elbow injuries and pathologies and corresponding preclinical models that have been used to recapitulate each disease are reviewed. Preclinical models considered and discussed herein were included based upon the model’s relevance to pathologic conditions of the elbow specifically; and, any therapeutic strategy using these preclinical models is not discussed.
Trauma, immobilization, and post-traumatic contracture of the elbow
Elbow trauma (e.g., dislocation and soft-tissue injury) and subsequent complications (e.g., pain, joint immobilization, contracture, stiffness, and arthritis) are some of the most common elbow pathologies observed clinically (5,21-29). Unfortunately, injuries are poorly tolerated in the elbow, leading to debilitating consequences for the patient (5,21-29). The elbow can experience various types of injuries including intra-articular fractures, soft tissue and ligament damage, as well as simple and complex dislocations (without or with concurrent bony fracture, respectively) (5,21-29). Typically, the elbow is immobilized post-injury to stabilize the joint; however, extended joint immobilization post-injury could induced unwanted elbow contracture (23,26,28,30,31). Generally, the most common elbow complaints following injury are increased elbow pain, stiffness, or limited range of motion.
A full picture of the post-injury response of elbow tissues leading to elbow contracture/stiffness remains unclear. Some clinical evidence of pathological changes in the elbow synovial capsule suggests that the capsule becomes fibrotic and restricts the elbow range of motion (3,21,32,33). Furthermore, in fibrosis of the elbow (21,33-35) and other joints (31,34,36), fibroblasts within the joint capsule have been observed to take on a pro-fibrotic phenotype by transitioning to myofibroblasts. Myofibroblasts are the effector cells that release pro-fibrotic factors and over-produce extracellular matrix, leading to capsule stiffness and joint contracture. Indeed, studies have demonstrated the presence of myofibroblasts and high levels of matrix metabolism in the contracted human elbow capsule (3,33-35,37-39). In contrast, at least one study did not observe increased number of myofibroblasts at chronic-disease stages (40). This contradiction suggests that disease stage and patient variability may complicate human findings; preclinical models could allow for greater experimental control to study myofibroblasts temporally. Generally, the primary goal of preclinical models recapitulating post-traumatic elbow contracture is to cause similar cellular (i.e., myofibroblasts) and tissue-level (i.e., capsular) changes and permanent loss of elbow joint motion post-trauma.
Since the early 2000s, Hildebrand and colleagues have pioneered preclinical work in the context of post-traumatic joint contracture with respect to the elbow (41). In their preclinical model of joint contracture, a surgical procedure was performed on the rabbit knee to induce an intra-articular fracture combined with immobilization via Kirschner wire (K-wires) (Figure 1). After 8 weeks of immobilization, K-wires are removed, followed by a period of remobilization (0,8,16, and 32 weeks), mimicking the clinical treatment paradigm of elbow injuries of injury, immobilization, and then remobilization. Using this preclinical model, Hildebrand and colleagues identified a reduced range of motion following immobilization (~30°) and remobilization (~25° at 8 weeks; ~10° at 16 and 32 weeks), as well as increased myofibroblasts, mast cells, neuropeptides, and pro-fibrotic factors in the capsule (41-45). Results from their work demonstrated that this rabbit knee model could induce changes similarly seen in contracted elbows of humans.
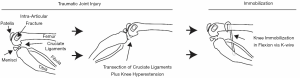
Building upon the rabbit animal model developed by Hildebrand and colleagues (41), Nesterenko and Abdel et al. developed a similar yet more severe and permanent joint contracture in the rabbit knee (46-48). In their model, the cruciate ligaments are transected and the knee is hyperextended in addition to an intra-articular fracture and K-wire immobilization (Figure 1). Indeed, their model produced more severe knee contracture and reduced range of motion at both the end of the 8 weeks immobilization (~76°) and 16 weeks of remobilization (~45°). Interestingly, Abdel et al. (47) observed a different pattern of myofibroblasts compared to Hildebrand et al. (41), which may be related to differences in model severity. Other groups have found similar findings using the more severe model by Nesterenko and Abdel et al. in the rat knee (49-51). While knowledge obtained by these models has been critical towards the understanding of post-traumatic joint contracture, each of these studies utilized the knee joint. Knee models are useful towards understanding general concepts related to joint contracture; however, due to anatomical and functional differences between the knee and elbow, results may be limited in understanding aspects of elbow-specific injury and contracture.
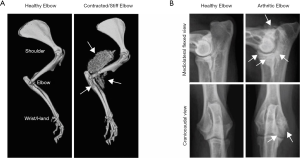
Nearly a decade after the initial work by Hildebrand et al. (41), our research group developed a preclinical model of post-traumatic elbow contracture specific to the elbow (52,53). In our model, we utilized the Long-Evans rat, which has similar anatomy and functionality (flexion-extension and pronation-supination movement) as the human elbow (19,20,52). To induce permeant post-traumatic elbow contracture, we performed an anterior capsulotomy and lateral collateral ligament transection, followed by 6 weeks of immobilization (IM) using wrapping/bandage, and then an additional 6 weeks of free mobilization (FM) (Figure 2A). Our model caused a time-dependent reduction in the range of motion of the elbow with a peak loss following 6-weeks of IM and with a less severe loss after 6-weeks of FM in both flexion-extension (~43° and ~26°, respectively) and pronation-supination (~40° and ~37°, respectively) motion (52-57). Ultimately, loss of motion caused permanent deficits in functional measures of grip strength and gait (58). We also confirmed the thickening of the capsule with increased cellularity and presence of myofibroblasts, as well as altered matrix integrity and composition of both the capsule and cartilage surfaces (52,53,55). Additionally, we determined that the major periarticular tissue contributors to contracture in our model were largely the anterior capsule and lateral ligament complexes, not periarticular muscles (56,57). Overall, our work has advanced the field forward by creating the first animal model of joint contracture using the elbow.
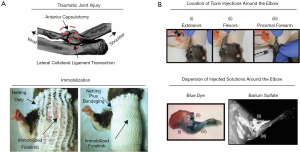
Soon after the development of our elbow-specific model, Moore-Lotridge et al. created a mouse model of post-traumatic joint contracture (stiffness) of the elbow (59). In this model, injury to the periarticular soft-tissues (i.e., capsule ligaments, and surrounding musculature) of the elbow was induced by local injections of cardiotoxin combined with researcher-imposed plasminogen deficiency (Figure 2B). After 28 days post-injury, this study showed a significant loss of elbow motion accompanied by an altered gait, muscle fibrosis and inflammation, thickening of the joint capsule, and heterotopic ossification in the muscle (59). Given these findings, this mouse model of post-traumatic elbow contracture replicates many of the same changes seen in human contracted elbows.
