Reverse shoulder arthroplasty for proximal humerus fractures
Introduction
Proximal humerus fractures account for about 5% of fractures in adults and are the third most common fracture type (1). Elderly patients are more likely to sustain these injuries with 1/3 of these fractures sustained in patients over 60 years of age or older (2). Optimal treatment of these fractures remain a constant source of debate and is dependent on patient age, activity level, fracture pattern, bone quality, and surgeon preference.
Patients with comminuted, displaced fractures and poor bone quality not amenable to open reduction and internal fixation were traditionally treated surgically with shoulder hemiarthroplasty. Studies showed that results of such cases were dependent on accurate restoration of anatomy, including prosthetic height, version and tuberosity reduction and healing (3). It is for these reasons that the results of hemiarthroplasty for fracture seemed to be either excellent or poor, dependent mainly on tuberosity healing (4,5).
Reverse shoulder arthroplasty provides a treatment option that is less dependent on tuberosity healing to achieve a satisfactory result (6). The function of the nonanatomic arthroplasty is to provide a semiconstrained, fixed fulcrum to allow the deltoid muscle to function without an intact rotator cuff. Medializing the glenohumeral center of rotation and lengthening the arm improve the moment arm of the deltoid (7). Reverse shoulder arthroplasty for fracture has demonstrated similar or improved outcomes when compared to hemiarthroplasty (8-10) and has been shown to be preferred by shoulder surgeons in the treatment of complex proximal humerus fractures in the elderly (11,12).
Indications
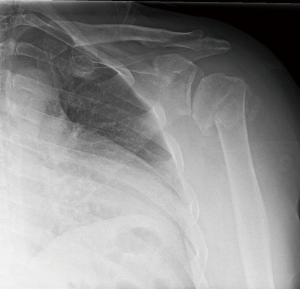
Reverse shoulder arthroplasty is indicated in elderly patients with 3 and 4 part proximal humerus fractures not amenable to plate fixation (Figure 1). Factors such as fracture comminution, rotator cuff tear, preexisting arthritis, fracture of the humeral head, and risks of osteonecrosis and tuberosity nonunion are all weighed in determining surgical technique and implant. Contraindications to reverse shoulder arthroplasty in the acute fracture include permanent axillary nerve injury, brachial plexus injury or deltoid dysfunction. The acromial process and scapular spine should be examined closely as an unrecognized fracture can further displace due to the tension placed on the deltoid after reverse shoulder arthroplasty. Caution should be exercised in cases of open fractures due to risk for infection and in high risk patients with medical comorbidities or inability to comply with postoperative rehabilitation instructions.
Surgical technique
Patients can be positioned in the beach chair or supine position. A regional anesthetic is administered preoperatively, and the operative extremity is confirmed to have full adduction and extension prior to draping. We prefer the deltopectoral approach as it minimizes risk of injury to the axillary nerve although an anterosuperior approach for these fractures has been utilized by some as it allows for a more direct approach to the glenoid in these cases.
The humeroscapular motion interface is released and the fracture is identified. The axillary nerve is palpated deep to the conjoint tendon and protected throughout the procedure. The long head of the biceps tendon is identified and dissected through the intertubercular groove to aid in identification of the tuberosities. We tenodese the biceps to the pectoralis tendon to prevent subsequent cosmetic deformity. The fracture can then be assessed to determine whether the fracture can be repaired. Bone quality, fracture comminution, rotator cuff tearing, condition/attachments to the humeral head, and calcar bone loss are all determining factors in whether to proceed with a reverse replacement.
