
Clinical and pathological perspectives on elbow arthritis and arthroplasty
Introduction
Total elbow joint replacement (TER) components were first developed for arthritis in the late 1960s at the same time as total joint replacement components for the hip, knee and shoulder.
There has been a continuing year on year increase in the numbers of total hip replacement (THR), total knee replacement (TKR) and total shoulder replacement (TSR) procedures performed since then but this has not been the case in the elbow. The annual numbers of TER’s carried out for arthritis has fallen particularly in European countries, since reaching a peak in the 1990s (1). The decline in the annual number of TERs performed in the UK is a concern of the British Elbow and Shoulder Society (BESS) which now supports the view that, for surgeons to be able to maintain an appropriate level of expertise, TER should be restricted to a relatively few surgeons working in regional centers.
The main indication for the development of TER in the 1970’s in Europe was to treat patients with rheumatoid arthritis (RA), particularly those who had developed severe erosive degenerative changes. Although estimates varied, most agreed that approximately half of all patients with RA had elbow joint involvement and in many the disease was bilateral (2).
Peterson and Jones when discussing surgery of the rheumatoid elbow in 1971 considered the elbow to be ‘merely a connecting joint between the hand and trunk, and restricted motion can be compensated for in other joints’ (3). We found however that this was not the case in our patients with severe elbow RA, most of whom reported considerable difficulties in performing the basic activities of daily life independently including washing, dressing, eating and attending to their own toileting needs.
Although arthrodesis was still considered in the 1970’s to be an option for treating severe degenerative changes in the other major limb joints, this was not a practical solution for the elbow as there is no single position of function in which to permanently fix the elbow joint. The importance of elbow movement in enabling an individual to live independently was clearly illustrated to us when talking with most of our patients with elbow RA. Whereas patients with arthritis involving the other major limb joints usually reported some loss of independence due to difficulties dressing and performing domestic tasks, those with elbow arthritis which compromised washing, eating and toileting reported loss of their dignity and self-esteem in addition. This of course was far more distressing and arguably therefore the need to develop components with which to replace an arthritic elbow joint was more pressing than that in the other major limb joints.
Nevertheless, many of the widely used designs of TER including the GSB, Souter-Strathclyde, Kudo and IBP, all of which proved capable of providing satisfactory clinical medium/long-term results in the 1980s/1990s (4-7) are no longer manufactured. Furthermore, the variety of TER implants manufactured and therefore available to orthopaedic surgeons has continued to reduce over recent years, although the performance of the currently available designs has not been demonstrated to be superior to those designs that have been discontinued (8).
The early developments in TER design
The hip and shoulder are both spheroidal (ball-and-socket) joints and consequently the shape of the articular surfaces of joint replacement components had also to be spheroidal in order to replicate the normal pattern of movement in the hip and shoulder joints. There was no debate about this, and it is perhaps therefore not surprising that the basic design of the components originally developed for hip and shoulder replacement first developed during the 1960s is identical to those used today.
The knee joint and elbow joints were both regarded as ‘hinge’ joints and consequently the components originally first developed in the 1960s for treating arthritis of the knee and elbow were designed as uniaxial hinges (9). However, a high incidence of early failure of these designs was then observed often accompanied by considerable loss and expansion of the cortical bone around the intramedullary stem of the components which consequently then presented technically difficult revision problems. By the beginning of the 1970’s it had become recognized that the design of both TKR and TER components had to replicate more closely the pattern of movement in the knee and the elbow, which is not that of a uniaxial hinge but also includes varus/valgus and rotatory movements (10).
TKR design then evolved throughout the 1970s from the original uniaxial hinges with a linking axle mechanism providing stability, briefly through unlinked ‘inlay’ type components which relied upon the knee ligaments for stability, before finally evolving into the condylar-shaped designs used today. This was a logical progression as condylar shaped components closely approximate to the anatomical shape of the articular surfaces of the knee and allow the natural rotatory movements to occur during flexion and extension of the joint with the minimum of constraint. These condylar shaped designs thereby avoided generating the rotational forces which had caused loosening of the original uniaxial designs of TKR.
No similar logical progression however occurred in the development of components for treating arthritis of the elbow during the 1970s. Although it had been generally agreed that the elbow joint could not be successfully replaced by a uniaxial hinge design of TER, opinion concerning the basic design needed for a successful TER however then diverged and there has been no reconciliation of opinion during the past 40 years.
Two distinct groups of TER designs began to be manufactured during the 1970s. Hinged (linked) designs, known as ‘sloppy-hinges’ comprising humeral and ulnar components linked by an axle mechanism which permitted a wider range of movements than the fully constrained original uniaxial hinge designs, were developed. Non-hinge (unlinked) designs in which the humeral and ulnar components were not linked by an axle mechanism were also developed.