In addition to using an animal model of post-traumatic joint contracture, Hildebrand and colleagues have explored other preclinical models of elbow contracture (60). Namely, they used an in vitro collagen gel contraction assay to mimic capsule contracture in the elbow (60). In this in vitro model, Hildebrand et al. isolated primary capsule cells from contracted human elbow capsule and resuspended them in a collagen matrix with mast cells, another cell type thought to be involved in joint contracture (23,33,60,61). In these studies, elbow capsule cells contracted the collagen gels, which was enhanced with the addition of mast cells. In a set of gel contraction studies by another group, Mattyasovszky and colleagues found that inflammatory cytokines could modulate gel contraction of human capsule cells obtained from elbows undergoing arthroplasty (62,63). Results of these collagen gel contraction models have shown that capsule cells from contracted human elbows are contractile and can be modulated by mast cells and inflammatory cytokines to influence the contraction of in vitro tissue analogs.
Overall, this section highlighted the progression of various preclinical models for post-traumatic elbow contracture. While each model uses different species, joints, injuries, and outcomes, they all each provided insights into the etiology and pathology of post-traumatic elbow contracture. Future work is necessary to fully elucidate the contributions of each elbow tissue, create new models to address other types of traumatic injuries, and determine the translatability of each preclinical model attempting to recapitulate the human condition.
Elbow overuse
Another common elbow pathology is elbow overuse, where repetitive use of the elbow can cause overstrain and microtrauma of ligaments around the elbow (64-66). Signs of elbow overuse are increased pain, point tenderness, and difficulty in grasping items. Elbow overuse is common in individuals whose jobs and household tasks are repetitive and require the elbow and other motions involving extensive wrist use (65-70). While many individuals are at risk for overusing the elbow, athletes such as pitchers, golfers, and tennis players are at exceptionally high risk (64-69). Elbow overuse is often referred to as tennis or golfer elbow, but clinically described as lateral and medial epicondylitis, respectively (64-69). While the terminology describing elbow overuse is still debated in the field, lateral epicondylitis is thought to occur in the absence of inflammation and resemble tendinosis (64-69). Significant progress has been made clinically to characterize the pathogenesis of elbow overuse, which has identified important structural and cellular changes in these tendons along with various neuropeptides thought to cause pain (64-69).
To date, only two preclinical models in the context of elbow overuse exist. One study by Nakama and colleagues used a rabbit model to induce medial epicondylitis (71). In this model, the flexor digitorum profundus muscle, which transmits forces through a tendon that inserts into the medial epicondyle, was stimulated repeatedly for 80 hours a week for 14 weeks (71). Results from this study demonstrated that the tendon at the insertion site of the medial epicondyle underwent significant increases in size, as well as an increase in the number and size of tears within the tendon (71).
Another group developed a rat model to explore repetitive, upper-extremity tasks required for reaching and grabbing with the wrist, elbow, and shoulder (72). In this model, rats repeatedly elevated their shoulder, fully extended their elbow, and gripped with their wrist to obtain food on cue, which caused repetitive muscular activation (72). While this group’s primary focus was on changes to the wrist, they did evaluate the elbow in this model and found that the elbow was unaffected (72).
Each of the aforementioned preclinical models of elbow overuse has provided preliminary insights into the etiology and pathogenesis of elbow overuse/epicondylitis/tendinopathy. Future investigations utilizing these established, as well as novel, preclinical models are warranted to elucidate the mechanisms involved fully with elbow overuse. Perhaps other preclinical models previously used to examine degenerative changes in other tendons, such as the Achilles or the rotator cuff in the shoulder, could provide insights to assist the study of elbow related tendon issues (73-75).
Elbow arthritis
Arthritis occurs in the elbow and can take the form of either idiopathic osteoarthritis (OA), post-traumatic osteoarthritis (PTOA), and rheumatoid arthritis (RA). All three types of arthritis ultimately lead to cartilage degeneration, pain, loss of joint function, and a need for arthroplasty. While OA in the elbow is rare (~2% of all joints with OA and of patients with elbow arthritis), the etiology and pathogenesis remain unclear (17,18,76-83). There are some risk factors for OA such as aging and overuse. A more common risk factor for developing elbow OA is a traumatic joint injury, which accelerates the development of OA; referred to as PTOA, this condition is also relatively uncommon in the elbow (17,18,76,77,79,84-86). Despite the rarity, OA and PTOA in the elbow are debilitating for patients once the disease initiates and progresses. Although reporting on prognosis is mixed, treated intra-articular elbow fractures can lead to at least a 50% chance of developing PTOA as early as 10 years post-injury (17,84), which are similar odds in other joints such as the knee and ankle (86,87). This high occurrence is concerning since elbow trauma frequently occurs in younger (28,29,79,85,88) and active individuals including athletes (29,79) and military service members (89,90). Besides OA and PTOA, the next most common form of arthritis affecting the elbow is RA, inflammatory-driven arthritis (17,76,91). RA isolated to the elbow alone occurs in ~5% of patients with RA (92); however, ~20–50% of patients with RA in other joints or organs also have signs RA in the elbow (76,92).
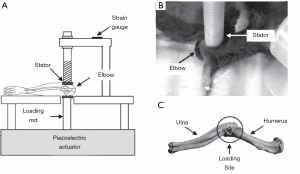
A few preclinical models exist addressing elbow arthritis. In the context of idiopathic OA, preclinical studies have evaluated cartilage damage, joint space narrowing, gait, and lameness in pig (93), cats (94-97), and dogs (98-106) with signs of elbow OA. Although informative, these studies recruited animals with naturally occurring OA and were unable to control the onset of degenerative changes. An alternative model with more experimental control is the mouse elbow/ulna loading models, which have historically been used to study the bone response of the elbow/ulna under controlled loading rate and magnitude (107,108). Recently, an elbow loading model was used to evaluate cartilage metabolism in the proximal ulna and distal humerus following mechanical stimuli (Figure 3) (109). Additional studies, however, are needed to validate the OA-like phenotype of the elbow in this model. In the context of PTOA, Dunham et al. minimally evaluated changes in the cellularity and matrix integrity of the cartilage (55). No preclinical models currently exist to study RA in the elbow. Although limited effort has been made to study elbow arthritis using preclinical models, the etiology and pathogenesis of elbow arthritis remain unclear.
Some knowledge about elbow arthritis and guidance on preclinical models could be inferred from arthritis in other joints. For instance, countless preclinical models have been created to study arthritis in the knee, including OA (110-112), PTOA (110,112-114), and RA (115-118). Most commonly, in vivo models of OA and PTOA include the use of chemicals, genetic manipulation, aging, or surgically and non-surgically induced traumatic injuries, while models of RA include injections of compounds inducing an immune response. To study interactions between various tissues of the joint that are otherwise challenging in vivo, researchers have turned to ex vivo and in vitro preclinical models of arthritis (112,119). While these established models of arthritis in other joints can provide insights for models of elbow arthritis, researchers should be aware that the cartilage structure and cellularity is different between joints, potentially causing differences in cartilage response to arthritic stressors (120).