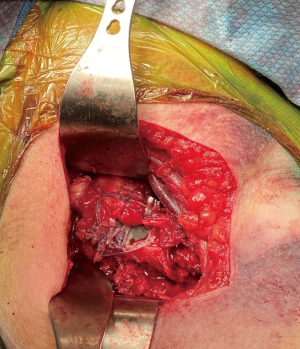
When reverse shoulder arthroplasty is selected, heavy, nonabsorbable sutures are then passed through the tendon-bone junction of the tuberosities. We typically pass four sutures through the greater tuberosity and two sutures through the lesser tuberosity (Figure 2). This allows for control of the tuberosities and aid in future repair. The humeral head is then removed from the surgical field and can be used to harvest autograft.
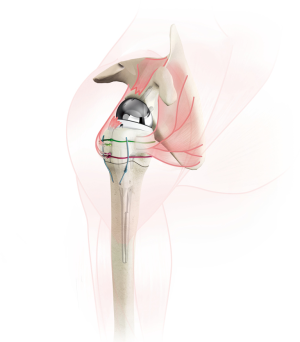
With control of the tuberosities, attention is turned to glenoid preparation. Preoperatively, computed tomography of the shoulder can be utilized for 3-dimensional planning, especially in cases of glenoid bone erosion. As in reverse shoulder arthroplasty for cuff tear arthropathy, we prefer to address any glenoid bone loss with metal augments on the glenoid baseplate (Figure 3). The labrum is resected while protecting the axillary nerve inferiorly. The baseplate guide is applied to the inferior glenoid rim with neutral or slight inferior tilt, and the guidewire is drilled into the glenoid. Reaming is performed to a concentric surface, leaving the subchondral bone intact when possible. The baseplate is then applied, with goal of achieving stable fixation. Glenosphere size is dependent on patient size, anatomy, concern for instability, preventing notching and range of motion without impingement.
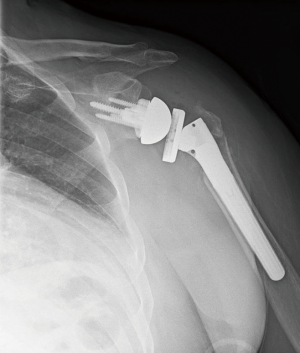
Once the glenosphere has been secured, the humeral diaphysis is prepared. Two drill holes are made in the proximal diaphysis for later suture passage. Traditionally, the humeral prosthesis is secured into the humeral canal with cement. A cement restrictor is placed distally to improve cementing technique, and bone autograft can be placed proximally in the humeral canal to prevent cement extravasation into the tuberosity-stem junction (13). When possible, we prefer not to apply cement into the humeral canal to diminish risks associated with cement including future loosening, extravasation and thermal necrosis. An uncemented humeral stem is only utilized if it is stable to rotational and axial forces. Fracture-specific humeral stems have been developed, featuring suture holes within the prosthesis and bony ingrowth surfaces proximally to aid tuberosity healing (Figure 4). The humeral prosthesis trial is applied, and the tuberosities and medial calcar are commonly used to gauge the proper height of the prosthesis. When there is significant bone loss/comminution, the landmark of the pectoralis tendon inserting 5.6 cm from the top of the humeral head (14) can be utilized, or traction can be applied on the arm after reducing the stem to judge proper tensioning. Our preference is to place the stem in 20 degrees of retroversion relative to the forearm.