An explanation for this divergence of opinion is that categorization of the elbow in terms of its anatomical and mechanical characteristics is not as simple as the knee which can be accurately categorized as a bi-condylar rotating hinge. Consequently, bi-condylar shaped components allowing flexion/extension together with a degree of rotation could be expected to closely replicate the normal pattern of knee joint movement, and this has subsequently proved to be the case. Categorizing the elbow joint in these terms is however less straightforward. The ulnohumeral articulation behaves mechanically as a hinge joint and the radiocapitellar articulation is anatomically a spheroidal (ball-and-socket) joint. Although the radiocapitellar joint (RCJ) has also been described as a ‘condyle-type’ joint (11) as it is constrained by the proximal radioulnar joint, thereby allowing only flexion/extension and pronation/supination. The proximal radioulnar articulation itself constitutes a separate ‘pivot joint’.
When inserting most of either the linked or unlinked designs of TER, degenerative changes in the RCJ were treated by excision of the radial head (RH), although some unlinked designs which included a RH replacement component notably the Sorbie-Questor (Wright Medical Technology Inc. Arlington, TN, USA), Pritchard ERS (DePuy, Warsaw, IN, USA) and Capitellocondylar (Codman and Shurtleff, Raynham, MA, USA) also became available. Nevertheless, none of the wide range of either linked or unlinked designs of TER which were developed during the 1970s and 1980s could be said to replicate both the anatomical and mechanical characteristics of the elbow joint as closely as had been achieved in the knee joint (Figure 1).

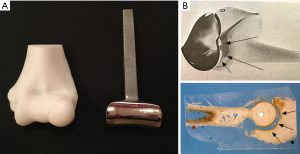
Experience of TER in our department began in the early 1980s with the Souter-Strathclyde design (Stryker, Howmedica, Osteonics Limerick). We chose this design because it appeared to us to resemble the normal anatomy of the ulnohumeral joint (UHJ) more closely than the other available designs (Figure 2). Furthermore, as it is an unlinked system in common with the designs of TKR which were being developed at the time we thought the Souter-Strathclyde TER was likely to provide a more normal pattern of elbow joint movement than the linked designs. The Souter-Strathclyde TER then provided satisfactory early results in our rheumatoid patients, but we remained concerned about the extent of bone resection required to insert the components at the time of surgery. We also later noted a uniform pattern of wear in the articular surfaces of the ulnar components of the Souter-Strathclyde TER we had retrieved during subsequent revision procedures. These components, manufactured from ultra-high molecular weight polyethylene (UHMP), demonstrated considerable wear of the UHMP on each side of the central ridge which itself remained well preserved. When these components were examined with a scanning electron microscope a pattern of surface wear was found which was consistent with a rocking motion between the humeral and ulnar components on elbow flexion and extension in response to varus/valgus forces (12) (Figure 3). We concluded that the articular surfaces of the components of the Souter-Strathclyde TER were too close-fitting and therefore the pattern of movement was essentially that of a uniaxial hinge.
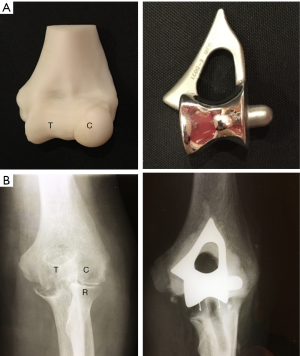
In 1989 we began to use the Kudo (MK IV) TER (Biomet UK Ltd., Bridgend, UK) which is also an unlinked design and had been demonstrated to provide satisfactory medium-long term results in RA (13). Although the Kudo TER did not resemble the normal external anatomical shape of the humeroulnar joint, we noted that the articular surfaces of the components were not as close-fitting as those of the Souter-Strathclyde TER. We thought that the articular surfaces of the Kudo TER closely replicated those of a series of normal elbow joints we had removed post-mortem and sectioned in the sagittal plane (Figure 4) and that this design would probably therefore allow a normal pattern of elbow movement to occur with the minimum of constraint (Figure 4).
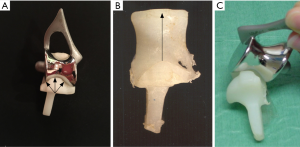
Our main concern about using linked designs of TER as a primary procedure was that the intramedullary stems of linked components are generally longer than those of the unlinked designs and would therefore be more difficult to remove during subsequent revision operations carried out for component wear and loosening. We considered the only advantage of linked designs of TER in comparison to unlinked designs was that linked designs are inherently more stable than the unlinked designs which rely to some extent upon the integrity of the soft tissue envelope for their stability. These tissues are often deficient, particularly in a rheumatoid elbow and dislocation of unlinked components was a recognized complication (14). We noted during surgery to insert Kudo TER components that aligning the humeral component parallel to the posterior cortex of the humerus, rather than the trans-epicondylar plane, reliably provided a stable concentric reduction of the components. We then investigated this intra-operative observation by carrying out a laboratory study on post-mortem material which demonstrated that the posterior humeral cortex proximal to the olecranon fossa is aligned with the isometric plane of elbow flexion and extension (15). This therefore provided a bony reference enabling us to develop instrumentation for bone preparation and component alignment which then led to the development of the instrumented bone preserving (IBP) TER (Biomet UK Ltd., Bridgend, UK) with articular surfaces identical to those of the Kudo TER (Figure 5). The IBP was subsequently demonstrated to be capable of providing the reliable stability we had postulated during its design (16).