Post-burn elbow contracture
Post-burn contracture is defined as a contracture of the skin or joint following a significant burn. Contracture can develop following a burn in many parts of the body, including the knee (121) and non-musculoskeletal tissues (122); however, the elbow is one of the most commonly affected joints (123-125). While the skin and other soft tissues may play a role in post-burn contracture, the pathogenesis and scope of tissues involved in this condition in the elbow remain unclear. One of the most common symptoms that occur following burns is the formation of heterotopic ossifications (see next section), which is thought to reduce the range of motion in the elbow (123-125).
Current preclinical models of post-burn injury include the use of various species such as mice and rats; however, no model has been used to address post-burn elbow contracture (126). Caution must be taken when choosing a species to study post-burn elbow contracture, partly because the structure of the skin and the post-burn severity and response in other species may not translate to humans (122,126). In the future, the development of preclinical models will be necessary to shed insights into the etiology and pathogenesis of post-burn elbow contracture.
Osteophyte formation and heterotopic ossification in elbow pathologies
Among many of the elbow pathologies clinically, there is the concurrent formation of osteophytes and heterotopic ossification (HO). Osteophytes are defined as bony outgrowths or spurs that form; in the elbow, these tend to form along the joint margins of the ulna, radius, and humerus (79,85,127-129). The formation of osteophytes ultimately causes significant pain and reduces the joint space, limiting the elbow range of motion (79,85,127-129). In addition to osteophyte formations, HO in the elbow has recently gained attention in the literature (27,130-133). HO is an aberrant bone formation that can contribute to elbow pain and reduction in elbow range of motion. Although osteophytes and HO are prevalent in diseased elbows, how osteophytes and HO form and what their full role in various elbow pathologies remains unknown.
Unfortunately, the preclinical evaluation of HO and osteophytes in the elbow has been limited. To date, only one preclinical study has addressed the presence of HO in elbow contracture/stiffness (Figure 4A) (59), and only a few have evaluated osteophytes in elbow osteoarthritis (Figure 4B) (96,97,100-103,105). To study osteophytes and HO in the elbow, researchers can study the current preclinical models of elbow pathologies mentioned above or other preclinical models that may be appropriate. For example, researchers have performed intra-articular injection of pro-fibrotic factors into mouse knees, causing the formation of osteophytes (134). Although these pro-fibrotic factors can induce osteophytes in the knee, it is not known whether the same effects would be seen in the elbow. Overall, a need exists for preclinical models to investigate HO and osteophytes in all elbow pathologies.
Osteochondritis dissecans
Another common clinical elbow pathology is osteochondritis dissecans (OCD) (17,135-137). OCD is the process by which the articular cartilage separates from the underlying subchondral bone, leading to fragmentations of cartilage in the joint space, pain, and a reduced range of motion in the elbow (17,29,135-137). In the elbow, OCD is typically observed in young, over-head throwing athletes at the humeral capitellum (17,29,135-137). The etiology of OCD formation and its role in elbow pathologies remains to be elucidated (17,29,135,136). It has been suggested that genetics, repetitive injury, subchondral bone abnormalities (e.g., loss of vasculature and becoming necrotic), and excessive force applied to the cartilage potentially contribute to OCD symptoms (17,29,135,136). Regardless of the etiology, OCD may make the surrounding cartilage prone to further degeneration, leading to arthritis (17).
Unfortunately, no preclinical study has investigated the etiology or pathogenesis of OCD in the elbow. OCD has been researched in preclinical models of other joints, such as the knee (138-140). However, it should be cautioned that the etiology and pathogenesis of OCD in other joints could be different than in the elbow, partly because of differences in biomechanical forces experienced (e.g., weight vs. non-weight bearing), structure, and cellularity of different joint’s cartilage (120,141). To investigate OCD in the elbow, preclinical models could be non-invasive, repeated motion overuse models; however, these models don’t exist for the elbow. Given that the etiology and pathogenesis of OCD in the elbow is unknown, current and novel preclinical models of the elbow, especially trauma and overuse models, may note that OCD lesions develop.
Elbow pain and innervation
Most elbow pathologies are associated with some degree of pain; however, the pathomechanisms causing pain are still up for debate in the elbow and have received little attention. One potential source of pain could arise from the extensive network of nerves and nerve endings throughout the elbow (4,8,10-13). While the nerve supply for each elbow tissue is different, neuroinflammatory pathways, which involve neuropeptides (substance-P) and mast cells (23,33,42,61), may play a role in elbow pain. It remains to be determined if other inflammatory and nerve cells are also involved with neuroinflammatory pathways. In addition to sensing pain, nerves of the elbow are prone to complications such as nerve entrapment and neuropathy, particularly after an injury, capsulectomy, or arthroplasty (22,142-145).
To date, no preclinical models of elbow pathologies exist that have directly evaluated pain and innervation. The most relevant preclinical studies addressing any degree of pain and innervation are those preclinical studies that have indirectly assessed the role of innervation through studying neuropeptides (substance-P) and mast cells in post-traumatic elbow contracture (23,33,42,61). However, a few studies have begun to directly address elbow innervation under non-pathological conditions in monkey (146) and rat elbows (147,148). Thus, future work is warranted to understand the etiology and pathogenesis of pain and innervation in every elbow pathology.
Future considerations for preclinical models of elbow diseases
This review has demonstrated and summarized in Table 1 that only a limited number of preclinical models exist to study elbow diseases. Future studies are warranted to refine and create new preclinical models to elucidate the etiology and pathogenesis of common elbow pathologies. Research groups interested in pursuing future work in elbow pathologies might consider looking to other joints with more mature research results to gain valuable guidance for developing new preclinical models of elbow pathologies. To help facilitate the implementation of novel preclinical elbow models, the following section will highlight some critical points to consider and will provide some suggestions for appropriate next steps.
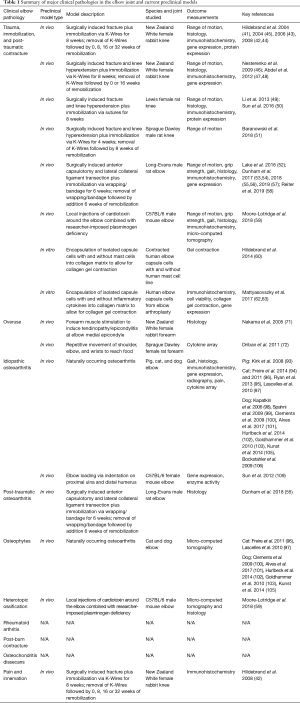
Full table
Considerations for refining and developing preclinical models of elbow pathologies
An important consideration when refining or developing a preclinical model for the elbow is to specify which elbow pathology will be studied. As highlighted previously, each elbow pathology involves different tissues and cells, as well as having a different time scales of etiology and pathogenesis; thus, a “gold-standard” for preclinical models would be to closely mimic the clinical observations of the specific elbow condition under investigation.