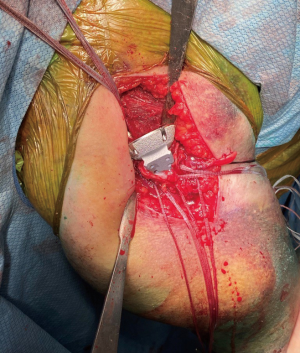
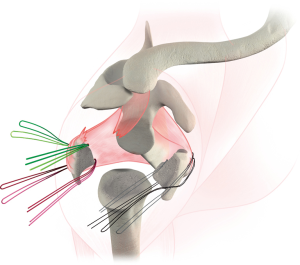
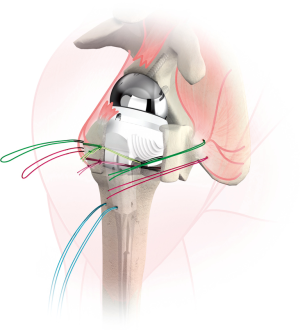
Once the humeral prosthesis is selected, it is secured to the humerus, recreating the height and version of the trial. The arthroplasty is then reduced. Tuberosity healing has been shown to improve rotational motion (15) and is accomplished by securing fractured fragments together and to the stem. The greater tuberosity is anatomically positioned, and two of the sutures through the bone tendon junction are passed through the stem, securing the greater tuberosity to the stem (Figure 5). The remaining two sutures passed through the greater tuberosity are passed through the lesser tuberosity creating a medial cerclage of the tuberosities (16). Bone graft can be inserted between the tuberosities and between the stem and tuberosities to augment healing. Finally, two sutures are passed through two drill holes of the proximal humeral shaft and then passed through the tuberosities to further secure tuberosity repair and impart vertical stability to the construct (Figures 6 and 7). Range of motion of the prosthesis is assessed and fluoroscopic images are taken. We prefer to utilize a deep drain to reduce risk of hematoma formation and the wound is closed in layered fashion and the arm is placed into an abduction sling.
Results
The clinical results of reverse shoulder arthroplasty for fracture are generally good. Multiple studies have compared results of shoulder hemiarthroplasty and reverse shoulder arthroplasty for fractures. In a comparative study examining results 17 patients undergoing hemiarthroplasty for fracture and 16 undergoing reverse shoulder arthroplasty, Gallinet et al. found increased forward flexion, abduction and better Constant scores in patients who had undergone reverse arthroplasty (17). Patients also demonstrated faster return to activity with active motion starting 35 days sooner than the hemiarthroplasty group. It should be noted that in this study, the hemiarthroplasty group demonstrated better external rotation when compared to the reverse shoulder arthroplasty group, likely because the tuberosities were not repaired during reverse shoulder arthroplasty. Fixation of the tuberosities is now recommended in these cases and has been shown to result in increased external rotation and shoulder functional scores (18).
A prospective study of 53 patients who underwent hemiarthroplasty and reverse shoulder arthroplasty for three and four part fractures demonstrated improved forward elevation and better ASES, SST and patient satisfaction scores in the reverse shoulder cohort with minimum 2 year follow up. Three patients who initially were treated with hemiarthroplasty were revised to reverse shoulder arthroplasty due to tuberosity resorption and subsequent shoulder instability (19). Similar findings demonstrating improved outcome measures and diminished complication rate of reverse shoulder arthroplasty compared to shoulder hemiarthroplasty for fractures have been published in other studies (20-22).
While the short term results of reverse shoulder arthroplasty for fracture are encouraging, there are still few long term studies in the literature. Cazeneuve et al. reported on average 6.5 year follow up and found an increase in complication rate and slightly lower strength and Constant scores when compared to short term follow up (21). Reverse shoulder arthroplasty for cuff tear arthropathy similarly demonstrated a decrease in function and Constant scores after 6 year follow up (23). Further studies are needed to examine how factors such as deltoid fatigue, scapular notching and shoulder instability affect long term results of reverse shoulder arthroplasty for fractures as they seem to for cuff tear arthropathy.
Complications
Scapular notching and glenoid loosening have been reported as the most common complication and occurs after reverse shoulder arthroplasty as a result of impaction of the humeral component against the native scapula and as a reaction to polyethylene wear debris. One study reported 73% of patients demonstrated radiologic evidence of glenoid loosening with the use of a Grammont style prosthesis for proximal humerus fractures with 6 year follow up (15). The predictors of scapular notching include superior glenosphere placement, glenosphere offset, patient BMI and altered anatomy in the setting of malunion (23). Lateralized baseplates have demonstrated lower rates of notching (24) although an increased shear force is seen at the baseplate. To date, there have not been any clinical studies comparing the results of lateralized and medialized reverse shoulder arthroplasty for proximal humerus fractures.