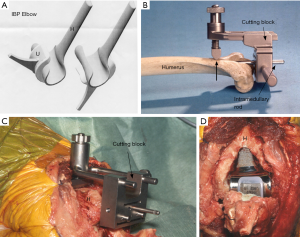
Both the linked and unlinked designs of TER however proved equally capable of providing satisfactory results (17,18) and the debate ‘linked versus unlinked’ designs for TER then continued throughout the 1990s.
TER developments in the 21st century
Although the debate concerning the most appropriate design for TER implants remained the same at the beginning of the 21st century as it had been for the previous 30 years developments have taken place since then which have profoundly changed our views.
- The pattern of the pathological changes in the elbows of the patients with arthritis referred to us for TER has changed significantly in recent years.
- Increasing use of the arthroscope has changed our understanding of the pattern and evolution of articular cartilage degeneration in osteoarthritis (OA).
Understanding the pattern and evolution of the degenerative changes in elbow arthritis
RA
The clinical onset of RA begins with pain and swelling due to synovitis, but radiographs at that stage usually demonstrate normal appearances. As the disease progresses subsequent radiographs reveal a degree of periarticular osteoporosis and perhaps some evidence of soft tissue changes before loss of the radiological joint space and characteristic subchondral bone erosions provide more definite evidence of RA. This if not effectively treated may then progress and result in ankylosis or, more commonly, loss of the normal bone architecture aptly described as ‘arthritis mutilans’ (Figure 6).
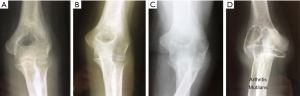
Lehtinen et al. prospectively studied the radiological course of elbow RA over a 15-year period (2). They pointed out that although most authors reported joint space narrowing as a typical radiological finding, opinion was divided on whether the disease process began in the ulnohumeral or radiocapitallar articulations and which was the more severely involved. They concluded that joint space narrowing is inevitable and occurs equally in both the ulnohumeral and RCJs, although they noted that only RCJ space narrowing was visible in the earlier stages, Larsen grade 1 disease (19). The bony erosions were most often observed on the capitellum (64% of elbows) and severe joint destruction was almost always bilateral. This predilection of rheumatoid disease for the RCJ had been reported earlier by others (20).
Although opinion had been divided about the exact pattern of the evolution of degenerative changes in the rheumatoid elbow, the impact of the newer disease-modifying drug for RA, etanercept and methotrexate for example, (21) was becoming increasingly obvious to us. By the mid-1990s we were rarely then seeing patients with severe erosive degenerative changes for whom the currently available TERs had provided a good predictable outcome, at least in the medium term. The newer drugs seemed to be very effective in preventing bone erosion, but they had not prevented loss of articular cartilage and consequent diminution of the radiological joint space in the patients referred to us for TER. We noted however that because the cortical bone outline had been preserved in these patients with treated RA, their radiographs were practically indistinguishable from those of patients with ‘hypotrophic’ OA, i.e., OA with little or no evidence of osteophyte formation.
By the end of the 1990s we were becoming aware that the results of TER in our patients with primary OA and treated RA were less satisfactory than in those patients with severe erosive RA we had treated in the past, and this observation was reported by others (22,23).
OA
There had been a general belief that the elbow joint is a ‘protected site’ against the development of primary (idiopathic) OA (24). Stanley examined radiographs of patients attending fracture clinics and concluded that the prevalence of primary OA of the elbow in the population was 2% (25). More recently however Oya et al. studied the prevalence of elbow OA in the ‘middle-aged’ population (mean age 67; range, 40–93 years) of a single village in Japan (26). Radiographic evidence of elbow OA was detected in 55% of the elbows and the prevalence of symptomatic OA was 22.6%, which we think is more in keeping with the size of the clinical problem we encounter in our practice rather than the previous much lower estimates of the prevalence of elbow OA.
Differences in opinion about the site of the development and progression of degenerative changes in elbow OA have been expressed, similarly to those about elbow RA. In the late 1960’s, Goodfellow and Bullough reported their observations of the articular surfaces of the elbows of elderly subjects examined post-mortem (27). They were surprised to find that extensive loss of the articular cartilage in the RCJ could be seen in subjects in whom the articular cartilage of the humeroulnar joint appeared to be entirely normal, and identical findings have since been reported by others (28-30).