After deciding an elbow pathology to study, the next likely consideration is which type of preclinical model to use: in vivo, in situ, ex vivo, or in vitro model systems. Each type of model has important trade-offs worth considering. For example, in vivo and in situ models allow for the study and interaction of all tissues in the body, including the full biological response, enabling for the closest translation of findings to the human disease. A critical consideration when choosing an in vivo and in situ model is how closely the anatomy and functionality of the animal of choice mimic the human elbow. Additionally, another consideration is whether to use an invasive or non-invasive approach to induce a traumatic elbow injury. An invasive model requires a surgical incision that may induce an unwanted and confounding biological response that would otherwise not be present in the human; thus, non-invasive animal models would be more clinically relevant. While in vivo models are more representative of the human situation, they require the use of animals, which are accompanied by ethical concerns and other requirements (149). Thus, it is critical to follow the recommended guidelines of animal research: reduction, refinement, and replacement (149).
Ex vivo and in vitro models can serve as alternatives to in vivo and in situ models of elbow pathologies and be used to achieve many samples while minimizing the need for many animals. Furthermore, ex vivo and in vitro allow the following: (I) more control of the experimental design and variables involved; (II) the ability to isolate and study the interaction of certain experimental variables between tissues/cells; and (III) the introduction of endogenous factors (e.g., growth factors and inflammatory cytokines) in a controlled manner. However, a downside to both ex vivo and in vitro models is that the tissues/cells are removed from the body, and thus, results may not necessarily translate to the in vivo condition. Nonetheless, all models have positive and negative aspects and can each help provide insight into the etiology and pathogenesis of elbow conditions.
Considerations for experimental outcomes and model variables
An essential aspect of any elbow preclinical model is the experimental outcomes used to assess a model's ability to mimic aspects of elbow pathologies. Importantly, it is critical for these outcome measures to be well defined and in-depth enough to understand the disease process, but also universal enough and not too specialized, such that outcome measures can be compared across research laboratories. Outcome measures can range from the molecular and cellular level to the tissue and functional level; such a multi-level approach will allow for easier translation to the clinic. Preclinical studies should continue to assess these outcome measures but implement additional approaches that have not been applied to the elbow. For instance, non-invasive imaging, such as micro-computed tomography, magnetic resonance, and ultrasound techniques, could be used to assess structural and compositional changes in the elbow. Additionally, local and systemic biochemical outcome measures could be defined, such as the establishment of synovial fluid and serum biomarkers of elbow pathology.
A limitation of this overview relates to the obvious challenge to discuss every type of elbow pathology and potential confounding variables in detail. The effects of species sex, age, and other conditions, such as diabetes, joint dysplasia, changes to the microbiome, and sedentary versus active lifestyle, could impact the progression of elbow pathologies and are worth considering. Furthermore, it’s worthwhile to consider clinical complications with post-surgical treatments, implants, devices, and infection.
Conclusion
The human elbow is one of the most complicated musculoskeletal joints and critical for many daily activities that require the upper extremity; yet, to date, the elbow is a relatively understudied musculoskeletal joint. Due to its complex nature, the elbow has high susceptibility to injury and pathologies, such as joint contracture, stiffness, and arthritis. Unfortunately, limited therapies exist to treat elbow pathologic conditions, which only offer limited clinical success. To develop alternative therapeutic strategies for elbow conditions, it is essential to understand the etiology and pathogenesis of common elbow pathologies better; preclinical models serve as ideal alternatives to pursue such topics. This review has highlighted major clinical elbow diseases and the preclinical models currently available to recapitulate these diseases, while also providing recommendations for future preclinical models. Overall, this review could serve as the foundation for preclinical models to study the etiology and pathogenesis of elbow pathologies with the goal of better understanding elbow function and joint health, and for developing therapeutic intervention strategies to improve treatment of elbow conditions.
Acknowledgments
Funding: This review was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR071444.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Spencer Lake) for the series “Emerging Trends in Elbow Injury, Pathology and Treatment” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj.2020.02.09). The series “Emerging Trends in Elbow Injury, Pathology and Treatment” was commissioned by the editorial office without any funding or sponsorship. MAD reports grants from National Institutes of Health, during the conduct of the study; AMC reports grants from Zimmer, grants from National Institute of Health, personal fees from DePuy, personal fees from Arthrex, personal fees from Wright Medical, outside the submitted work; SP served as the unpaid Guest Editor of the series and reports grants from National Institutes of Health, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- King GJW, Morrey BF, An KN. Stabilizers of the elbow. J Shoulder Elbow Surg 1993;2:165-74. [Crossref] [PubMed]
- Safran MR, Baillargeon D. Soft-tissue stabilizers of the elbow. J Shoulder Elbow Surg 2005;14:179S-85S. [Crossref] [PubMed]
- Cohen MS, Schimmel DR, Masuda K, et al. Structural and biochemical evaluation of the elbow capsule after trauma. J Shoulder Elbow Surg 2007;16:484-90. [Crossref] [PubMed]
- Martin S, Sanchez E. Anatomy and biomechanics of the elbow joint. Semin Musculoskelet Radiol 2013;17:429-36. [Crossref] [PubMed]
- Chalmers PN, Chamberlain AM. Biomechanics of the Elbow. Unstable Elb., Cham: Springer International Publishing, 2017:13-26.