While tuberosity healing is not as vital to the success of reverse shoulder arthroplasty as it is for hemiarthroplasty, healing of the tuberosities is associated with improved rotation. Healing rates of the tuberosities has been reported to be 47–100% (25) and nonunion of the tuberosities is associated with diminished range of motion in forward elevation, abduction and external rotation with the arm at the side (26). Tuberosity fixation may also prevent dislocation of the prosthesis as well (27). Reverse specific fracture stems now feature bony ingrowth surfaces and holes for suture passage to aid in tuberosity healing. To our knowledge no clinical studies exist examining whether this technology results in a radiographic or clinical difference in comparison to previous generations.
Neurologic complications have been reported after reverse arthroplasty for fracture at similar rates to other indications. In their series of 43 patients, Bufquin et al. noted that 5 patients demonstrated nerve complications and 3 patients developed reflex sympathetic dystrophy (28). As a result, some have recommended against lengthening the arm more than 2.5 cm in order to prevent nerve injury as well as other complications such as acromial stress fracture and deltoid fatigue (29).
Conclusion
Reverse shoulder arthroplasty is now commonly used in the treatment of complex proximal humerus fractures. When compared to hemiarthroplasty, clinical outcomes are more consistent because success of reverse shoulder arthroplasty is less dependent on tuberosity healing. Advancements in surgical technique and implant design have overall led to good short term results, but there remains a need for further long term studies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Adam Seidl) for the series “Management of Fractures Around the Shoulder” published in Annals of Joint. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aoj-20-15). The series “Management of Fractures Around the Shoulder” was commissioned by the editorial office without any funding or sponsorship. BKL reports other from Arthrex, outside the submitted work. JMI reports other from Shoulder innovations, other from Acumed, other from Arthrex, other from DJO, other from Tornier/Wright Medical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim SH, Szabo RM, Marder RA. Epidemiology of humerus fractures in the United States: nationwide emergency department sample, 2008. Arthritis Care Res 2012;64:407-14. [Crossref] [PubMed]
- Green A, Norris T, Browner B, et al. Proximal humerus fractures and fracture dislocations. Skeletal trauma. 3rd ed. Philadelphia: Saunders. 2003:1532-624.
- Huffman GR, Itamura JM, McGarry MH, et al. Neer Award 2006: biomechanical assessment of inferior tuberosity placement during hemiarthroplasty for four-part proximal humeral fractures. J Shoulder Elbow Surg 2008;17:189-96. [Crossref] [PubMed]
- Sirveaux F, Roche O, Molé D. Shoulder arthroplasty for acute proximal humerus fracture. Orthop Traumatol Surg Res 2010;96:683-94. [Crossref] [PubMed]
- Boileau P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg 2002;11:401-12. [Crossref] [PubMed]
- Klein M, Juschka M, Hinkenjann B, et al. Treatment of comminuted fractures of the proximal humerus in elderly patients with the Delta III reverse shoulder prosthesis. J Orthop Trauma 2008;22:698-704. [Crossref] [PubMed]
- Boileau P, Watkinson DJ, Hatzidakis AM, et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S-161S. [Crossref] [PubMed]
- van der Merwe M, Boyle MJ, Frampton CM, et al. Reverse shoulder arthroplasty compared with hemiarthroplasty in the treatment of acute proximal humeral fractures. J Shoulder Elbow Surg 2017;26:1539-45. [Crossref] [PubMed]