This evidence that the earliest degenerative changes of elbow OA develop in the RCJ seems however to have been later discarded following a radiographic study reported by Minami (31) who concluded that elbow OA begins with osteophyte formation in the coronoid, coronoid fossa, olecranon and olecranon fossa. These observations then led to the development of the Outerbridge Kashiwagi (OK) procedure (32) and other variations of ‘ulnohumeral arthroplasty’ (33). Although these procedures proved satisfactory in some patients, in others the outcome was found to be unsatisfactory and some of these patients reported that their elbow pain was worse postoperatively (34). Forster et al. in 2001 classified the outcome as ‘good’ in only one-third of their patients following the OK procedure and postulated that pain felt at rest in the others had persisted because RCJ pathology was not treated in these operations (35).
When we reviewed a consecutive series of 117 arthroscopies on our patients with intrusive elbow pain but with little or no abnormality shown on radiographs we found degenerative changes in the articular cartilage in 68 elbows, and in 60 of these the RCJ surfaces only were involved, the humeroulnar joint articular surfaces were normal (36). The pattern of articular cartilage degeneration we had observed in our younger symptomatic patients was therefore the same as that first observed by Goodfellow and Bullough in their post-mortem studies on elderly subjects.
Because of our concerns about the relatively poor results of TER towards the end of the 1990’s, we had by then reverted to performing arthrolysis and debridement procedures rather than TER on painful, stiff osteoarthritic elbows. During these procedures we found that the pattern of articular cartilage degeneration was identical to that we had seen in the patients we had examined arthroscopically. Considerable loss of articular cartilage from the RCJ contrasted with relatively normal appearances of the UHJ. We found that this pattern of articular cartilage degeneration was present irrespective of the cause, either primary OA, post-traumatic OA (PTOA) or treated RA (Figure 7).
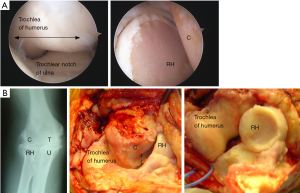
We would agree that it was entirely reasonable in the late 1970’s to infer the pattern of degenerative changes in elbow OA from the location of osteophytes seen on radiographs, as this would explain the impingement symptoms. However, as the very earliest degenerative changes in OA begin in articular cartilage and osteophytes develop as a secondary feature, we consider that the location of osteophytes in the elbow has proved to be misleading. The clinical and pathological evidence both now point to the conclusion that degenerative changes in the elbow in both RA and OA begin in the articular cartilage of the RCJ. The studies on post-mortem material indicate that degenerative changes may then remain confined to the RCJ in some patients, in others however the disease progresses to involve the articular surfaces of the humeroulnar joint.
An explanation for the pattern of degenerative change in the elbow joint
Radiological evidence would support the view that the degenerative changes in any synovial joint, to which we refer collectively as ‘arthritis’, begin in the hyaline cartilage covering the articular surfaces. Initially the degenerative process results in loss of the surface layers of the articular cartilage recognized on radiographs as narrowing of the ‘radiological joint space’. Further progression of the disease process may then lead to complete loss of articular cartilage, resulting in bone on bone contact and usually increasing levels of pain. Radiological evidence of the reaction of the underlying bone may also develop in addition to progressive loss of the radiological joint space. This is characterized by varying degrees of subchondral bone sclerosis, peripheral osteophytes and subchondral bone cyst formation. In those joints in which the bone reaction is pronounced, particularly osteophyte formation, the condition is categorized as ‘hypertrophic OA’ to differentiate it from those joints in which the radiological changes are largely confined to loss of the radiological joint space, categorized as ‘hypotrophic OA’.
The radiological changes may develop spontaneously with advancing age and the condition is then referred to as ‘primary’, ‘age-related’ or ‘idiopathic’ OA. Identical radiological changes can also occur as a result of trauma causing fractures or dislocations and the condition is then called ‘secondary’ OA or, more specifically, PTOA. Other causes of secondary OA include inflammatory arthropathies, the most common of which is rheumatoid disease.
As the articular cartilage degeneration progresses, inflammatory changes occur in the synovial membrane, the joint capsule, and intrinsic ligaments causing eventual fibrosis, thickening and contractures. This results in progressive joint stiffness and limitation of movement, which in the elbow joint characteristically causes progressive loss of extension, i.e., a fixed flexion contracture.
The question we have had to ask ourselves about the elbow however is why degenerative changes occur earlier and progress more rapidly in the RCJ rather than in the UHJ, particularly as the area of articular cartilage covering the UHJ surfaces is much greater than that of the RCJ?