- LaStayo PC, Lee MJ. The Forearm Complex: Anatomy, Biomechanics and Clinical Considerations. J Hand Ther 2006;19:137-44. [Crossref] [PubMed]
- Kapandji A. Biomechanics of pronation and supination of the forearm. Hand Clin 2001;17:111-22. vii. [PubMed]
- Alcid JG, Ahmad CS, Lee TQ. Elbow anatomy and structural biomechanics. Clin Sports Med 2004;23:503-17. [Crossref] [PubMed]
- An KN, Hui FC, Morrey BF, et al. Muscles across the elbow joint: A biomechanical analysis. J Biomech 1981;14:659-61, 663-9. [Crossref] [PubMed]
- Gardner E. The innervation of the elbow joint. Anat Rec 1948;102:161-74. [Crossref] [PubMed]
- Bekler H, Riansuwan K, Vroeman JC, et al. Innervation of the Elbow Joint and Surgical Perspectives of Denervation: A Cadaveric Anatomic Study. J Hand Surg Am 2008;33:740-5. [Crossref] [PubMed]
- Cavalheiro CS, Filho MR, Rozas J, et al. Anatomical study on the innervation of the elbow capsule. Rev Bras Ortop 2015;50:673-9. [Crossref] [PubMed]
- Nourbakhsh A, Hirschfeld AG, Schlatterer DR, et al. Innervation of the elbow joint: A cadaveric study. J Hand Surg Am 2016;41:85-90. [Crossref] [PubMed]
- Yamaguchi K, Sweet FA, Bindra R, et al. The Extraosseous and Intraosseous Arterial Anatomy of the Adult Elbow. J Bone Joint Surg Am 1997;79:1653-62. [Crossref] [PubMed]
- Fornalski S, Gupta R, Lee TQ. Anatomy and Biomechanics of the Elbow Joint. Tech Hand Up Extrem Surg 2003;7:168-78. [Crossref] [PubMed]
- Morrey BF, Askew LJ, Chao EY. A biomechanical study of normal functional elbow motion. J Bone Joint Surg Am 1981;63:872-7. [Crossref] [PubMed]
- Heijink A, Vanhees M, van den Ende K, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the elbow. Knee Surg Sports Traumatol Arthrosc 2016;24:2313-8. [Crossref] [PubMed]
- Kroonen LT, Piper SL, Ghatan AC. Arthroscopic Management of Elbow Osteoarthritis. J Hand Surg Am 2017;42:640-50. [Crossref] [PubMed]
- Whishaw IQ, Gorny B, Foroud A, et al. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav Brain Res 2003;145:221-32. [Crossref] [PubMed]
- Sacrey L-AR, Alaverdashvili M, Whishaw IQ. Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav Brain Res 2009;204:153-61. [Crossref] [PubMed]
- Charalambous CP, Morrey BF. Posttraumatic Elbow Stiffness. J Bone Joint Surg Am 2012;94:1428-37. [Crossref] [PubMed]
- Mellema JJ, Lindenhovius ALC, Jupiter JB. The posttraumatic stiff elbow: an update. Curr Rev Musculoskelet Med 2016;9:190-8. [Crossref] [PubMed]
- Monument MJ, Hart DA, Salo PT, et al. Posttraumatic elbow contractures: targeting neuroinflammatory fibrogenic mechanisms. J Orthop Sci 2013;18:869-77. [Crossref] [PubMed]
- Myden C, Hildebrand K. Elbow joint contracture after traumatic injury. J Shoulder Elbow Surg 2011;20:39-44. [Crossref] [PubMed]
- Englert C, Zellner J, Koller M, et al. Elbow Dislocations: A Review Ranging from Soft Tissue Injuries to Complex Elbow Fracture Dislocations. Adv Orthop 2013;2013:951397. [Crossref] [PubMed]
- Lindenhovius ALC, Jupiter JB. The Posttraumatic Stiff Elbow: A Review of the Literature. J Hand Surg Am 2007;32:1605-23. [Crossref] [PubMed]
- Bruno RJ, Lee ML, Strauch RJ, et al. Posttraumatic elbow stiffness: evaluation and management. J Am Acad Orthop Surg 2002;10:106-16. [Crossref] [PubMed]
- Lu X, Yan G, Lu M, et al. Epidemiologic features and management of elbow dislocation with associated fracture in pediatric population. Medicine (Baltimore) 2017;96:e8595. [Crossref] [PubMed]
- Andelman S, DiPrinzio E, Hausman M. Elbow injuries in the pediatric athlete. Ann Joint 2018;3:21. [Crossref]
- Schippinger G, Seibert FJ, Steinböck J, et al. Management of simple elbow dislocations: Does the period of immobilization affect the eventual results? Langenbecks Arch Surg 1999;384:294-7. [Crossref] [PubMed]
- Wong K, Trudel G, Laneuville O. Noninflammatory Joint Contractures Arising from Immobility: Animal Models to Future Treatments. Biomed Res Int 2015;2015:848290. [Crossref] [PubMed]
- Gallay SH, Richards RR, O’Driscoll SW. Intraarticular capacity and compliance of stiff and normal elbows. Arthroscopy 1993;9:9-13. [Crossref] [PubMed]
- Hildebrand KA. Posttraumatic Elbow Joint Contractures: Defining Pathologic Capsular Mechanisms and Potential Future Treatment Paradigms. J Hand Surg Am 2013;38:2227-33. [Crossref] [PubMed]
- Mattyasovszky SG, Hofmann A, Brochhausen C, et al. The effect of the pro-inflammatory cytokine tumor necrosis factor-alpha on human joint capsule myofibroblasts. Arthritis Res Ther 2010;12:R4. [Crossref] [PubMed]
- Germscheid NM, Hildebrand KA. Regional Variation Is Present in Elbow Capsules after Injury. Clin Orthop Relat Res 2006.219-24. [PubMed]
- Usher KM, Zhu S, Mavropalias G, et al. Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res 2019;7:9. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, van Snellenberg W, et al. Myofibroblast Numbers are Elevated in Human Elbow. Clin Orthop Relat Res 2004.189-97. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Hart DA. High Rate of Joint Capsule Matrix Turnover in Chronic Human Elbow Contractures. Clin Orthop Relat Res 2005.228-34. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Hart DA. Myofibroblast Upregulators are Elevated in Joint Capsules in Posttraumatic Contractures. Clin Orthop Relat Res 2007.85-91. [Crossref] [PubMed]
- Doornberg JN, Bosse T, Cohen MS, et al. Temporary Presence of Myofibroblasts in Human Elbow Capsule After Trauma. J Bone Joint Surg Am 2014;96:e36. [Crossref] [PubMed]