- Mata-Fink A, Meinke M, Jones C, et al. Reverse shoulder arthroplasty for treatment of proximal humeral fractures in older adults: a systematic review. J Shoulder Elbow Surg 2013;22:1737-48. [Crossref] [PubMed]
- Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am 2013;95:2050-5. [Crossref] [PubMed]
- Acevedo DC, Mann T, Abboud JA, et al. Reverse total shoulder arthroplasty for the treatment of proximal humeral fractures: patterns of use among newly trained orthopedic surgeons. J Shoulder Elbow Surg 2014;23:1363-7. [Crossref] [PubMed]
- Savin DD, Zamfirova I, Iannotti J, et al. Survey study suggests that reverse total shoulder arthroplasty is becoming the treatment of choice for four-part fractures of the humeral head in the elderly. Int Orthop 2016;40:1919-25. [Crossref] [PubMed]
- Levy JC. Avoiding cement bone necrosis effect on tuberosity healing: the “black-and-tan” technique. Tech Shoulder Elbow Surg 2013;14:81-4. [Crossref]
- Murachovsky J, Ikemoto RY, Nascimento LG, et al. Pectoralis major tendon reference (PMT): A new method for accurate restoration of humeral length with hemiarthroplasty for fracture. J Shoulder Elbow Surg 2006;15:675-8. [Crossref] [PubMed]
- Gallinet D, Adam A, Gasse N, et al. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:38-44. [Crossref] [PubMed]
- Frankle MA, Ondrovic LE, Markee BA, et al. Stability of tuberosity reattachment in proximal humeral hemiarthroplasty. J Shoulder Elbow Surg 2002;11:413-20. [Crossref] [PubMed]
- Gallinet D, Clappaz P, Garbuio P, et al. Three or four parts complex proximal humerus fractures: hemiarthroplasty versus reverse prosthesis: a comparative study of 40 cases. Orthop Traumatol Surg Res 2009;95:48-55. [Crossref] [PubMed]
- Lenarz C, Shishani Y, McCrum C, et al. Is reverse shoulder arthroplasty appropriate for the treatment of fractures in the older patient?: early observations. Clin Orthop Rel Res 2011;469:3324-31. [Crossref] [PubMed]
- Garrigues GE, Johnston PS, Pepe MD, et al. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics 2012;35:e703-8. [Crossref] [PubMed]
- Boyle MJ, Youn SM, Frampton CMA, et al. Functional outcomes of reverse shoulder arthroplasty compared with hemiarthroplasty for acute proximal humeral fractures. J Shoulder Elbow Surg 2013;22:32-7. [Crossref] [PubMed]
- Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br 2010;92:535-9. [Crossref] [PubMed]
- Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am 2006;88:1742-7. [Crossref] [PubMed]
- Cheung E, Willis M, Walker M, et al. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2011;19:439-49. [Crossref] [PubMed]
- Cuff D, Pupello D, Virani N, et al. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am 2008;90:1244-51. [Crossref] [PubMed]
- Formaini NT, Everding NG, Levy JC, et al. Tuberosity healing after reverse shoulder arthroplasty for acute proximal humerus fractures: the “black and tan” technique. J Shoulder Elbow Surg 2015;24:e299-306. [Crossref] [PubMed]
- Jain NP, Mannan SS, Dharmarajan R, et al. Tuberosity healing after reverse shoulder arthroplasty for complex proximal humeral fractures in elderly patients—does it improve outcomes? A systematic review and meta-analysis. J Shoulder Elbow Surg 2019;28:e78-91. [Crossref] [PubMed]
- Levy JC., Badman B. Reverse shoulder prosthesis for acute four-part fracture: tuberosity fixation using a horseshoe graft. J Orthop Trauma 2011;25:318-24. [Crossref] [PubMed]
- Bufquin T, Hersan A, Hubert L, et al. Reverse shoulder arthroplasty for the treatment of three-and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br 2007;89:516-20. [Crossref] [PubMed]
- Henninger HB, Barg A, Anderson AE, et al. Effect of deltoid tension and humeral version in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 2012;21:483-90. [Crossref] [PubMed]
Cite this article as: Lee BK, Itamura JM. Reverse shoulder arthroplasty for proximal humerus fractures. Ann Joint 2021;6:24.