We would suggest that the answer to this includes the facts that the RCJ transmits more load than the UHJ during normal activities, it is more vulnerable to trauma particularly impact injuries, and also the articular surfaces of the RCJ are moved much more than those of the UHJ.
It has long been established that when the elbow joint is extended and axially loaded, almost 60% of the load is borne by the RCJ despite its much smaller surface area than that of the UHJ through which to transmit loads (37).
As there is no direct bony contact between the wrist joint and the distal ulna, impact forces applied to the wrist as a result of falls onto the outstretched hand for example are inevitably transmitted directly to the radius and then along the radius to the surfaces of the RCJ. The vulnerability of the RCJ surfaces to trauma compared with the more closely constrained UHJ surfaces is illustrated by the fact that RH fractures account for approximately 33% of all elbow fractures (38).
It is self-evident that during flexion and extension of the elbow both the RCJ and UHJ surfaces move simultaneously through the same arc of motion. However, during activities which require pronation and supination (rotatory movements of the forearm and hand) only the articular surfaces of the RCJ move in relation to each other, the articular surfaces of the UHJ remain stationary.
Although we are aware of no published studies of elbow movement which would verify the fact that during the day-to-day activities we perform many more tasks which require pronation and supination than those requiring elbow flexion and extension, we believe that the reader would probably agree from personal experience that this is the case.
We think it is evident therefore that the articular surfaces of the RCJ are moved much more in relation to each other during our daily activities than those of the UHJ.
The articular surfaces of the RCJ are therefore required to bear greater loads, they are subjected to more impact trauma and they are required to move greater distances in relation to each other during the course of the day than those of the UHJ. It is perhaps therefore not surprising that when articular cartilage degeneration begins in the elbow joint, irrespective of the cause, the articular surfaces of the RCJ ‘wear out’ earlier than those of their ‘near neighbour’ the UHJ.
Why has TER proved to be more successful in severe RA than treated RA or OA?
We suggested above that perhaps the divergences of opinion about the design required for successful TER is that the elbow joint is not as simple to categorize in terms of its anatomical and mechanical characteristics as the other major limb joints. Whereas, this is true when the anatomy of the elbow is preserved, the pattern of bone destruction in severe RA however simplified matters considerably. In primary OA, OA secondary to trauma or treated RA, the normal bi-compartmental configuration of the elbow is preserved. This was not however the case in most of the elbows of our patients with untreated RA who underwent TER procedures. In these patients the disease process had resulted in erosion of the distal humerus to the extent that the definition between the trochlea and capitellum was lost. The RH was largely destroyed, and the ulna not only migrated proximally but also aligned with the long axis of the humerus. The elbow joint therefore had effectively become uni-compartmental and consequently could be appropriately treated by inserting either a TER designed as ‘sloppy hinge’ or an unlinked design in which the humeral component was aligned with the ulnar component (Figure 8).
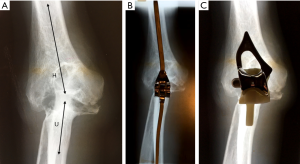
This would also explain why these designs of TER, when used to treat radiologically well-preserved elbow joints which still retain their normal bi-compartmental configuration comprising separate hinge (ulnohumeral), spheroidal (radiocapitellar) and pivot (proximal radioulnar) articulations, have proved to be less successful.
What is the future for TER?
Whereas the use of TER for arthritis surgery has declined over the past 20 years, the opposite has occurred in trauma surgery where there has been a gradual increase in the number of TERs used to treat distal humeral fractures, particularly in the elderly (39). Our review of literature published since the mid-1990s identified a reverse of opinion between that expressed by Ray et al. (40) who considered TER as a ‘last-ditch’ attempt and ‘salvage’ procedure, to that expressed by Argintar et al. (41) who considered that TER had become the ‘gold standard’. TER now seems therefore to have established a place in the treatment of elbow trauma. There is debate about whether it should be used as a hemiarthroplasty in the trauma setting, and we think it is likely that new designs of TER will now be developed specifically for fracture treatment.
Because of the relatively poor outcome of TER for arthritis surgery and a change in our understanding of the pathology of elbow arthritis we began to consider other implant solutions. Our experience of the ‘non-implant’ surgical procedures (arthrolysis/debridement) had been the same as those of the published studies which had reported disappointing and unpredictable outcomes, particularly in providing pain relief (42). We therefore set out to design components with which to resurface the RH and capitellum intended initially for our relatively young patients in whom arthroscopy had revealed complete loss of the articular cartilage from the RCJ. This led us to develop the lateral resurfacing elbow (LRE) arthroplasty (Formally Biomet Ltd., Bridgend, now LRE system Ltd., Oxon) which we began to use in 2005 and then combined with arthrolysis/debridement for patients with more advanced degenerative changes (Figure 9).