- Hildebrand KA, Sutherland C, Zhang M. Rabbit knee model of post-traumatic joint contractures: The long-term natural history of motion loss and myofibroblasts. J Orthop Res 2004;22:313-20. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Hart DA. Joint capsule mast cells and neuropeptides are increased within four weeks of injury and remain elevated in chronic stages of posttraumatic contractures. J Orthop Res 2008;26:1313-9. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Hart DA. Joint capsule matrix turnover in a rabbit model of chronic joint contractures: Correlation with human contractures. J Orthop Res 2006;24:1036-43. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Germscheid NM, et al. Cellular, matrix, and growth factor components of the joint capsule are modified early in the process of posttraumatic contracture formation in a rabbit model. Acta Orthop 2008;79:116-25. [Crossref] [PubMed]
- Hildebrand KA, Holmberg M, Shrive N. A New Method to Measure Post-Traumatic Joint Contractures in the Rabbit Knee. J Biomech Eng 2003;125:887. [Crossref] [PubMed]
- Nesterenko S, Morrey ME, Abdel MP, et al. New rabbit knee model of posttraumatic joint contracture: Indirect capsular damage induces a severe contracture. J Orthop Res 2009;27:1028-32. [Crossref] [PubMed]
- Abdel MP, Morrey ME, Barlow JD, et al. Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J Orthop Res 2012;30:713-9. [Crossref] [PubMed]
- Abdel MP, Morrey ME, Grill DE, et al. Effects of joint contracture on the contralateral unoperated limb in a rabbit knee contracture model: A biomechanical and genetic study. J Orthop Res 2012;30:1581-5. [Crossref] [PubMed]
- Li F, Liu S, Fan C. Lentivirus-mediated ERK2 siRNA reduces joint capsule fibrosis in a rat model of post-traumatic joint contracture. Int J Mol Sci 2013;14:20833-44. [Crossref] [PubMed]
- Sun Y, Li F, Fan C. Effect of pERK2 on extracellular matrix turnover of the fibrotic joint capsule in a post-traumatic joint contracture model. Exp Ther Med 2016;11:547-52. [Crossref] [PubMed]
- Baranowski A, Schlemmer L, Förster K, et al. A novel rat model of stable posttraumatic joint stiffness of the knee. J Orthop Surg Res 2018;13:185. [Crossref] [PubMed]
- Lake SP, Castile RM, Borinsky S, et al. Development and use of an animal model to study post-traumatic stiffness and contracture of the elbow. J Orthop Res 2016;34:354-64. [Crossref] [PubMed]
- Dunham CL, Castile RM, Havlioglu N, et al. Persistent motion loss after free joint mobilization in a rat model of post-traumatic elbow contracture. J Shoulder Elbow Surg 2017;26:611-8. [Crossref] [PubMed]
- Dunham CL, Castile RM, Chamberlain AM, et al. Pronation-Supination Motion Is Altered in a Rat Model of Post-Traumatic Elbow Contracture. J Biomech Eng 2017;139:1-7. [Crossref] [PubMed]
- Dunham CL, Castile RM, Havlioglu N, et al. Temporal Patterns of Motion in Flexion-extension and Pronation-supination in a Rat Model of Posttraumatic Elbow Contracture. Clin Orthop Relat Res 2018;476:1878-89. [Crossref] [PubMed]
- Dunham CL, Chamberlain AM, Meyer GA, et al. Muscle does not drive persistent posttraumatic elbow contracture in a rat model. Muscle and Nerve 2018;58:843-51. [Crossref] [PubMed]
- Dunham CL, Castile RM, Chamberlain AM, et al. The Role of Periarticular Soft Tissues in Persistent Motion Loss in a Rat Model of Posttraumatic Elbow Contracture. J Bone Joint Surg Am 2019;101:e17. [Crossref] [PubMed]
- Reiter A, Kivitz GJ, Castile RM, et al. Functional Measures of Grip Strength and Gait Remain Altered Long-Term in a Rat Model of Post-Traumatic Elbow Contracture. J Biomech Eng 2019;141:071001. [Crossref] [PubMed]
- Moore-Lotridge SN, Oelsner WK, Ihejirika Y, et al. Novel preclinical murine model of trauma-induced elbow stiffness. J Exp Orthop 2018;5:36. [Crossref] [PubMed]
- Hildebrand KA, Zhang M, Befus AD, et al. A myofibroblast-mast cell-neuropeptide axis of fibrosis in post-traumatic joint contractures: An in vitro analysis of mechanistic components. J Orthop Res 2014;32:1290-6. [Crossref] [PubMed]
- Monument MJ, Hart DA, Salo PT, et al. Neuroinflammatory Mechanisms of Connective Tissue Fibrosis: Targeting Neurogenic and Mast Cell Contributions. Adv Wound Care (New Rochelle) 2015;4:137-51. [Crossref] [PubMed]
- Mattyasovszky SG, Mausbach S, Ritz U, et al. Influence of the anti-inflammatory cytokine interleukin-4 on human joint capsule myofibroblasts. J Orthop Res 2017;35:1290-8. [Crossref] [PubMed]
- Mattyasovszky SG, Mausbach S, Ritz U, et al. Cytokine Interferon-γ suppresses the function of capsule myofibroblasts and induces cell apoptosis. J Orthop Res 2017;35:2524-33. [Crossref] [PubMed]
- Inagaki K. Current concepts of elbow-joint disorders and their treatment. J Orthop Sci 2013;18:1-7. [Crossref] [PubMed]
- Fedorczyk JM. Tennis Elbow: Blending Basic Science with Clinical Practice. J Hand Ther 2006;19:146-53. [Crossref] [PubMed]
- Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am 1999;81:259-78. [Crossref] [PubMed]
- Waseem M, Nuhmani S, Ram CS, et al. Lateral epicondylitis: A review of the literature. J Back Musculoskelet Rehabil 2012;25:131-42. [Crossref] [PubMed]
- Linnman C, Catana C, Svardsudd K, et al. Decreased brain neurokinin-1 receptor availability in chronic tennis elbow. PLoS One 2016;11:e0161563. [Crossref] [PubMed]
- Fedorczyk JM. Tendinopathies of the elbow, wrist, and hand: Histopathology and clinical considerations. J Hand Ther 2012;25:191-200. [Crossref] [PubMed]
- Lee HS, Park HY, Yoon JO, et al. Musicians’ Medicine: Musculoskeletal Problems in String Players. Clin Orthop Surg 2013.155-60. [Crossref] [PubMed]
- Nakama LH, King KB, Abrahamsson S, et al. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res 2005;23:1199-205. [Crossref] [PubMed]
- Driban JB, Barr AE, Amin M, et al. Joint Inflammation and Early Degeneration Induced by High-Force Reaching Are Attenuated by Ibuprofen in an Animal Model of Work-Related Musculoskeletal Disorder. J Biomed Biotechnol 2011;2011:691412. [Crossref] [PubMed]
- Warden SJ. Animal models for the study of tendinopathy. Br J Sports Med 2007;41:232-40. [Crossref] [PubMed]
- Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil 2008;30:1530-41. [Crossref] [PubMed]