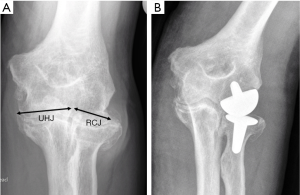
We were encouraged by our early results with the LRE arthroplasty (43) and further reassured when these were then replicated by other groups who performed the procedure on a wider range of patients including manual workers (44). Our early results with the LRE have been maintained in the longer term, up to 10 years (45), and this has also now been the experience of others (46). Consequently, the LRE has now replaced TER as our primary implant option for patients with arthritis other than those relatively rare patients we see with severe erosive RA.
Although ‘convertible’ designs of TER have been developed in recent years (e.g., the Latitiude, Tornier, Stafford, TX, USA) no exclusively unlinked designs are now commercially available and the number of linked designs has fallen to 3 or 4. A recent review of the Latitude TER [at a mean 4.7 (range: 1–7.5) years] following surgery reported complications in 33% of patients and revision procedures in 25%, but nevertheless concluded that it provides patients with favorable clinical outcomes and a complication rate comparable to other total elbow arthroplasty implants (8). More disturbingly however, Somerson and Matsen having reviewed the adverse events reports of the US Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database found that it revealed a ‘higher relative frequency of mechanical dissociation of elbow implants than what has been represented in the literature’ (47). We would agree therefore with the comments of colleagues that the decision to discontinue some designs of TER seems to have been made on commercial rather than on clinical grounds as there is no evidence that the newer currently commercially available designs of TER provide a better clinical outcome.
Summary and conclusions
The success of THR, TKR and TSR can be explained by the fact that it proved possible to develop a total joint replacement design which replaces the excised bone and closely replicated the normal anatomy of these joints. It has however not proved possible to develop a single total joint replacement design which replaces each of the very different articulations in the elbow and replicate their normal anatomy.
The pathological changes of OA appear to begin in the surface layers of the specialized hyaline cartilage which covers the articular surfaces of all synovial joints. Subsequent changes, including reaction of the subchondral bone and inflammatory changes in the synovial membrane and periarticular soft tissues then occur secondary to this.
We believe therefore that the ultimate goal in treating OA of the elbow is to find a way with which to resurface with viable hyaline cartilage those areas in which the articular cartilage has been lost, by either developing cartilage transplantation techniques or developing methods to stimulate the growth of healthy cartilage cells in situ within the degenerate areas.
Developing a ‘biological solution’ for the effects of elbow arthritis is not a new concept. Raunio in the mid-1980s when discussing surgical treatment for RA stated that ‘there are two competitors available: replacement arthroplasty and ‘auto-arthroplasty’ which he defined as using a patient’s own tissues or synthetic materials in the form of sheets in order to resurface the degenerate joint surfaces (48). Unfortunately, however the results of such ‘biological resurfacing’ procedures proved to be unpredictable and no satisfactory long-term results were reported (49).
At the present time we consider that there is a growing need to continue to develop replacement elbow implants for trauma and other conditions which result in loss of bone tissue. We think it is likely that future designs will more closely replicate normal elbow anatomy, perhaps based on pre-operative 3D CT imaging of each individual patient’s contralateral normal elbow joint. We also think it is likely that future designs will incorporate replaceable bearing surfaces in order to prevent the need to revise the stems of the primary components if these are found to have remained firmly fixed at the time of revision surgery. However, our experience of arthritis surgery has taught us to view the elbow differently. Rather than considering the humeroulnar and radiocapitellar articulations as simply the medial and lateral ‘compartments’ of the same joint, we now regard these as two distinctly different joints occupying the same synovial cavity. We think therefore it is likely that the emphasis for implant development in arthritis surgery will move away from replacement and towards resurfacing technologies in order to preserve the normal anatomical relationships of the separate articulations to which we refer collectively as ‘the elbow joint’.
We consider that the LRE system is a step in the right direction, but we anticipate continuing developments in both materials and resurfacing implant design.