- Dirks RC, Warden SJ. Models for the study of tendinopathy. J Musculoskelet Neuronal Interact 2011;11:141-9. [PubMed]
- Guitton TC, Ring D. Elbow arthritis. Curr Orthop Pract 2008;19:626-32. [Crossref]
- Wysocki RW, Cohen MS. Primary Osteoarthritis and Posttraumatic Arthritis of the Elbow. Hand Clin 2011;27:131-7. [Crossref] [PubMed]
- Heijink A, Gomoll AH, Madry H, et al. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 2012;20:423-35. [Crossref] [PubMed]
- Ravalli S, Pulici C, Binetti S, et al. An Overview of the Pathogenesis and Treatment of Elbow Osteoarthritis. J Funct Morphol Kinesiol 2019;4:30. [Crossref]
- Cushnaghan J, Dieppe P. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis 1991;50:8-13. [Crossref] [PubMed]
- Gramstad GD, Galatz LM. Management of Elbow Osteoarthritis. J Bone Joint Surg Am 2006;88:421-30. [Crossref] [PubMed]
- Doherty M, Preston B. Primary osteoarthritis of the elbow. Ann Rheum Dis 1989;48:743-7. [Crossref] [PubMed]
- Morrey BF. Primary degenerative arthritis of the elbow. Treatment by ulnohumeral arthroplasty. J Bone Joint Surg Br 1992;74:409-13. [Crossref] [PubMed]
- Chammas M. Post-traumatic osteoarthritis of the elbow. Orthop Traumatol Surg Res 2014;100:S15-24. [Crossref] [PubMed]
- Sears BW, Puskas GJ, Morrey ME, et al. Posttraumatic elbow arthritis in the young adult: Evaluation and management. J Am Acad Orthop Surg 2012;20:704-14. [Crossref] [PubMed]
- Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res 2011;29:802-9. [Crossref] [PubMed]
- Brown TD, Johnston RC, Saltzman CL, et al. Posttraumatic Osteoarthritis: A First Estimate of Incidence, Prevalence, and Burden of Disease. J Orthop Trauma 2006;20:739-44. [Crossref] [PubMed]
- McAuliffe JA. Surgical alternatives for elbow arthritis in the young adult. Hand Clin 2002;18:99-111. [Crossref] [PubMed]
- Rivera JC, Wenke JC, Buckwalter JA, et al. Posttraumatic Osteoarthritis Caused by Battlefield Injuries. J Am Acad Orthop Surg 2012;20:S64-9. [Crossref] [PubMed]
- Bilic R, Kolundzic R, Bicanic G, et al. Elbow arthrodesis after war injuries. Mil Med 2005;170:164-6. [PubMed]
- Studer A, Athwal GS. Rheumatoid Arthritis of the Elbow. Hand Clin 2011;27:139-50. [Crossref] [PubMed]
- Kauffman JI, Chen AL, Stuchin S, et al. Surgical Management of the Rheumatoid Elbow. J Am Acad Orthop Surg 2003;11:100-8. [Crossref] [PubMed]
- Kirk RK, Jørgensen B, Jensen HE. The impact of elbow and knee joint lesions on abnormal gait and posture of sows. Acta Vet Scand 2008;50:5. [Crossref] [PubMed]
- Freire M, Meuten D, Lascelles D. Pathology of Articular Cartilage and Synovial Membrane From Elbow Joints With and Without Degenerative Joint Disease in Domestic Cats. Vet Pathol 2014;51:968-78. [Crossref] [PubMed]
- Ryan JM, Lascelles BDX, Benito J, et al. Histological and molecular characterisation of feline humeral condylar osteoarthritis. BMC Vet Res 2013;9:110. [Crossref] [PubMed]
- Freire M, Robertson I, Bondell HD, et al. Radiographic Evaluation of Feline Appendicular Degenerative Joint Disease vs. Macroscopic Appearance of Articular Cartilage. Vet Radiol Ultrasound 2011;52:239-47. [Crossref] [PubMed]
- Lascelles BDX, Henry JB III, Brown J, et al. Cross-Sectional Study of the Prevalence of Radiographic Degenerative Joint Disease in Domesticated Cats. Vet Surg 2010;39:535-44. [Crossref] [PubMed]
- Kapatkin AS, Tomasic M, Beech J, et al. Effects of electrostimulated acupuncture on ground reaction forces and pain scores in dogs with chronic elbow joint arthritis. J Am Vet Med Assoc 2006;228:1350-4. [Crossref] [PubMed]
- Spahni AI, Schawalder P, Rothen B, et al. Immunohistochemical Localization of RANK, RANKL and OPG in Healthy and Arthritic Canine Elbow Joints. Vet Surg 2009;38:780-6. [Crossref] [PubMed]
- Clements DN, Fitzpatrick N, Carter SD, et al. Cartilage gene expression correlates with radiographic severity of canine elbow osteoarthritis. Vet J 2009;179:211-8. [Crossref] [PubMed]
- Alves JC, Santos AM. Evaluation of the Effect of Mesotherapy in the Management of Osteoarthritis-Related Pain in a Police Working Dog Using the Canine Brief Pain Inventory. Top Companion Anim Med 2017;32:41-3. [Crossref] [PubMed]
- Hurlbeck C, Einspanier R, Pfeil I, et al. Evaluation of biomarkers for osteoarthritis caused by fragmented medial coronoid process in dogs. Res Vet Sci 2014;96:429-35. [Crossref] [PubMed]
- Goldhammer MA, Smith SH, Fitzpatrick N, et al. A comparison of radiographic, arthroscopic and histological measures of articular pathology in the canine elbow joint. Vet J 2010;186:96-103. [Crossref] [PubMed]
- Kapatkin AS, Nordquist B, Garcia T, et al. Effect of single dose radiation therapy on weight-bearing lameness in dogs with elbow osteoarthritis. Vet Comp Orthop Traumatol 2016;29:338-43. [Crossref] [PubMed]
- Kunst CM, Pease AP, Nelson NC, et al. Computed Tomographic Identification of Dysplasia and Progression of Osteoarthritis in Dog Elbows Previously Assigned OFA Grades. Vet Radiol Ultrasound 2014;55:511-20. [Crossref] [PubMed]
- Bockstahler BA, Vobornik A, Müller M, et al. Compensatory load redistribution in naturally occurring osteoarthritis of the elbow joint and induced weight-bearing lameness of the forelimbs compared with clinically sound dogs. Vet J 2009;180:202-12. [Crossref] [PubMed]
- Lee KCL, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone 2002;31:407-12. [Crossref] [PubMed]
- Zhang P, Yokota H. Elbow loading promotes longitudinal bone growth of the ulna and the humerus. J Bone Miner Metab 2012;30:31-9. [Crossref] [PubMed]
- Sun HB, Zhao L, Tanaka S, et al. Moderate joint loading reduces degenerative actions of matrix metalloproteinases in the articular cartilage of mouse ulnae. Connect Tissue Res 2012;53:180-6. [Crossref] [PubMed]
- Pelletier JP, Kapoor M, Martel-Pelletier J. Animal models of osteoarthritis. Rheumatol Sixth Ed 2014;2-2:1454-61.