However, the best material with which to resurface the worn surfaces of any synovial joint is of course hyaline cartilage and to achieve this will require a continuing collaborative effort involving not only orthopaedic surgeons and bioengineers but also cell biologists and related basic scientists.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Spencer P. Lake) for the series “Emerging Trends in Elbow Injury, Pathology and Treatment” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-19-178). The series “Emerging Trends in Elbow Injury, Pathology and Treatment” was commissioned by the editorial office without any funding or sponsorship. JP reports other from LRE systems Ltd., outside the submitted work. D Van der Linden reports non-financial support from LRE system Ltd., outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krukhaug Y, Hallan G, Dybvik E, et al. A survivorship study of 838 total elbow replacements: a report from the Norwegian Arthroplasty Register 1994-2016. J Shoulder Elbow Surg 2018;27:260-9. [Crossref] [PubMed]
- Lehtinen JT, Kaarela K, Belt EA, et al. Radiographic joint space in rheumatoid elbow joints. A 15 year prospective follow-up study in 74 patients. Rheumatology 2001;40:1141-5. [Crossref] [PubMed]
- Peterson LF, Janes JM. Surgery of the rheumatoid elbow. Orthop Clin North Am 1971;2:667-77. [PubMed]
- Cesar M., Roussanne Y, Bonnel F, et al. GSB III total elbow replacement in rheumatoid arthritis. J Bone Joint Surg Br 2007;89:330-4. [Crossref] [PubMed]
- Ikävalko M, Tiihonen R, Skyttä ET, et al. Long-term survival of the Souter-Strathclyde total elbow replacement in patients with rheumatoid arthritis. J Bone Joint Surg Br 2010;92:656-60. [Crossref] [PubMed]
- Qureshi F, Draviaraj KP, Stanley D. The Kudo 5 total elbow replacement in the treatment of the rheumatoid elbow: results at a minimum of ten years. J Bone Joint Surg Br 2010;92:1416-21. [Crossref] [PubMed]
- Kleinlugtenbelt IV, Bakx PA, Huij J. Instrumented bone preserving elbow in rheumatoid arthritis: 2-8 year follow up. J Shoulder Elbow Surg 2010;19:923-8. [Crossref] [PubMed]
- Cinats D, Bois AJ, Hildebrand KA. Clinical outcomes and complications following primary total elbow arthroplasty using the Latitude prosthesis. Shoulder and Elbow 2019;11:359-71. [Crossref] [PubMed]
- Dee R. Total arthroplasty of the elbow for rheumatoid arthritis. J Bone Joint Surg Br 1972;54:88-95. [Crossref] [PubMed]
- Morrey BF, Bryan RS. Complications of total elbow arthroplasty. Clin Orthop Relat Res 1982.204-12. [PubMed]
- Vaquero-Picado A, Nunez de Armas J, Antuna M, et al. Morphometry of the radiocapitellar joint: is humeral condyle diameter a reliable predictor of the radial head prosthesis? J Shoulder Elbow Surg 2018;27:1092-6. [Crossref] [PubMed]
- Robinson E, Burke N, Douglas P, et al. Mechanism of loosening in the Souter-Strathclyde total elbow replacement evidence from revision surgery. Acta Orthop Belg 2010;76:27-9. [PubMed]
- Kudo H, Iwano K. Total elbow arthroplasty with a non-constrained surface-replacement prosthesis in patients who have rheumatoid arthritis. A long-term follow-up study. J Bone Joint Surg Am 1990;72:355-62. [Crossref] [PubMed]
- Gschwend N, Simmen B, Matejovsky Z. Late complications in elbow arthroplasty. J Shoulder Elbow Surg 1996;5:86-96. [Crossref] [PubMed]
- Blewitt N, Pooley J. An anatomic study of the axis of elbow movement in the coronal plane: Reference to component alignment in elbow arthroplasty. J Shoulder Elbow Surg 1994;3:151-8. [Crossref] [PubMed]
- Kalogrianitis S, Sinopidis C, El Meligy M, et al. Unlinked elbow arthroplasty as primary treatment for fractures of the distal humerus. J Shoulder Elbow Surg 2008;17:287-92. [Crossref] [PubMed]
- Kelly EW, Coghlan J, Bell S. Five- to thirteen-year follow-up of the GSB III total elbow arthroplasty. J Shoulder Elbow Surg 2004;13:434-40. [Crossref] [PubMed]
- Malone AA, Taylor AJ, Fyfe IS. Successful outcome of the Souter-Strathclyde elbow arthroplasty. J Shoulder Elbow Surg 2004;13:548-554. [Crossref] [PubMed]
- Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh) 1977;18:481-91. [Crossref] [PubMed]
- Stein H, Dickson RA, Bentley G. Rheumatoid arthritis of the elbow. Pattern of joint involvement, and results of synovectomy with excision of the radial head. Ann Rheum Dis 1975;34:403-8. [Crossref] [PubMed]
- Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586-93. [Crossref] [PubMed]
- Wright TW, Hastings H. Total elbow arthroplasty failure due to overuse, C-ring failure, and/or bushing wear. J Shoulder Elbow Surg 2005;14:65-72. [Crossref] [PubMed]
- Schneeberger AG, Meyer DC, Yian EH. Coonrad-Morrey total elbow replacement for primary and revision surgery: a 2- to 7.5-year follow-up study. J Shoulder Elbow Surg 2007;16:S47-54. [Crossref] [PubMed]
- Doherty M, Preston B. Primary osteoarthritis of the elbow. Ann Rheum Dis 1989;48:743-7. [Crossref] [PubMed]
- Stanley D. Prevalence and etiology of symptomatic elbow osteoarthritis. J Shoulder Elbow Surg 1994;3:386-9. [Crossref] [PubMed]
- Oya N, Tajika T, Ichinose T, et al. The prevalence of osteoarthritis in Japanese middle-aged and elderly populations; the relationship between risk factors and function. J Shoulder Elbow Surg 2018;27:1086-91. [Crossref] [PubMed]
- Goodfellow JW, Bullough PG. The pattern of aging of the articular cartilage of the elbow joint. J Bone Joint Surg Br 1967;49:175-81. [Crossref] [PubMed]
- Murata H, Ikuta Y, Murakami T. An anatomic investigation of the elbow joint, with special reference to aging of the articular cartilage. J Shoulder Elbow Surg 1993;2:175-81. [Crossref] [PubMed]
- Debouck C, Rooze M. A topographical study of cartilaginous lesions to the elbow. Surg Radiol Anat 1995;17:301-5. [Crossref] [PubMed]
- Ahrens PM, Redfern DR, Forester AJ. Patterns of articular wear in the cadaveric elbow joint. J Shoulder Elbow Surg 2001;10:52-6. [Crossref] [PubMed]
- Minami M. Roentgenological studies of osteoarthritis of the elbow joint. J Jpn Orthop Assoc 1977;51:1223-6.