- McCoy AM. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet Pathol 2015;52:803-18. [Crossref] [PubMed]
- Cope PJ, Ourradi K, Li Y, et al. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage 2019;27:230-9. [Crossref] [PubMed]
- Blaker CL, Clarke EC, Little CB. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J Orthop Res 2017;35:424-39. [Crossref] [PubMed]
- Christiansen BA, Guilak F, Lockwood KA, et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015;23:1627-38. [Crossref] [PubMed]
- Dekkers JS, Schoones JW, Huizinga TW, et al. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: a systematic literature review and meta-analysis. Ann Rheum Dis 2017;76:458-67. [Crossref] [PubMed]
- Caplazi P, Baca M, Barck K, et al. Mouse Models of Rheumatoid Arthritis. Vet Pathol 2015;52:819-26. [Crossref] [PubMed]
- Hegen M, Keith JC, Collins M, et al. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis 2008;67:1505-15. [Crossref] [PubMed]
- Asquith DL, Miller AM, McInnes IB, et al. Animal models of rheumatoid arthritis. Eur J Immunol 2009;39:2040-4. [Crossref] [PubMed]
- Johnson CI, Argyle DJ, Clements DN. In vitro models for the study of osteoarthritis. Vet J 2016;209:40-9. [Crossref] [PubMed]
- Novakofski KD, Berg LC, Bronzini I, et al. Joint-dependent response to impact and implications for post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015;23:1130-7. [Crossref] [PubMed]
- Tang J, Xu M, Wu W, et al. Treatment and Rehabilitation of Knee Joints Straight Stiffness After Burns. Indian J Surg 2015;77:1088-93. [Crossref] [PubMed]
- Nguyen A V, Soulika AM. The Dynamics of the Skin’s Immune System. Int J Mol Sci 2019;20:1811. [Crossref] [PubMed]
- Medina A, Shankowsky H, Savaryn B, et al. Characterization of Heterotopic ossification in burn patients. J Burn Care Res 2014;35:251-6. [Crossref] [PubMed]
- Balumuka DD, Galiwango GW, Alenyo R. Recurrence of post burn contractures of the elbow and shoulder joints: experience from a ugandan hospital. BMC Surg 2015;15:103. [Crossref] [PubMed]
- Manske MC, Hanel DP. Postburn Contractures of the Elbow and Heterotopic Ossification. Hand Clin 2017;33:375-88. [Crossref] [PubMed]
- Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014;71:3241-55. [Crossref] [PubMed]
- Lim YW, van Riet RP, Mittal R, et al. Pattern of osteophyte distribution in primary osteoarthritis of the elbow. J Shoulder Elbow Surg 2008;17:963-6. [Crossref] [PubMed]
- Nishiwaki M, Willing R, Johnson JA, et al. Identifying the Location and Volume of Bony Impingement in Elbow Osteoarthritis by 3-Dimensional Computational Modeling. J Hand Surg Am 2013;38:1370-6. [Crossref] [PubMed]
- Kawanishi Y, Miyake J, Omori S, et al. The association between cubital tunnel morphology and ulnar neuropathy in patients with elbow osteoarthritis. J Shoulder Elbow Surg 2014;23:938-45. [Crossref] [PubMed]
- Hastings H, Graham TJ. The classification and treatment of heterotopic ossification about the elbow and forearm. Hand Clin 1994;10:417-37. [PubMed]
- Cholok D, Chung MT, Ranganathan K, et al. Heterotopic ossification and the elucidation of pathologic differentiation. Bone 2018;109:12-21. [Crossref] [PubMed]
- Meyers C, Lisiecki J, Miller S, et al. Heterotopic Ossification: A Comprehensive Review. JBMR Plus 2019;3:e10172. [Crossref] [PubMed]
- Foruria AM, Augustin S, Morrey BF, et al. Heterotopic ossification after surgery for fractures and fracture-dislocations involving the proximal aspect of the radius or ulna. J Bone Joint Surg Am 2013;95:e66. [Crossref] [PubMed]
- van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage 2007;15:237-44. [Crossref] [PubMed]
- van Bergen CJA, van den Ende KIM, ten Brinke B, et al. Osteochondritis dissecans of the capitellum in adolescents. World J Orthop 2016;7:102-8. [Crossref] [PubMed]
- van den Ende KIM, McIntosh AL, Adams JE, et al. Osteochondritis Dissecans of the Capitellum: A Review of the Literature and a Distal Ulnar Portal. Arthroscopy 2011;27:122-8. [Crossref] [PubMed]
- Maruyama M, Takahara M, Satake H. Diagnosis and treatment of osteochondritis dissecans of the humeral capitellum. J Orthop Sci 2018;23:213-9. [Crossref] [PubMed]
- Pfeifer CG, Kinsella SD, Milby AH, et al. Development of a large animal model of osteochondritis dissecans of the knee: A pilot study. Orthop J Sports Med 2015;3:2325967115570019. [Crossref] [PubMed]
- Tóth F, Johnson CP, Mills B, et al. Evaluation of the Suitability of Miniature Pigs as an Animal Model of Juvenile Osteochondritis Dissecans. J Orthop Res 2019;37:2130-7. [Crossref] [PubMed]
- Wang L, Nissi MJ, Toth F, et al. Quantitative susceptibility mapping detects abnormalities in cartilage canals in a goat model of preclinical osteochondritis dissecans. Magn Reson Med 2017;77:1276-83. [Crossref] [PubMed]
- Schub DL, Frisch NC, Bachmann KR, et al. Mapping of Cartilage Depth in the Knee and Elbow for Use in Osteochondral Autograft Procedures. Am J Sports Med 2013;41:903-7. [Crossref] [PubMed]
- Shin R, Ring D. The Ulnar Nerve in Elbow Trauma. J Bone Joint Surg Am 2007;89:1108-16. [Crossref] [PubMed]
- Isaacs J, Ugwu-Oju O. High Median Nerve Injuries. Hand Clin 2016;32:339-48. [Crossref] [PubMed]
- Pederson WC. Median Nerve Injury and Repair. J Hand Surg Am 2014;39:1216-22. [Crossref] [PubMed]
- Blonna D, Wolf JM, Fitzsimmons JS, et al. Prevention of Nerve Injury During Arthroscopic Capsulectomy of the Elbow Utilizing a Safety-Driven Strategy. J Bone Joint Surg Am 2013;95:1373-81. [Crossref] [PubMed]
- Wiberg M, Widenfalk B. An anatomical study of the origin of sympathetic and sensory innervation of the elbow and knee joint in the monkey. Neurosci Lett 1991;127:185-8. [Crossref] [PubMed]
- Baron R, Jänig W, With H. Sympathetic and afferent neurones projecting into forelimb and trunk nerves and the anatomical organization of the thoracic sympathetic outflow of the rat. J Auton Nerv Syst 1995;53:205-14. [Crossref] [PubMed]
- Widenfalk B, Elfvin L-G, Wiberg M. Origin of sympathetic and sensory innervation of the elbow joint in the rat: A retrograde axonal tracing study with wheat germ agglutinin conjugated horseradish peroxidase. J Comp Neurol 1988;271:313-8. [Crossref] [PubMed]
- Allen MJ, Hankenson KD, Goodrich L, et al. Ethical use of animal models in musculoskeletal research. J Orthop Res 2017;35:740-51. [Crossref] [PubMed]
Cite this article as: David MA, Chamberlain AM, Lake SP. Preclinical models of elbow injury and pathology. Ann Joint 2021;6:12.