- Minami M, Kato S, Kashiwagi D, et al. Outerbridge-Kashiwagi's method for arthroplasty of osteoarthritis of the elbow—44 elbows followed for 8-16 years. J Orthop Sci 1996;1:11-5. [Crossref]
- Wada T, Isogai S, Ishii S, et al. Debridement arthroplasty for primary osteoarthritis of the elbow. J Bone Joint Surg Am 2004;86:233-41. [Crossref] [PubMed]
- Antuña SA, Morrey BF, Adams RA, et al. Ulnohumeral arthroplasty for primary degenerative arthritis of the elbow: long-term outcome and complications. J Bone Joint Surg Am 2002;84:2168-73. [Crossref] [PubMed]
- Forster MC, Clark DI, Lunn PG. Elbow osteoarthritis prognostic in ulnohumeral debridement-the Outerbridge-Kashiwagi procedure. J Shoulder Elbow Surg 2001;10:557-60. [Crossref] [PubMed]
- Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg 2009;75:37-40. [PubMed]
- Pooley J, Salvador Carreno J. Total elbow joint replacement for fractures in the elderly-functional and radiological outcomes. Injury 2015;46:S37-42. [Crossref] [PubMed]
- Halls AA, Travill A. Transmission of pressures across the elbow joint. Anat Rec 1964;150:243-7. [Crossref] [PubMed]
- Morrey BF. editor. The elbow and its disorders. 2nd ed. Philadelphia: WB Saunders, 1993:383.
- Ray PS, Kakarlapudi K, Rajsekhar C, et al. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. Injury 2000;31:687-92. [Crossref] [PubMed]
- Argintar E, Berry M, Narvy SJ, et al. Hemiarthroplasty for the treatment of distal humeral fractures: short-term clinical results. Orthopedics 2012;35:1042-5. [Crossref] [PubMed]
- Pooley J. Total elbow replacement - patient selection and perspectives. Orthop Res Rev 2019;11:23-40. [Crossref] [PubMed]
- Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. Tech Shoulder Elbow Surg 2007;8:204-12. [Crossref]
- Giannicola G, Angeloni R, Mantovani A, et al. Open debridement and radiocapitellar replacement in primary and post-traumatic arthritis of the elbow: a multicenter study. J Shoulder Elbow Surg 2012;21:456-63. [Crossref] [PubMed]
- Watkins CEL, Elson DW, Harrison JWK, et al. Long-term results of the lateral resurfacing elbow arthroplasty. Bone Joint J 2018;100-B:338-345. [Crossref] [PubMed]
- Giannicola G, Calella P, Bigazzi P, et al. Midterm results of radiocapitellar arthroplasty of the elbow. Bone Joint J 2019;101-B:1362-9. [Crossref] [PubMed]
- Somerson JS, Matsen FA. Timely recognition of total elbow and radial head arthroplasty adverse events: an analysis of reports to the US Food and Drug Administration. J Shoulder Elbow Surg 2019;28:510-9. [Crossref] [PubMed]
- Raunio P. The role of non-prosthetic surgery in the treatment of rheumatoid arthritis by fusions and auto-arthroplasties. Current practice at the Rheumatism Foundation Hospital, Heinola. Ann Chir Gynaecol Suppl 1985;198:96-102. [PubMed]
- Pooley J, Prasad MG. Arthritis of the elbow: surgical treatment other than by joint replacement. Curr Orthop 1997;11:236-41. [Crossref]
Cite this article as: Pooley J, Van der Linden D. Clinical and pathological perspectives on elbow arthritis and arthroplasty. Ann Joint 2021;6:9.


